Abstract
OBJECTIVE
Cross-sectional studies link both depressive symptoms (DS) and diabetes-related distress (DRD) to diabetes self-management and/or glycemic control. However, longitudinal studies of these variables are rare, and their results are somewhat conflicting. The study objective was to compare DS and DRD as longitudinal predictors of medication adherence, self-care behavior, and glycemic control in type 2 diabetes.
RESEARCH DESIGN AND METHODS
Primary care patients with type 2 diabetes reported DS, DRD, and other variables at baseline were studied. Medication adherence, self-care behaviors (diet, physical activity, and glucose testing), and glycemic control (HbA1c) were assessed 6 months later (n = 253). Cross-sectional and longitudinal regression analyses were used to model behavioral and medical outcomes as a function of baseline confounders, DS, and DRD.
RESULTS
Adjusted cross-sectional and longitudinal analyses yielded very similar results. In the latter, only DS were significantly associated with future diet behavior (P = 0.049), physical activity (P = 0.001), and glucose testing (P = 0.018). In contrast, only DRD predicted future glycemic control (P < 0.001) and medication adherence (P = 0.011).
CONCLUSIONS
Distress-outcome associations seem to vary by type of distress under consideration. Only DS predicts future lifestyle-oriented self-management behaviors. In contrast, only DRD predicts glycemic control, perhaps by decreasing medication adherence. Clinical assessment and intervention should encompass both types of distress, unless the goal is to narrowly target a highly specific outcome.
Depressive symptoms (DS) are prevalent in type 2 diabetes (1,2) and are associated with a wide variety of deficits in diabetes self-management behavior and glycemic control (3,4). Although most existing studies are cross-sectional, only the more powerful longitudinal design can demonstrate temporal priority, which is a necessary (but not sufficient) precondition for establishing causality. One longitudinal study linked DS to poor medication adherence and poorer self-management (diet, exercise, and foot care) that was measured 9 months later (5), whereas a second study indicated that persistent or worsening DS were associated with poor self-management in terms of diet and exercise over the subsequent 5 years (6). Turning to glycemic control, multiple clinical trials indicate when depression accompanying diabetes is successfully treated, metabolic control does not tend to improve (7). Moreover, one longitudinal study concluded that DS do not predict future glycemic control, but rather that glycemic control actually predicts future DS (8). However, a small meta-analysis of depression treatment trials found that DS reduction was associated with glycemic improvement in two of the three studies analyzed (4). To summarize, DS may predict self-management behavior, whereas the nature of their longitudinal association with glycemic control remains unclear.
In contrast to the generalized concept of DS, diabetes-related distress (DRD) refers to significant negative psychological reactions that are specific to one’s diabetes diagnosis, potential or actual complications, self-management burdens, difficult patient–provider relationships, and problematic interpersonal relationships (9). Due to its illness-specific nature, DRD is conceptually and empirically distinct from DS that accompany diabetes (10,11). Cross-sectional studies have consistently indicated that, like DS, DRD is associated with concurrent diabetes self-management (12–15) and glycemic control (12,15). Although no longitudinal studies have shown a link between DRD and self-management, two studies demonstrated that DRD predicts subsequent glycemic control (16,17).
Given the variation and gaps in reported associations, the most recent wave of studies has directly compared the independent predictive value of DS versus DRD when levels of both variables are accounted for. This strategy helps to disentangle which type of distress has the most substantial association, which helps to identify underlying biobehavioral mechanisms and determine priorities for clinical assessment and modification. Two such cross-sectional studies indicated that DRD, but not DS, is associated with self-management behaviors (11,16,18), whereas a third study concluded that only DS are associated with self-management (10). Three cross-sectional studies indicated that only DRD is independently related to concurrent (11,17,19) glycemic control. Of these three studies, one additionally demonstrated that only DRD was related to concurrent control (17). Moreover, although neither DS nor DRD predicted future glycemia, DRD and glycemic control varied together across 18 months.
To summarize, several studies link DS and DRD to diabetes self-management, glycemic control, or both. Longitudinal studies are rare, have yielded conflicting findings, and have not evaluated whether DRD predicts future self-management. Of the few studies that simultaneously evaluate these two types of distress, all but one are cross-sectional. The goal of this study was therefore to clarify the longitudinal associations between two types of psychological distress (DS and DRD) and key outcomes (self-management behaviors and glycemic control) measured 6 months later.
