Abstract
OBJECTIVE
Physical activity (PA), even at low intensity, promotes health and improves hyperglycemia. However, the effect of low-intensity PA captured with accelerometery on glucose variability in healthy individuals and patients with type 1 diabetes has not been examined. Quantifying the effects of PA on glycemic variability would improve artificial endocrine pancreas (AEP) algorithms.
RESEARCH DESIGN AND METHODS
We studied 12 healthy control subjects (five males, 37.7 ± 13.7 years of age) and 12 patients with type 1 diabetes (five males, 37.4 ± 14.2 years of age) for 88 h. Participants performed PA approximating a threefold increase over their basal metabolic rate. PA was captured using a PA-monitoring system, and interstitial fluid glucose concentrations were captured with continuous glucose monitors. In random order, one meal per day was followed by inactivity, and the other meals were followed by walking. Glucose and PA data for a total of 216 meals were analyzed from 30 min prior to meal ingestion to 270 min postmeal.
RESULTS
In healthy subjects, the incremental glucose area under the curve was 4.5 mmol/L/270 min for meals followed by walking, whereas it was 9.6 mmol/L/270 min (P = 0.022) for meals followed by inactivity. The corresponding glucose excursions for those with type 1 diabetes were 7.5 mmol/L/270 min and 18.4 mmol/L/270 min, respectively (P < 0.001).
CONCLUSIONS
Walking significantly impacts postprandial glucose excursions in healthy populations and in those with type 1 diabetes. AEP algorithms incorporating PA may enhance tight glycemic control end points.
Diabetes has reached epidemic proportions, especially in the U.S., and is classified mainly into type 1 and type 2 (1). Although type 2 diabetes constitutes ∼90% of the population burden of diabetes and is classically associated with a BMI that is >27 kg/m2, modern society and improvements in multiple technologies have transformed type 1 diabetes into a disorder that is increasingly associated with obesity (2).
Glycemic control remains a challenge in type 1 diabetes and is associated with extreme glucose variability of hypo- and hyperglycemia (3). An artificial endocrine pancreas (AEP) would represent a significant advance for patients with type 1 diabetes. Closed-loop algorithms for type 1 diabetes are currently being developed (4). Two major reasons for glucose excursions are food and physical activity (PA). How these factors interrelate, especially on an hour-to-hour basis and postprandially, is poorly documented. Incorporation of data from PA sensors into the AEP has the potential to improve the efficacy and safety of the system. Therefore, we examined the impact of levels of PA akin to activities of daily living on glycemic excursions.
Low-cost motion sensors, such as accelerometers, exploit micro-electromechanical systems technology, making it possible to measure daily PA using miniature sensors worn underneath regular clothing. These accelerometers have been proven valid when compared with measurements of total daily energy expenditure performed using doubly labeled water (5). PA data thus captured along with the daily glucose profiles recorded by continuous glucose monitoring (CGM) sensors would enable us to better understand the underlying relationship.
We studied healthy control subjects and patients with type 1 diabetes with the hypothesis that in patients with type 1 diabetes, glucose excursions are blunted by low-intensity PA.
RESEARCH DESIGN AND METHODS
In this article, we discuss certain end points of a prospective study of integrated carbohydrate physiology in healthy people and people with type 1 diabetes. The data presented here are obtained from a larger study that sought to determine the existence of a diurnal pattern of postprandial glucose tolerance in subjects with and without type 1 diabetes (6). The Mayo Clinic Institutional Review Board approved the study. Informed consent was obtained from all participants prior to the initiation of study procedures.
