Abstract
OBJECTIVE
The Diabetes Prevention Program (DPP) reported no racial/ethnic differences in the incidence of diabetes in individuals with impaired glucose tolerance (IGT). Therefore, it has been hypothesized that factors associated with racial/ethnic disparities act prior to the development of IGT. Because impaired fasting glucose (IFG) and obesity were also very prevalent in the DPP, we examined IGT, IFG, and obesity as effect modifiers of ethnic disparities in the San Antonio Heart Study.
RESEARCH DESIGN AND METHODS
Participants were 3,015 Mexican Americans and non-Hispanic whites aged 25–64 years. The median follow-up period was 7.8 years. IGT, IFG, and diabetes were defined by the 2003 American Diabetes Association criteria, and obesity was defined as BMI ≥30 kg/m2.
RESULTS
Mexican Americans had an excess risk of incident IGT (odds ratio 1.48 [95% CI 1.16–1.89]) and incident IFG (1.71 [1.31–2.23]) compared with non-Hispanic whites. Mexican Americans also had a higher incidence of diabetes among individuals who had normal 2-h glucose (2.20 [1.48–3.29]) and IGT (1.72 [1.08–2.74]) at baseline. There was an interaction of obesity on the relationship between ethnicity and progression to IGT or diabetes (P = 0.034), with Mexican Americans having a greater risk among the nonobese (1.73 [1.36–2.21]) and a comparable risk among the obese (1.08 [0.75–1.56]).
CONCLUSIONS
Ethnic differences can be detected at both the early and later stages of the diabetes disease process. However, non-Hispanic whites lose much of the ethnic advantage once they have developed obesity.
Minority populations are at increased risk of developing diabetes (1–4). Obesity and fat distribution do not fully account for racial/ethnic disparities in the development of diabetes (1,2). Obesity and ethnicity influence the development of diabetes in individuals with impaired glucose tolerance (IGT) (5). However, the rate of conversion from IGT to type 2 diabetes was similar across racial/ethnic groups in the Diabetes Prevention Program (DPP) (6). The DPP was designed as a large, randomized clinical trial involving adults who were at very high risk of future diabetes (rate of conversion to diabetes was 11.0% per year). Consequently, Dagogo-Jack et al. (7) recently hypothesized that factors associated with racial/ethnic disparities act prior to the development of IGT (i.e., at early stages). A longitudinal study is underway to explore this hypothesis in African Americans and non-Hispanic whites (7).
A closer look at the DPP eligibility criteria suggests that factors other than IGT may also have contributed to the very high rate of progression to diabetes (6). DPP criteria for enrollment included fasting plasma glucose 5.3–6.9 mmol/L (≤6.9 mmol/L in Native Americans) and BMI ≥24 kg/m2 (≥22 kg/m2 in Asians). Therefore, it is also plausible that the lack of racial/ethnic differences in the risk of diabetes could have been influenced by the fact that mean fasting glucose was 5.92 mmol/L and mean BMI was 34.2 kg/m2. To clarify these assumptions, the objective of this study was to assess ethnic disparities proximal to and during the IGT and impaired fasting glucose (IFG) stages in nonobese and obese participants in the San Antonio Heart Study (SAHS).
RESEARCH DESIGN AND METHODS
The SAHS is a longitudinal, epidemiological study designed to study type 2 diabetes and cardiovascular disease among Mexican Americans and non-Hispanic whites living in San Antonio, Texas. Protocols were approved by the institutional review board of the University of Texas Health Science Center at San Antonio. A detailed description of the methods has previously been published (8). Briefly, all Mexican Americans and non-Hispanic whites (men and nonpregnant women) aged 25–64 years who resided in randomly selected households from low-, middle-, and high-income census tracts were invited to participate. A total of 5,158 individuals (response rate 65.3%) were enrolled in two phases: cohort 1, from January 1979 to December 1982, and cohort 2, from January 1984 to December 1988. Cohort 1 participants were reexamined between January 1984 and December 1988 and cohort 2 participants between October 1991 and October 1996. The median follow-up period was 7.8 years (range 6.3–10.7). All subjects gave written informed consent.
Diabetes status was ascertained in 3,228 of 4,429 participants who were nondiabetic at the baseline examination. Relevant data were missing in 213 individuals. Therefore, this study presents data on 3,015 individuals.
Anthropometric measurements and blood specimens were obtained by trained personnel using identical, standardized protocols at the baseline and follow-up examinations. Family history of diabetes was positive if a first-degree relative (parents or siblings) had been previously diagnosed with type 2 diabetes. An oral glucose tolerance test was administered at the baseline and follow-up visits to assess glucose tolerance status. Blood specimens were collected prior (0 min) and 120 min after a 75-g oral glucose load (Orangedex; Custom Laboratories, Baltimore, MD). Blood specimens were also collected at 30 and 60 min post–glucose load only at the baseline visit in cohort 2 participants. Plasma glucose concentration was measured by conventional methods and serum insulin concentration by a radioimmunoassay (Diagnostic Products, Los Angeles, CA). In this assay, the cross-reactivity with proinsulin was high (70–100%).