RESEARCH DESIGN AND METHODS
Participants
Potential participants were identified from the administrative and clinical databases of a large Midwestern urban healthcare system. Eligible patients were required to have type 2 diabetes as indicated by at least one of the following: 1) at least one hospitalization with a diabetes-related ICD-9 code (250.×, 357.2, 362.0, or 366.41), 2) at least two outpatient visits with a diabetes-related ICD-9 code or at least one prescription for a glucose-control medication or monitoring supplies, 3) between 18 and 80 years of age, and 4) able to complete self-report instruments. Type 1 diabetes was subsequently ruled out through telephone screening.
Procedure
Following an institutional review board–approved protocol, eligible patients were mailed a study invitation followed by a telephone call for screening. Following informed consent, participants attended two research appointments separated by 6 months for the assessment of adherence, glycemic control, and other variables.
Measurements
Depressive symptoms were assessed with the widely used and well-validated Patient Health Questionnaire-9 (PHQ-9), which includes only depressive symptoms per se, unlike most other DS measures. Although it was indeed developed as a screening measure, it is well-validated as a measure of DS severity (20). DRD was measured using the Problem Areas in Diabetes scale (PAID) (16), a well-validated 20-item self-report questionnaire. Items are rated on a 5-point scale ranging from 0 (not a problem) to 4 (a serious problem). Summed scores are converted to a 0–100 scale by multiplying by 1.25. Medication adherence was assessed using the Morisky scale, which elicits information about the presence or absence of various forms of nonadherence (e.g., “In general, are you careless at times about taking your antidepressant medication?”) (21). Each item is in a yes or no format with a maximum possible score of four. It has demonstrated concurrent and predictive validity and adequate internal consistency (21) and has been used for >20 years with numerous chronic diseases. For the detection of nonadherence, sensitivity is 0.81, and specificity is 0.44. In type 2 diabetes, the measure has good reliability and predictive validity, and its scores are associated with a 10% increase in concurrent HbA1c (22) as well as negative attitudes toward diabetes medication (23). Diabetes self-care behavior frequencies were assessed with the Summary of Diabetes Self-Care Activities (SDSCA) (24). Respondents rate how many days of the past week they engaged in self-care behaviors reflecting diet, exercise, glucose testing, foot care, and cigarette smoking. The SDSCA has adequate internal and test–retest reliability, is sensitive to change, and correlates appropriately with other measures of adherence and related constructs (24). Glycemic control (HbA1c) was measured with the DCA 2000 (GMI, Inc., Ramsey, MN), which analyzes capillary blood samples through a monoclonal antibody method. Comorbid medical illness was assessed by abstracting electronic medical records using a checklist of common medical illnesses (asthma, chronic obstructive lung disease, congestive heart failure, osteoarthritis, rheumatoid arthritis, arthritis associated with lupus [systemic lupus erythematosus] or scleroderma, peripheral vascular disease, cirrhosis, chronic hepatitis, coronary artery disease thyroid disease, Addison disease, and Cushing syndrome) (25,26). Presence of diabetes complications was measured using a standard self-report checklist of visual, cardiovascular, kidney, genitourinary, and other common diabetes complications taken from the Diabetes Care Profile (27). Participants classified themselves using U.S. Census racial/ethnic categories. Socioeconomic status (SES) was assessed using the U.S. Census Bureau Index of Socioeconomic Status adjusted for the regional Consumer Price Index (28).
Data analysis
Data were analyzed using STATA 11.2 software (StataCorp, College Station, TX). Descriptive analyses were conducted to characterize the sample, and distributions were visually and quantitatively examined for normality of distribution. A matrix of zero-order Pearson correlations were examined to identify bivariate relationships between potential demographic and medical confounders of Month 6 outcomes, using the criterion of two-tailed P < 0.05. The associations of both DS and DRD variables (measured at baseline) and Month 6 outcomes were analyzed using ordinary least squares regression, with potential confounders included in the model and also using the criterion of two-tailed P < 0.05. Missing data were not imputed. Because DRD and DS scores are moderately interrelated (10,15), they were evaluated both alone and when the other was adjusted for, because their multicollinearity may distort their individual regression coefficients.
RESULTS
Enrollment and retention
Of the 420 patients screened by telephone, 332 met entry criteria, 287 (86%) of whom provided consent and baseline data. Thirty-four participants (12%) dropped out after baseline, leaving 253 study completers. Attrition was significantly associated with being <60 years of age (85% of dropouts vs. 74% of nondropouts; P < 0.014) and being African American (74 vs. 55%; P = 0.036). However, attrition was not significantly related to gender, SES, medication adherence, or poor glycemic control. Consent was unrelated to age and sex, although African Americans were more likely to consent than Caucasians (62 vs. 52%; P = 0.025).