Subjects
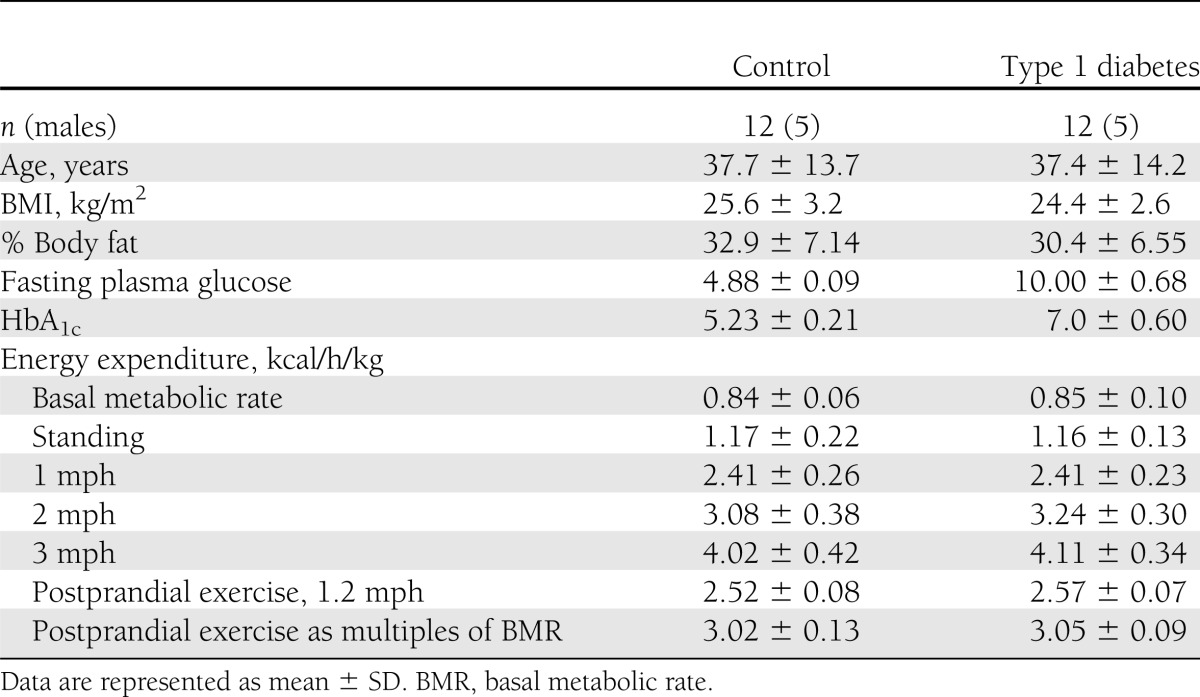
Table 1 shows demographic characteristics of subjects. Each subject underwent two screening visits described below.
Table 1.
Baseline characteristics of study subjects
Screening visit 1.
Subjects reported to our clinical research unit (CRU) in the morning after an overnight fast. A detailed history and physical examination was performed and blood collected to ensure normal/acceptable values for complete blood count, glucose, serum creatinine, electrolytes, thyroid function, and HbA1c. All women of child-bearing potential had a negative pregnancy test within 24 h of the study visit. All subjects met with a dietitian at the time of screening to ensure adherence to a weight-maintaining (fluctuation <2 kg) diet consisting of at least 200 g of carbohydrates per day and meeting American Dietetic Association guidelines for protein, fat, and carbohydrate intake. We also performed a standard urinalysis, resting electrocardiogram, body composition measurement using dual X-ray absorptiometry, and in controls, a 75-g oral glucose tolerance test to ensure normal glucose tolerance. We used a validated gastrointestinal symptoms questionnaire to maximize enrollment of individuals with normal gastrointestinal physiology. Subjects were asked to refrain from unaccustomed PA during this period.
Screening visit 2.
All subjects meeting the inclusion/exclusion criteria after screening visit 1 underwent a gastric emptying study by established scintigraphic techniques to ensure normal gastric motility of solids and liquids. All subjects meeting inclusion/exclusion criteria were studied further within 3 weeks of this screening visit.
Experimental design
All subjects spent 3 days and 4 nights in the CRU. Subjects reported at ∼1600 h on the evening prior to the first study day. Throughout the in-patient stay, DexCom SevenPlus continuous glucose sensor (CGM) and triaxial accelerometer devices were used. The subjects consumed a standard 10 kcal/kg meal (55% carbohydrate, 15% protein, and 30% fat) between 1700 and 1730 h. No additional food was consumed until the next morning. Meal times were fixed at 0700 h for breakfast, 1300 h for lunch, and 1900 h for dinner for the three study days.
CGM calibration
CGM was calibrated per the manufacturer’s instructions with reflectance meter glucose measurements: twice after 2 h of equilibration (1845 h on day 1 of admission) and every 12 h thereafter for the duration of the study.
PA protocol
Subjects wore a PA-monitoring system (PAMS) suit, which allowed body posture and PA to be measured every half second continuously. PAMS has been validated against both room calorimetry and doubly labeled water (5) and consists of a lycra-spandex undergarment with four integrated inclinometers that measure body angle on the right and left lateral aspect of the torso in duplicate and the right and left lateral aspect of the midthigh. Two accelerometers placed at the base of the spine connected to two data loggers (Crossbow Technology, Inc., San Francisco, CA) were worn around the waist. The PAMS suit weighed approximately 1 kg.