Sample characteristics
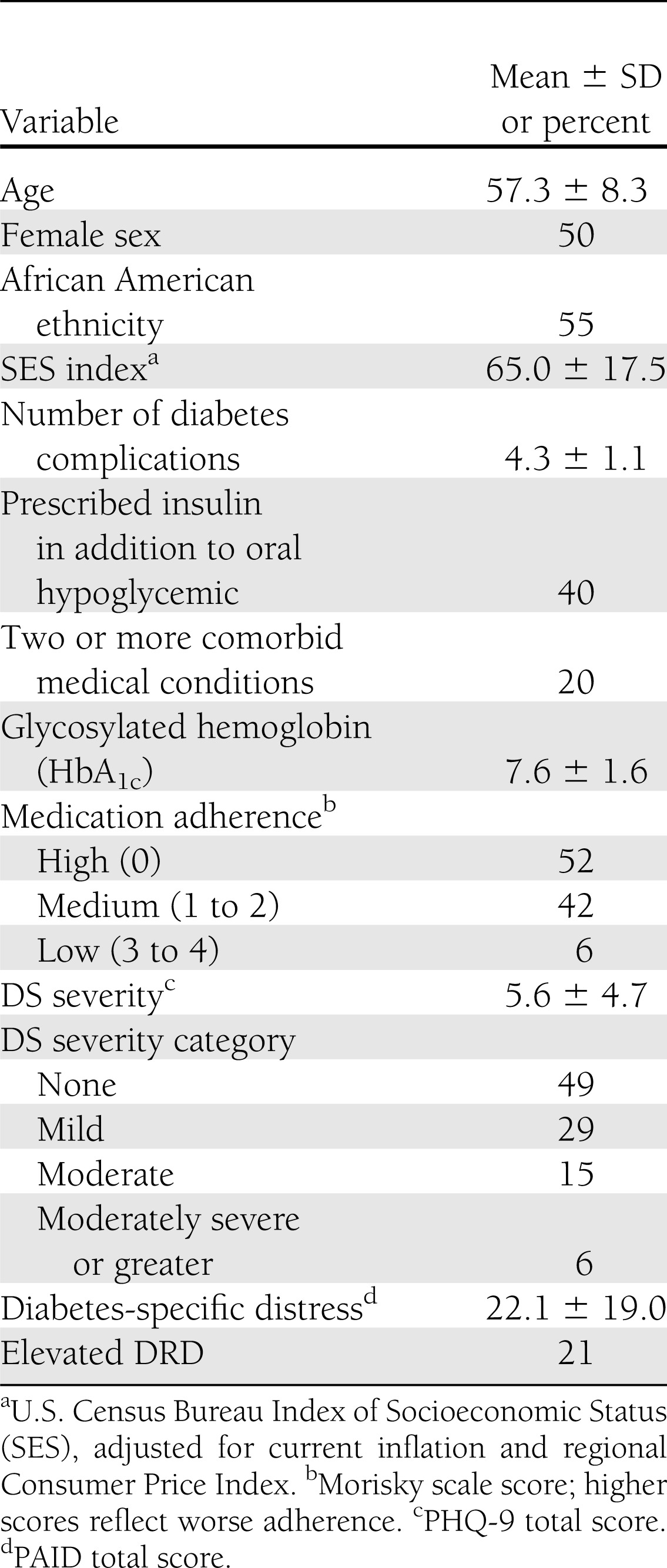
The characteristics of study completers are shown in Table 1. Half were female, 55% were African American, and age ranged from 27–88 years (mean 57.3 ± 8.3). Forty percent were prescribed insulin in addition to an oral hypoglycemic. Glycemic control (HbA1c) was generally poor (mean 7.6 ± 1.6), complications were common (mean 4.3 ± 1.1), and 20% had at least two significant comorbid medical conditions. Based upon Morisky scores, 52% percent of the participants could be classified with high adherence (i.e., score of 0), 42% with medium adherence (scores of 1 to 2), and 6% with low adherence (score of 3 to 4). Approximately half had a baseline PHQ-9 total >5 (indicating at least mild DS), with 15% reporting moderate and 6% reporting moderately severe DS. The PAID score had a mean of 22.1 ± 19, with 21% of participants scoring above the distressed cutoff.
Table 1.
Sample characteristics (n = 253)
Preliminary analysis to identify confounders
Bivariate and multiple regression analyses revealed that higher HbA1c was reliably associated with younger age (r = 0.30; P < 0.001), male sex (r = 0.16; P = 0.006), and being on insulin (r = 0.16; P = 0.006). Poor medication adherence was associated with being younger (r = 0.15; P = 0.012) and having fewer comorbid medical conditions (r = 0.16; P = 0.013). Poor dietary adherence was associated with not being on insulin (r = 0.14; P = 0.020). Lower frequency of blood glucose self-testing was associated with being male and not being on insulin (r = 0.31; P < 0.001).
Cross-sectional analyses at baseline
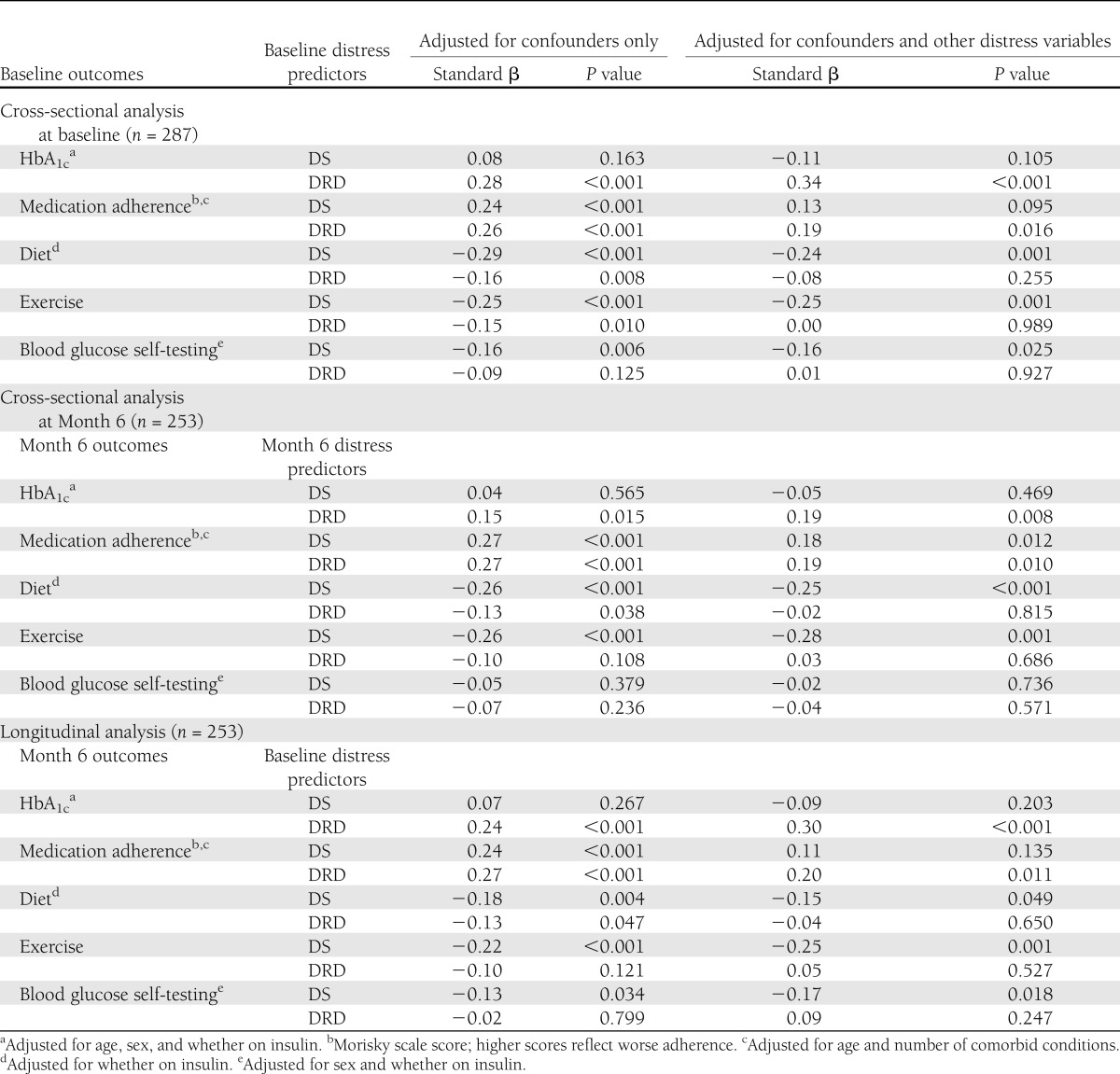
Regression analysis, adjusted for the confounders noted above, was used to evaluate the relationships among DS, DRD, and concurrent outcomes observed at baseline (Table 2, top section). Although DS were not significantly associated with HbA1c, it was associated with poorer medication adherence (β = 0.24; P < 0.001) and lower frequencies of diet (β = −0.29; P < 0.001), exercise (β = −0.25; P < 0.001), and testing behavior (β = −0.16; P = 0.006). These associations were unchanged after adjusting for DRD, except that DS were no longer significantly associated with medication adherence. Next, DRD was significantly associated with higher HbA1c (β = 0.28; P < 0.001), lower medication adherence (β = 0.26; P < 0.001), and lower frequencies of both diet (β = −0.16; P = 0.008) and exercise behaviors (β = −0.15; P = 0.010), but not of blood glucose testing behavior. Only the associations with HbA1c and medication adherence remained statistically significant after adjusting for DS.