The participants performed a carefully planned PA protocol, the adherence to which was captured using PAMS. PAMS data were analyzed using a validated and published method (5). Participants walked 5–6 h each day during the 3-day study period. A walking velocity of 1.9 kmph (1.2 mph) was chosen as consistent with median free living walking velocity (7,8). Each activity bout required the participants to walk at 1.9 kmph for 33.5 min followed by 26.5 min of sitting still, totaling ∼5.6–6.7 kms (3.5–4.2 miles) walked during a 24-h period. The distribution of active and nonactive time during each 24-h period varied depending upon labeled meal schedules, as determined by a random Latin square model whereby one meal per day was followed by the subjects lying in bed for 6 h, and the other two meals were followed by the subjects performing PA. As per protocol, all subjects were asked to retire to bed at 2200 h every night.
Indirect calorimetry
Basal metabolic rate was measured for 30 min using indirect calorimetry in the morning at 0630 h on day 2. Subjects had slept uninterrupted overnight in the CRU, had not moved prior to measurements, and had not eaten since 1900 h the night before. Relaxed subjects lay supine and awake with their head at a 10° tilt. To measure the energy expenditure associated with daily PA, the subjects were seated in a backed, armed office chair with their back, arms, and legs supported.
Indirect calorimetry was performed for 10 min each using a high-precision indirect calorimeter (Columbus Instruments, Columbus, OH) as described previously under the following conditions: 1) standing motionless, relaxed, and still with arms hanging by their sides and feet spaced 6 inches apart; and 2) walking energy expenditure was measured for 10 min each while walking at 1.6, 3.2, and 4.8 kmph (1, 2, and 3 mph) on a calibrated treadmill (True 600, O’Fallon, MO).
Expired air was collected using a full-face transparent dilution mask (Scott Aviation, Lancaster, NY) connected to the calorimeter by leak-proof tubing (Vacumed, Ventura, CA). Breakfast was served at 0700 h, and the energy expenditure measurement started at 1130 h, which is sufficiently spaced from the breakfast to avoid the thermic effect of food.
Study meals
The CRU metabolic kitchen provided all meals over study periods. Study participants received 3 days of weighed meals, three meals each day (at 0700, 1300, and 1900 h), with each meal comprising 33% of total estimated calorie intake based on Harris Benedict calorie requirements. Every meal, irrespective of whether it was an unlabeled mixed meal or a triple-tracer mixed meal, included ∼50 g of carbohydrates because this is the amount used during our triple-isotope tracer studies (9). Each meal thus had a higher fat and protein content compared with a balanced meal. Thus, the overall macronutrients for the labeled as well as unlabeled meals were identical, comprised of 30% carbohydrate, 40% fat, and 30% protein (Supplementary Table 1). One meal daily was randomly selected per Latin square design to include 50 g glucose enriched with [1-13C]glucose at 4% flavored with Jell-O as the sole carbohydrate component. Although the source of carbohydrate had somewhat more variety in the unlabeled mixed meals, there was no difference in the amount of carbohydrate between labeled and unlabeled meals (Supplementary Table 1). All the carbohydrates for the day came from the three meals, and no additional carbohydrates were ingested apart from the meals. As a part of Latin square design, each individual had each meal (breakfast, lunch, and dinner) once followed by a period of inactivity and twice followed by a period of PA equivalent to activities of daily living.
Data analysis
Glucose variability.
Using symmetrized blood glucose data, we calculated low and high blood glucose index. Computing low blood glucose index is of particular importance as it is a good predictor of hypoglycemia (10–12). In the preprandial period, we defined normoglycemia as 3.9–5.6 mmol/L, and in the postprandial period as 5.6–7.8 mmol/L. CGM data for healthy controls as well as participants with type 1 diabetes and insulin infusion data for participants with type 1 diabetes were further analyzed to measure the effect of insulin boluses and PA on premeal and postmeal blood glucose concentrations. The postmeal blood glucose data were further analyzed to measure interday variability between similar meals in control subjects and participants with type 1 diabetes.
To determine the effect of PA on glycemic variability (GV), data were analyzed from the CGM and the PAMS from 30 min prior to meal ingestion until 4.5 h after meal consumption. Basal glucose was defined as the average glucose measurements from the CGM for 30 min prior to meal ingestion to the start of the meal at time zero. CGM and PA data from the three meals spanning three study days were analyzed. As there was no PA after one of the meals per day, the CGM data were grouped based on the presence or absence of PA after meal ingestion. The area above basal calculations was computed for PA and CGM levels. The coefficient of variation (COV) was also used to quantify glucose variability after meal consumption. The COV was computed as the standard deviation of the CGM data measured over the 270 min after the meal divided by the mean blood glucose measurement over the same period of time.