Table 2.
Results of cross-sectional and longitudinal regression analyses
Cross-sectional analyses at Month 6
When the above cross-sectional analyses were repeated on Month 6 data, the results were nearly identical (Table 2, middle section). That is, the pattern of significance for the fully adjusted models was the same for 8 of 10 coefficients. The two exceptions were that the concurrent association between DS and poor medication adherence became significant at Month 6 (β = 0.18; P = 0.012), and the relationship between DS and testing frequency was no longer significant.
Longitudinal analysis
The above multiple regression analyses were repeated to evaluate the associations of baseline DS and DRD with Month 6 glycemic control after adjusting for age, sex, and regimen and both before and after adjusting for each other. Baseline DRD had a significant association with Month 6 HbA1c after adjusting for confounders (β = 0.30; P < 0.001) as well as for DS (β = 0.30; P < 0.001), whereas baseline DS did not have a significant association with Month 6 HbA1c in either model (Table 2).
Next, Month 6 medication adherence was analyzed after adjusting for age and number of comorbid conditions. As found in both cross-sectional analyses, baseline DS were a significant predictor before adjusting for DRD (β = 0.24; P < 0.001). However, as seen in baseline (but not Month 6) cross-sectional analyses, this effect became nonsignificant after adjusting for DRD. As found in both cross-sectional analyses, DRD had a significant effect both before adjusting for DS (β = 0.27; P < 0.001) as well as after (β = 0.20; P = 0.011). The valence of this latter association indicated that higher DRD at baseline was associated with poorer medication adherence at Month 6.
In agreement with the results of both cross-sectional analyses, baseline DS were a significant predictor of Month 6 diet behavior (adjusted for regimen), before adjusting for DRD (β = −0.18; P = 0.004) as well as after (β = −0.15; P = 0.049). Also, as found in both cross-sectional analyses, the significant longitudinal association between DRD and diet behavior (β = −0.13; P = 0.047) was attenuated after adjusting for DS (β = −0.04; P = 0.650).
For the prediction of Month 6 exercise, baseline DS had a significant effect (β = −0.22; P < 0.001) after adjusting for sex and regimen, and this was unaffected by adjusting for DRD (β = −0.25; P = 0.001). This result was consistent with the results of both cross-sectional analyses, as was the finding that DRD was not significantly related to exercise after adjustment for DS.
Finally, the longitudinal findings for Month 6 glucose testing showed that after adjusting for sex and regimen, baseline DS had a significant association with testing behavior both before (β = −0.13; P = 0.034) and after (β = −0.17; P = 0.018) the model was adjusted for DRD. The valences of these coefficients indicated that higher baseline DS were associated with lower levels of self-management behavior at a Month 6. In contrast, DRD was not a significant predictor in either the unadjusted or adjusted model.
Additional analyses
Because it is possible that DS and DRD may correspond more closely at low levels of distress, a scatter plot of DS and DRD was examined. The linear and lowest fitted values corresponded very closely, and the residuals appeared to be constant across all fitted values. Additionally, the Pearson correlations of DS with DRD did not vary when separately computed below and above the DRD median (0.33 vs. 0.35) or below and above the DS median (0.35 vs. 0.38). To explore the related possibility that the detected associations may vary by whether DS were clinically elevated or not, each model with a significant effect was retested with an interaction term of DS elevation (dichotomous) × significant predictor of interest. However, none of these interactions reached significance, suggesting that the associations do not depend upon the presence of elevated DS.
Because the sample varied substantially across age, regimen, and medical comorbidity, additional analyses were conducted to explore whether the above relationships varied across these characteristics. For example, perhaps being on insulin affects the association between DS and medication adherence (8,29). To achieve this, multiplicative interaction terms corresponding to DS or DRD times the potential moderator (age >60 years, sex, regimen, and having two or more medical comorbidities) were computed and tested separately for each potential moderator in each of the above five longitudinal models. Of the resulting 40 interaction terms tested, only one significant moderator effect was detected (i.e., baseline DS × regimen predicting Month 6 exercise frequency) (P = 0.026). Specifically, the association between baseline DS and subsequent exercise frequency was only significant among participants who were prescribed oral medication. However, this result might be spurious, because 2 (5%) of 40 terms might be expected to be significant through chance alone.
CONCLUSIONS
To summarize the findings, only DS predicted lifestyle-oriented self-management behaviors 6 months later, such as diet, glucose testing, and possibly exercise. In contrast, only DRD predicted future medication adherence and glycemic control. This new information adds to the growing literature pointing to a fundamental difference between DS and DRD. The findings may also help elucidate key underlying biobehavioral mechanisms and have implications for clinical practice.
As reviewed above, a growing evidence base supports the conceptual and empirical distinctions between DS and DRD (10,11,16–19). This is not surprising, given that a parallel distinction has been suggested to exist among patients with cancer (30,31), HIV/acquired immunodeficiency syndrome (32), chronic pain (32), multiple sclerosis (33,34), and other serious or chronic medical conditions. If DS and illness-specific distress are each associated with different behavioral and medical outcomes, then they probably operate according to different underlying mechanisms. For example, in the case of diabetes, DS might disrupt the lifestyle-oriented health behaviors recommended for good diabetes control. This may occur because depression reduces general motivation and disrupts activities that are complex, energy-intensive, effortful, or time-consuming. Examples would be depression-related difficulty avoiding unhealthy comfort food and eating at regular intervals, increased downtime leading to difficulty maintaining a healthy exercise routine, poor appointment attendance leading to inadequate medical care, and functional restrictions due to another medical condition associated with depression. Perhaps DS are relatively less likely to disrupt simpler habitual activities, such as taking oral medication as directed. As a moderately complex behavior, self-monitoring of blood glucose may fall somewhere in between these two extremes, with the present findings indicating that it is probably more disrupted by DS than by DRD.
Extending this reasoning further, perhaps DRD tends to selectively disrupt activities that are highly specific to diabetes (e.g., taking diabetes medication). For example, if the relatively simple act of taking an oral hypoglycemic happens to serve as a frequent and emotionally stressful reminder of negative aspects of having diabetes, then high DRD could be expected to reduce adherence. This could plausibly explain the observed link between DRD and glycemic control. In this respect, the findings agree with clinical trials indicating when DS are successfully reduced in diabetes, there is apparently no accompanying improvement of metabolic control (7). Another interpretation is that DRD measured at baseline is actually a psychological reaction to, rather than a cause of, concurrent or previous levels of glycemic control, because both factors may be somewhat stable within individuals and across time.
As summarized in the introduction, the most stringent studies to date have either used a longitudinal design to help eliminate the influence of concurrent associations or have included both DS and DRD so that their associations with outcomes of interest could be directly compared. Only one prior study was both longitudinal and included both DS and DRD, and it showed that DRD was related to future glycemic control, whereas DS were not (17). Although that result conflicts with the present findings, this might be due to the prior study’s longer assessment interval of 9 months or its inclusion of three time points rather than two. Another possible source of method variance may be the use of different DRD measures; Fisher et al. (15) used the recently developed Diabetes Distress Scale, whereas the current study used the PAID. Although both instruments are well-validated, and they probably intercorrelate highly, recent data suggest that they capture different aspects of diabetes distress (35). Perhaps future studies might consider this potential measurement issue. Building upon the limited existing work, this was the first longitudinal study to compare DS and DRD in terms of their unique longitudinal associations with self-management behaviors.
Study limitations
One important limitation of this study is that its correlational design cannot conclusively rule out alternative explanations for the findings. As noted above, perhaps hyperglycemia, DS, and DRD are each so stable that their association artifactually extends over time. However, this seems unlikely to be the case, given that the findings varied by type of distress (DS versus DRD) and because average glycemia does vary with diabetes severity. Related to this, although the design was longitudinal, it was also naturalistic insofar as no variables were experimentally manipulated. Therefore, it is impossible to definitively conclude that the observed longitudinal associations were causal. Because of practical limitations in the number of variables collected, it cannot be ruled out that DS, DRD, self-management, and glycemic control may be related to some important but unidentified common factor. The findings should be cautiously generalized, because enrollment occurred within a single health system and although African Americans were oversampled, not all minority groups were well-represented. Although DS were not verified by a structured psychiatric interview and therefore may have been misclassified, syndromal depression was not a study variable, and the measure of DS was originally validated against psychiatric interviews in medical patient samples. Related to this, for the few participants who reported suicidal ideation, protocol-related crisis intervention may have helped reduce depressive symptoms for that subgroup. Finally, the SDSCA may not comprehensively capture self-care, especially for patients who have unclear lifestyle recommendations.