Statistical considerations.
Standard descriptive statistics were used to describe sample characteristics. To test for difference in glucose variability attributable to diabetes status and activity level after meal (inactive vs. active), a randomized, complete-block ANOVA model was fit using SAS PROC MIXED (SAS System, version 9.3, Cary, NC). Model-based means (LSMeans) were estimated to compare across main effects (diabetes status and activity level) as well as to produce comparisons of interest while controlling for the repeated measurements within a participant and the potential interaction effect of diabetes status and activity level. All P values reported are unadjusted for multiple comparisons. Statistical significance was defined as P < 0.05 (two sided).
RESULTS
Patient demographics
Twenty-four subjects equally divided into two groups as healthy control subjects and those with type 1 diabetes successfully completed the study procedures (Table 1).
Energy expenditure
The resting as well as walking energy expenditure is expressed corrected for body weight (Table 1). When corrected for body weight, the energy expenditure showed a significant linear response to progressive increases in walking velocity for all the participants.
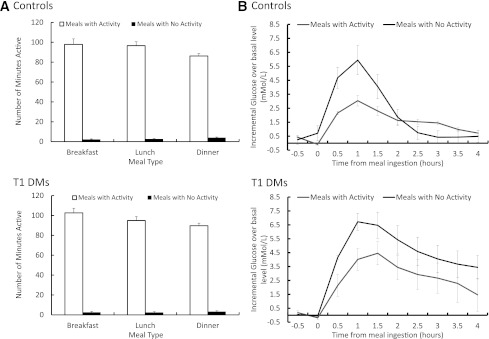
The basal (premeal) PA levels were similar between type 1 diabetic participants and control subjects prior to meal ingestion in the presence (P = 0.60) or absence of PA 6 h prior (P = 0.22). Although the number of minutes spent walking after meals with activity was identical between groups, there was slightly less time spent walking after dinner than breakfast (Fig. 1A). This was because all subjects were encouraged to retire to bed at 2200 h every night.
Figure 1.
A: Average PA in minutes after meals with activity and meals without activity for control and type 1 diabetic participants. B: Incremental CGM over basal levels in mmol/L with and without PA, for healthy control and type 1 diabetic participants. Data are represented in mean ± SE (n = 24). T1 DMs, type 1 diabetic participants.
CGM glucose excursions
Baseline CGM glucose concentration in the control subjects was 5.61 ± 0.11 mmol/L during meals with activity and 5.58 ± 0.29 mmol/L during meals without activity. The peak CGM glucose concentration in the same subjects was 8.25 ± 0.23 mmol/L during meals with activity and 11.99 ± 0.67 mmol/L during meals without activity. In the type 1 diabetic subjects, baseline CGM glucose concentration was 10.00 ± 0.78 mmol/L at meals with activity and 8.10 ± 0.34 mmol/L during meals without activity (P < 0.001), and also varied based on PA 6 h prior (P = 0.01 for interaction effect). The peak CGM glucose concentration in the type 1 diabetic subjects was 14.46 ± 0.47 mmol/L at meals with activity and 15.43 ± 0.53 mmol/L during meals without activity. This supported the analysis of the incremental changes in glucose levels (i.e., incremental area over basal levels).
In the control subjects, for the meals followed by PA, the incremental glucose area above basal (iAUC) was estimated to be 4.5 mmol/L/270 min (95% CI 0.9–8.1 mmol/L/270 min) and 9.6 mmol/L/270 min (95% CI 6.0–13.2 mmol/L/270 min) for meals followed by inactivity, which is an increase of 113% (P = 0.024) (Figs. 1B and 2B) for meals followed by PA.
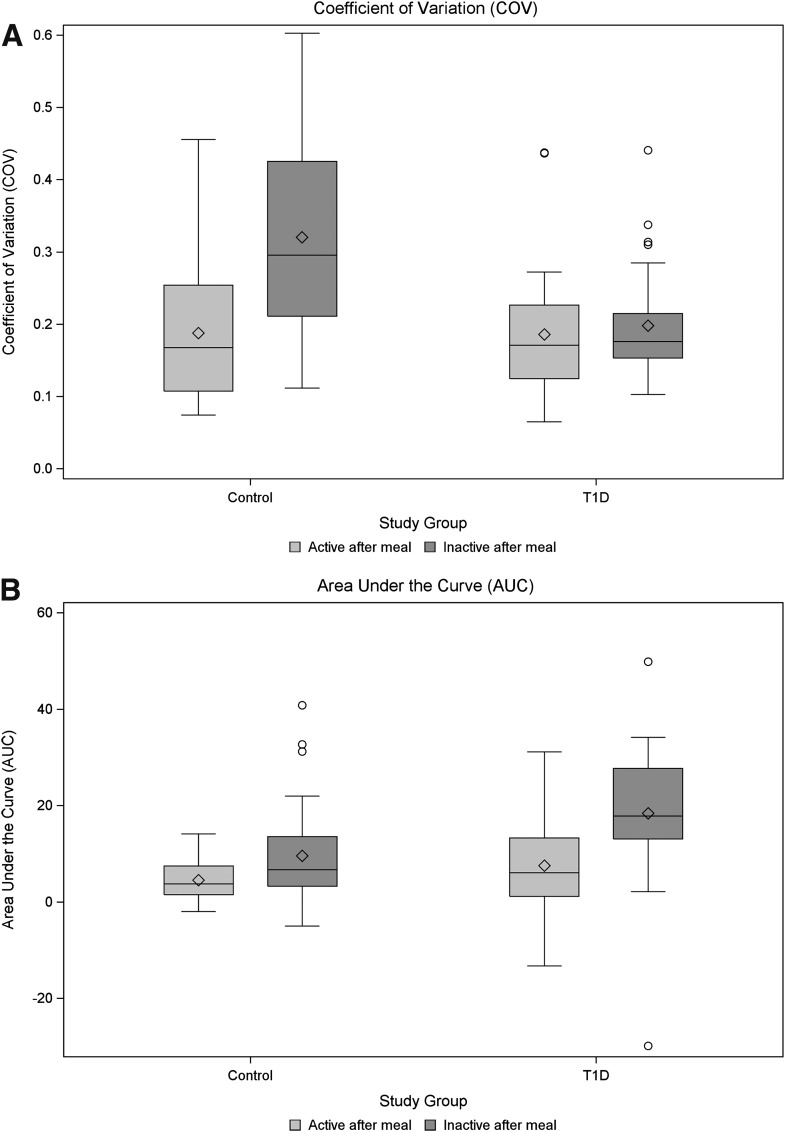
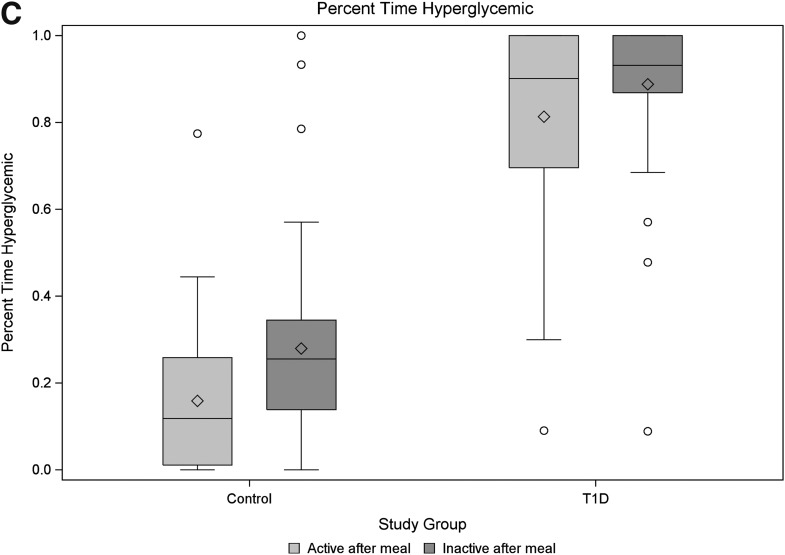
Figure 2.
Box plots of area under the curve (A), COV (B), and percentage time hyperglycemic (C) between control and type 1 diabetic participants by activity status after the standardized meals. P values reported are based on comparisons of model-based means from a mixed model consisting of fixed effects of diabetes status and activity level after meal and their interaction, along with a random effect for participant (blocking factor). (Fig. 2 continues on p. 2498.)