Clinical implications
One primary clinical implication is that DS may be less important than DRD is for the achievement and preservation of medication adherence and glycemic control. At the same time, perhaps DS is more important to lifestyle-oriented behaviors and blood glucose self-testing. Therefore, implications may vary by the type and complexity of whatever outcome is of greatest clinical concern. Second, DS and DRD predict different outcomes. Therefore, elevated DS may be a prognostic indicator for impending decline in the lifestyle aspects of diabetes self-management, whereas high DRD may predict reduced medication adherence and poor glycemic control. Clinical assessment has traditionally emphasized DS and therefore may need to broaden to encompass both issues in order to gain a complete prognostic picture. Additionally, the findings suggest that clinical efforts to reduce DS without also addressing DRD, whether through medication or narrowly focused cognitive behavioral therapy, will not by themselves improve medication adherence or glycemic control (7). Likewise, clinical strategies aimed at specifically reducing DRD without addressing DS may not lead to improved self-management in terms of lifestyle behaviors. The most effective clinical interventions will be those that are broadly designed to improve the management of both DS and illness-specific distress, and interventionists need to think broadly and creatively in this regard. Otherwise, if both DS and DRD are elevated, a change in merely one of them may not improve all of the important outcomes. Movement in this direction is already evident in recent success with integrated nurse care management for comorbid diabetes and depression (36). Additional related efforts are underway to combine brief cognitive behavioral therapies with Web-based diabetes self-management support (37) and the direct enhancement of medication adherence (7).
Conclusion
This study provides longitudinal support for the conceptual and empirical distinctions between DS and DRD in type 2 diabetes. DS may selectively suppress lifestyle-oriented self-management behaviors such as healthy eating, glucose testing, and exercise. In contrast, diabetes-specific distress may impact subsequent medication adherence and glycemic control. Clinical assessment and intervention should encompass both factors.
Acknowledgments
This research was supported by National Institutes of Health grants R01-DK-066016 and P60-DK-020572 (to Michigan Diabetes Research and Training Center), both from the National Institute of Diabetes and Digestive and Kidney Diseases.
No potential conflicts of interest relevant to this article were reported.
J.E.A. conceptualized the study, analyzed data, and wrote the manuscript. J.E.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The author thanks Dr. John D. Piette of the University of Michigan and VA Ann Arbor Healthcare System and Denise White-Perkins of the Henry Ford Health System for providing assistance in developing and conducting the parent study from which these secondary data were drawn.
References
- 1.Nouwen A, Winkley K, Twisk J, et al. European Depression in Diabetes (EDID) Research Consortium Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia 2010;53:2480–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campayo A, Gómez-Biel CH, Lobo A. Diabetes and depression. Curr Psychiatry Rep 2011;13:26–30 [DOI] [PubMed] [Google Scholar]
- 3.Lustman PJ, Clouse RE. Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complications 2005;19:113–122 [DOI] [PubMed] [Google Scholar]
- 4.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 2000;23:934–942 [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez JS, Safren SA, Delahanty LM, et al. Symptoms of depression prospectively predict poorer self-care in patients with Type 2 diabetes. Diabet Med 2008;25:1102–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katon WJ, Russo JE, Heckbert SR, et al. The relationship between changes in depression symptoms and changes in health risk behaviors in patients with diabetes. Int J Geriatr Psychiatry 2010;25:466–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markowitz SM, Gonzalez JS, Wilkinson JL, Safren SA. A review of treating depression in diabetes: emerging findings. Psychosomatics 2011;52:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aikens JE, Perkins DW, Lipton B, Piette JD. Longitudinal analysis of depressive symptoms and glycemic control in type 2 diabetes. Diabetes Care 2009;32:1177–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez JS, Fisher L, Polonsky WH. Depression in diabetes: have we been missing something important? Diabetes Care 2011;34:236–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez JS, Delahanty LM, Safren SA, Meigs JB, Grant RW. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia 2008;51:1822–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care 2010;33:1034–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang R-H, Wu L-C, Hsu H-Y. A path model of health-related quality of life in type 2 diabetic patients: a cross-sectional study in Taiwan. J Adv Nurs 2011;67:2658–2667 [DOI] [PubMed] [Google Scholar]