In the type 1 diabetic participants, the iAUC was 7.5 mmol/L/270 min (95% CI 3.9–11.1 mmol/L/270 min) for the meals followed by PA and 18.4 mmol/L/270 min (95% CI 14.8–22.0 mmol/L/270 min) for meals followed by inactivity, representing an increase of 145% (P < 0.001) (Figs. 1B and 2B).
In meals followed by inactivity, the iAUC was statistically higher for type 1 diabetic participants compared with controls (estimated difference, 8.8 mmol/L/270 min; 95% CI 3.7–13.9; P = 0.001), whereas in meals that were followed by activity, the iAUC did not differ statistically between study groups (estimated difference, 3.0 mmol/L/270 min; 95% CI −2.1 to 8.1; P = 0.24).
Participants with type 1 diabetes responded differently than the controls to PA after meals in terms of GV, as quantified by COV. Although the estimated mean COV was higher for meals followed by inactivity relative to those with activity (P < 0.001), this finding was confounded by a significant study group by activity interaction term (P < 0.001). Thus, although the healthy controls showed increased variability due to inactivity (P < 0.001), the participants with type 1 diabetes did not demonstrate the same pattern. Notably, the COV did not differ in the participants with type 1 diabetes across activity levels (P = 0.54) (Fig. 2A), and the COV for type 1 diabetic participants was, on average, lower than the controls (P = 0.036). This finding was assumed to be the result of a “ceiling” effect (lack of variability due to maximal blood glucose readings in the type 1 diabetic participants). To address this potential limitation, the percentage of time in the hyperglycemic range was also compared using the same methodology. Using this outcome measure, type 1 diabetic participants were estimated to have 85% of their blood glucose readings as hyperglycemic vs. only 22% in the controls (P < 0.001). Inactivity after a meal was found to increase the percentage of time hyperglycemic by 10% (P = 0.001); however, as with the COV, the effects due to inactivity were blunted in the type 1 diabetic participants (Fig. 2C).
The type 1 diabetic participants prior to the labeled meals received an average of 1.61 (range, 0–3) insulin boluses between 3 and 1 h prior to each meal in addition to their mealtime insulin boluses. Insulin boluses were provided to cover a meal based on each individual’s bolus insulin program. The insulin-to-carb ratio and insulin sensitivity factor used to dose bolus insulin were uniform throughout the study period for each participant. The average insulin bolus for labeled meals was 6.15 ± 0.70 units, whereas it was 4.84 ± 0.27 units for an unlabeled meal (P = 0.009).
CONCLUSIONS
Public health guidelines suggest 30 min of PA every day (13,14). Even half the recommended PA has been shown to improve mortality rate (15). In patients with cardiovascular disease, rehabilitation with moderate PA has been shown to improve overall quality of life (16). Therefore, PA has well-documented health-associated benefits. PA has a direct impact on glucose excursions. We wanted to quantify the effect of PA on GV in the postprandial state. The results from this study indicate that performing low-grade PA after meals, such as immediately attending to dishes and chores of daily living, equivalent to taking a short walk, has a potential benefit in participants by lowering postprandial glucose excursions.
Past studies examining the effect of PA on glucose control often use %VO2 max as a predictive measure of PA. Aerobic capacity as measured by %VO2 max, although precise, is not practical in quantifying daily free-living activities (17,18). Accelerometers constitute a practical way to quantify PA. Recent studies have used accelerometers during low and moderate PA in healthy populations and attempted correlation with changes in postprandial glucose concentrations (19,20). Neither study performed CGM but attempted correlation with plasma glucose. This approach is the accepted standard but does not allow translation to AEP protocols. Dunstan et al. (20) report that light and moderate PA has a glucose-lowering effect. Stephens et al. (19) report that short bursts of sitting also change the rate of glucose disappearance. The results from these studies are consistent with our findings that low-grade PA results in increased time spent in the euglycemic range. To our knowledge, ours is the first study to report this in healthy control subjects and age- and sex-matched individuals with type 1 diabetes and to simultaneously use CGM with accelerometer, thus permitting translation to AEP algorithms in the near future. The effect of low-grade PA on glucose status in type 1 diabetes raises the question of such activity contributing significantly to episodes of hypoglycemia during free living. This will need to be tested in prospective studies.
The current study does not permit direct evaluation of the relative contributions of physiological variables (e.g., changes in peripheral insulin sensitivity and hepatic insulin sensitivity), modulated by activities of daily living on postprandial glucose excursions. However, with ongoing and future studies, using state-of-the-art isotope dilution techniques, we hope to tease out the aforementioned physiological mechanisms that underlie these observations. To our knowledge, there are no available prior studies that have investigated these variables using modern methodologies, especially during low-grade PA.