- 13.Weinger K, Butler HA, Welch GW, La Greca AM. Measuring diabetes self-care: a psychometric analysis of the Self-Care Inventory-Revised with adults. Diabetes Care 2005;28:1346–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delahanty LM, Grant RW, Wittenberg E, et al. Association of diabetes-related emotional distress with diabetes treatment in primary care patients with Type 2 diabetes. Diabet Med 2007;24:48–54 [DOI] [PubMed] [Google Scholar]
- 15.Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care 2005;28:626–631 [DOI] [PubMed] [Google Scholar]
- 16.Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care 1995;18:754–760 [DOI] [PubMed] [Google Scholar]
- 17.Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care 2010;33:23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care 2007;30:542–548 [DOI] [PubMed] [Google Scholar]
- 19.van Bastelaar KMP, Pouwer F, Geelhoed-Duijvestijn PHLM, et al. Diabetes-specific emotional distress mediates the association between depressive symptoms and glycaemic control in Type 1 and Type 2 diabetes. Diabet Med 2010;27:798–803 [DOI] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67–74 [DOI] [PubMed] [Google Scholar]
- 22.Krapek K, King K, Warren SS, et al. Medication adherence and associated hemoglobin A1c in type 2 diabetes. Ann Pharmacother 2004;38:1357–1362 [DOI] [PubMed] [Google Scholar]
- 23.Mann DM, Ponieman D, Leventhal H, Halm EA. Predictors of adherence to diabetes medications: the role of disease and medication beliefs. J Behav Med 2009;32:278–284 [DOI] [PubMed] [Google Scholar]
- 24.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care 2000;23:943–950 [DOI] [PubMed] [Google Scholar]
- 25.Cass AR, Volk RJ, Nease DE., Jr Health-related quality of life in primary care patients with recognized and unrecognized mood and anxiety disorders. Int J Psychiatry Med 1999;29:293–309 [DOI] [PubMed] [Google Scholar]
- 26.Aikens JE, Nease DE, Jr, Nau DP, Klinkman MS, Schwenk TL. Adherence to maintenance-phase antidepressant medication as a function of patient beliefs about medication. Ann Fam Med 2005;3:23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzgerald JT, Davis WK, Connell CM, Hess GE, Funnell MM, Hiss RG. Development and validation of the Diabetes Care Profile. Eval Health Prof 1996;19:208–230 [DOI] [PubMed] [Google Scholar]
- 28.U.S. Bureau of the Census Methodology and Scores of the Socioeconomic Status, Working Paper No. 15. Fed Regist 2002;67:6931–6933 [Google Scholar]
- 29.Aikens JE, Perkins DW, Piette JD, Lipton B. Association between depression and concurrent Type 2 diabetes outcomes varies by diabetes regimen. Diabet Med 2008;25:1324–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golden-Kreutz DM, Andersen BL. Depressive symptoms after breast cancer surgery: relationships with global, cancer-related, and life event stress. Psychooncology 2004;13:211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kandasamy A, Chaturvedi SK, Desai G. Spirituality, distress, depression, anxiety, and quality of life in patients with advanced cancer. Indian J Cancer 2011;48:55–59 [DOI] [PubMed] [Google Scholar]
- 32.Strine TW, Hootman JM, Chapman DP, Okoro CA, Balluz L. Health-related quality of life, health risk behaviors, and disability among adults with pain-related activity difficulty. Am J Public Health 2005;95:2042–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janssens ACJW, van Doorn PA, de Boer JB, van der Meché FGA, Passchier J, Hintzen RQ. Impact of recently diagnosed multiple sclerosis on quality of life, anxiety, depression and distress of patients and partners. Acta Neurol Scand 2003;108:389–395 [DOI] [PubMed] [Google Scholar]
- 34.Janssens ACJW, van Doorn PA, de Boer JB, van der Meché FGA, Passchier J, Hintzen RQ. Perception of prognostic risk in patients with multiple sclerosis: the relationship with anxiety, depression, and disease-related distress. J Clin Epidemiol 2004;57:180–186 [DOI] [PubMed] [Google Scholar]
- 35.Graue M, Haugstvedt A, Wentzel-Larsen T, Iversen MM, Karlsen B, Rokne B. Diabetes-related emotional distress in adults: reliability and validity of the Norwegian versions of the Problem Areas in Diabetes Scale (PAID) and the Diabetes Distress Scale (DDS). Int J Nurs Stud 2012;49:174–182 [DOI] [PubMed] [Google Scholar]
- 36.Katon WJ, Lin EHB, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Bastelaar K, Cuijpers P, Pouwer F, Riper H, Snoek FJ. Development and reach of a web-based cognitive behavioural therapy programme to reduce symptoms of depression and diabetes-specific distress. Patient Educ Couns 2011;84:49–55 [DOI] [PubMed] [Google Scholar]