Although the overall effects of PA on glycemic variability are evident from the results shown here, they have potential implications for patients with diabetes. Glucose control in diabetes remains suboptimal (21). Reasons for suboptimal control include inability to adhere to energy intake for several reasons, inability to increase PA optimally, and fear of hypoglycemia interfering with tight glycemic goals and optimal PA (22).
Therefore, current and future approaches will have to be safer. A convenient and accurate system that records and displays PA in real time would enable better adherence of patients with diabetes to healthy lifestyle measures. This approach could therefore constitute logical “therapeutic” modalities for the immediate future. Establishing a predictable temporal relationship between PA and glucose excursion is of particular importance for building physiological models that could help prevent events related to hyperglycemia and hypoglycemia. These models could then be incorporated into more optimal closed-loop systems of glucose: insulin management.
Our PA capture system is accurate and correlates with GV, thus setting the stage for more intensive trials in the near future. PAMS in its current configuration is designed specifically for laboratory-based studies and is bulky and cumbersome for use during free living. New sensor systems have been developed that allow us to incorporate other techniques for quantifying PA, such as heart rate monitors (23,24).
We acknowledge that the study has limitations. First, it was conducted in a controlled environment, and the PA protocol was tightly controlled. Second, the sample size was small. Furthermore, it is noteworthy that type 1 diabetic participants received insulin boluses not infrequently (30 out of a possible 36 times) prior to their labeled meals in an effort to get their premeal glucose concentrations <8.33 mmol/L, as per the study design. It is likely that these insulin preboluses could have dampened postprandial CGM excursions after the labeled meals. Hence, the net effect on the size of our observations reported here is very likely an underestimate of the differences in postprandial glucose excursions between labeled meals followed by inactivity and unlabeled meals followed by activity in type 1 diabetic participants where no insulin preboluses were provided. Therefore, the findings from this study are even more significant, and further studies will expand on these findings with respect to free-living interventions. In addition, peak CGM glucose concentrations were elevated more than expected in the control subjects.
Differences between our data and that of previous reports (25,26) could be explained by factors that could be related to study design, subject biologic issues, and technical factors.
Study design
To our knowledge, ours is the first study capturing data for a 72-h period. Feeding the same individual 33% of their energy needs in each of three meals may produce a different pattern compared with a single meal. Second, previous reports may have prior exercise resulting in more efficient handling of the meal (25,26). For instance, the study by Short et al. (26) included 75% VO2 HRmax exercise within 24 h of the mixed meal. Third, caloric intake, carbohydrate content, and fat content may be different in previous reports.
Study population
Previous studies enrolled younger subjects with leaner BMI. Decrease in insulin action with age and increased BMI is most likely responsible for higher peak glucose concentrations in our dataset.
Technical factors
CGM sensors are not as accurate and precise as laboratory measurements and may provide higher measurements but are an essential component for field research studies (studies during ambulatory care and closing the loop for type 1 diabetes).
In conclusion, we demonstrate for the time, to our knowledge, that slow-pace walking improves postprandial glucose excursion in healthy controls and people with type 1 diabetes. These findings have relevance for algorithms being developed for AEP and could also be tested as a therapeutic modality in multiple metabolic contexts.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-085516 (to A.B. and Y.C.K.) and by the National Institutes of Health (NIH)/National Center for Research Resources Clinical and Translational Science Award (CTSA) UL1-RR-024150.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
No potential conflicts of interest relevant to this article were reported.
C.M. and R.E.C. contributed to writing the manuscript and were primarily responsible for data collection, integrity, and analysis. J.A.L., R.B., A.B., and Y.C.K. contributed to writing the manuscript. D.K.N., A.S., and S.K.M.-S. participated in recruitment and conducted the research study. C.D.M. and C.C. were primarily responsible for data collection, integrity, and analysis. Y.C.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the staff at the CTSA and research volunteers and their families.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2381/-/DC1.
References
- 1.Genuth S, Alberti KG, Bennett P, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–3167 [DOI] [PubMed] [Google Scholar]
- 2.Orchard TJ. The impact of gender and general risk factors on the occurrence of atherosclerotic vascular disease in non-insulin-dependent diabetes mellitus. Ann Med 1996;28:323–333 [DOI] [PubMed] [Google Scholar]
- 3.Kovatchev BP. Is glycemic variability important to assessing antidiabetes therapies? Curr Diab Rep 2006;6:350–356 [DOI] [PubMed] [Google Scholar]
- 4.Cobelli C, Renard E, Kovatchev B. Artificial pancreas: past, present, future. Diabetes 2011;60:2672–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine JA, Lanningham-Foster LM, McCrady SK, et al. Interindividual variation in posture allocation: possible role in human obesity. Science 2005;307:584–586 [DOI] [PubMed] [Google Scholar]
- 6.Saad A, Dalla Man C, Nandy DK, et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes. 29 June 2012 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conover CA, Mason MA, Levine JA, Novak CM. Metabolic consequences of pregnancy-associated plasma protein-A deficiency in mice: exploring possible relationship to the longevity phenotype. J Endocrinol 2008;198:599–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ezekowitz MD, Levine JA. Preventing stroke in patients with atrial fibrillation. JAMA 1999;281:1830–1835 [DOI] [PubMed] [Google Scholar]
- 9.Basu R, Di Camillo B, Toffolo G, et al. Use of a novel triple-tracer approach to assess postprandial glucose metabolism. Am J Physiol Endocrinol Metab 2003;284:E55–E69 [DOI] [PubMed] [Google Scholar]
- 10.Cox DJ, Gonder-Frederick LA, Kovatchev BP, Julian DM, Clarke WL. Understanding error grid analysis. Diabetes Care 1997;20:911–912 [DOI] [PubMed] [Google Scholar]
- 11.Kovatchev BP, Cox DJ, Farhy LS, Straume M, Gonder-Frederick L, Clarke WL. Episodes of severe hypoglycemia in type 1 diabetes are preceded and followed within 48 hours by measurable disturbances in blood glucose. J Clin Endocrinol Metab 2000;85:4287–4292 [DOI] [PubMed] [Google Scholar]
- 12.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke W. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care 1998;21:1870–1875 [DOI] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans, 2008. Available from http://www.health.gov/paguidelines/ Accessed 25 June 2012
- 14.World Health Organization. Global Recommendations on Physical Activity for Health Global Strategy on Diet, Physical Activity and Health, 2011. Available from http://www.who.int/dietphysicalactivity/factsheet_recommendations/en/ Accessed 25 June 2012
- 15.Wen CP, Wai JP, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet 2011;378:1244–1253 [DOI] [PubMed] [Google Scholar]
- 16.Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med 2004;116:682–692 [DOI] [PubMed] [Google Scholar]
- 17.Lazzer S, Boirie Y, Bitar A, Petit I, Meyer M, Vermorel M. Relationship between percentage of VO2max and type of physical activity in obese and non-obese adolescents. J Sports Med Phys Fitness 2005;45:13–19 [PubMed] [Google Scholar]
- 18.Dvorak RV, Tchernof A, Starling RD, Ades PA, DiPietro L, Poehlman ET. Respiratory fitness, free living physical activity, and cardiovascular disease risk in older individuals: a doubly labeled water study. J Clin Endocrinol Metab 2000;85:957–963 [DOI] [PubMed] [Google Scholar]
- 19.Stephens BR, Granados K, Zderic TW, Hamilton MT, Braun B. Effects of 1 day of inactivity on insulin action in healthy men and women: interaction with energy intake. Metabolism 2011;60:941–949 [DOI] [PubMed] [Google Scholar]
- 20.Dunstan DW, Kingwell BA, Larsen R, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care 2012;35:976–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergenstal RM, Tamborlane WV, Ahmann A, et al. STAR 3 Study Group Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010;363:311–320 [DOI] [PubMed] [Google Scholar]
- 22.Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 1987;10:617–621 [DOI] [PubMed] [Google Scholar]
- 23.Man CD, Breton MD, Cobelli C. Physical activity into the meal glucose-insulin model of type 1 diabetes: in silico studies. J Diabetes Sci Tech 2009;3:56–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breton MD. Physical activity-the major unaccounted impediment to closed loop control. J Diabetes Sci Tech 2008;2:169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen E, Høstmark AT. Effect of a single bout of resistance exercise on postprandial glucose and insulin response the next day in healthy, strength-trained men. J Strength Cond Res 2007;21:487–491 [DOI] [PubMed] [Google Scholar]
- 26.Short K, Pratt L, Teague A. The Acute and Residual Effect of a Single Exercise Session on Meal Glucose Tolerance in Sedentary Young Adults. J Nutr Metab 2012;2012:278678 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.