Obesity was defined as BMI ≥30 kg/m2. We used the 2003 American Diabetes Association criteria to define diabetes (fasting glucose ≥7.0 mmol/L and/or 2-h glucose ≥11.1 mmol/L), IFG (fasting glucose ≥5.6 and <7 mmol/L), and IGT (2-h glucose ≥7.8 and <11.1 mmol/L). Subjects who reported current treatment with glucose-lowering medications were considered to have diabetes. Homeostasis model assessment of insulin resistance (HOMA-IR) was determined according to the formula of Matthews et al.: HOMA-IR = fasting insulin (μIU/mL) × fasting glucose (mmol/L)/22.5 (9). In cohort 2 participants, we also computed Matsuda index and insulinogenic index as follows:
Matsuda index = 104/(fasting glucose × fasting insulin × mean glucose × mean insulin)0.5 (10). Mean glucose and mean insulin indicate mean glucose (mg/dL) and mean insulin concentrations (μIU/mL) based on sampling times at 0, 30, 60, and 120 min. We did not measure insulin and glucose concentrations at 90 min. This may be of relative importance to the original Matsuda index, which is calculated using insulin and glucose values at 0, 30, 60, 90, and 120 min (10). Matsuda index calculated from glucose and insulin levels at 0, 30, and 120 min has been validated against directly measured insulin sensitivity by clamp studies (11).
Insulinogenic index = 30-min insulin − fasting insulin/30-min glucose − fasting glucose (12).
Statistical analyses
Statistical analyses were performed with the SAS statistical software (version 9.2; SAS Institute, Cary, NC). We assessed differences in anthropometric and metabolic variables between glucose tolerance categories by one-way ANCOVA and logistic regression analysis. We examined the ethnic difference in the incidence of IFG, IGT, or diabetes by multiple logistic regression analysis. We used log-transformed values of fasting insulin, HOMA-IR, Matsuda index, and insulinogenic index in all analyses to improve discrimination and calibration of the models and to minimize the influence of extreme observations. We considered a P value <0.05 statistically significant.
RESULTS
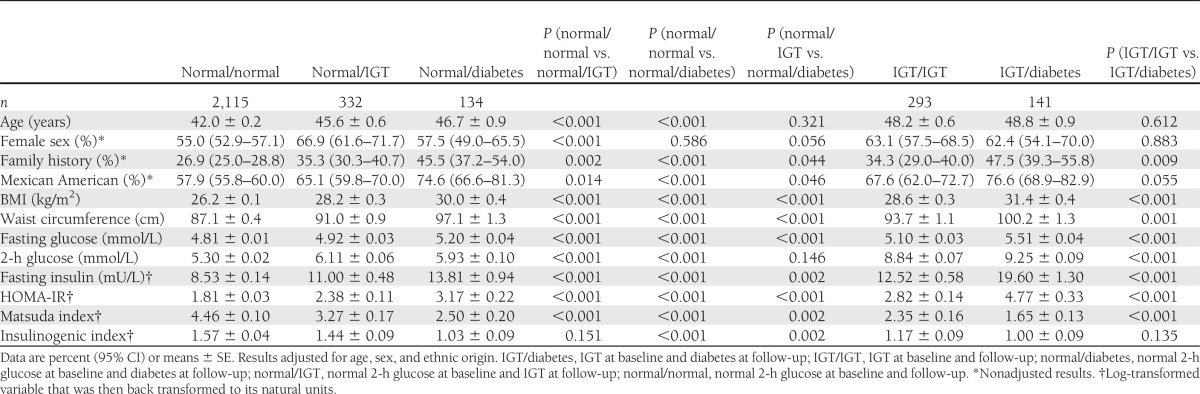
Table 1 presents baseline characteristics by IGT and diabetes status at the baseline and follow-up visits. Progression to either IGT or diabetes was more frequent in Mexican Americans and older individuals. Progression to IGT was more common in women. Furthermore, individuals who progressed to IGT and diabetes differ from those who did not in terms of family history of diabetes, adiposity, plasma glucose levels, and insulin resistance. Lower insulinogenic index was associated with rapid development of diabetes in individuals who had normal 2-h glucose at baseline. However, insulinogenic index was not associated with incident IGT and incident diabetes in individuals with normal 2-h glucose and IGT at baseline, respectively.
Table 1.
Baseline characteristics by glucose tolerance status at the baseline and follow-up visits
During the 7.8-year follow-up period, 67 of 1,168 (5.7%) non-Hispanic whites and 208 of 1,847 (11.3%) Mexican Americans developed diabetes. The number of persons who progress to diabetes by baseline glucose tolerance status was as follows: 92 of 2,350 (4.1%) with normal fasting and 2-h glucose, 42 of 231 (18.2%) with isolated IFG, 67 of 296 (22.6%) with isolated IGT, and 74 of 138 (53.6%) with both IFG and IGT. Among individuals who did not convert to diabetes and had normal 2-h glucose at baseline, 116 of 1,039 (11.2%) non-Hispanic whites and 216 of 1,533 (14.1%) Mexican Americans developed IGT.
The age- and sex-adjusted odds of developing diabetes were 2.33 times higher in Mexican Americans (95% CI 1.74–3.11). The ethnic difference between Mexican Americans and non-Hispanic whites decreased to 1.53 (1.09–2.14) after the additional adjustment for IGT, IFG, obesity, and family history of diabetes. In this model, IGT (odds ratio [OR] 4.69 [95% CI 3.49–6.31]), IFG (4.07 [2.98–5.57]), family history of diabetes (1.73 [1.29–2.31]), and BMI (OR × 1 kg/m2 increase: 1.10 [1.07–1.13]) were independent predictors of incident diabetes.
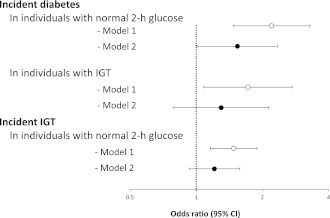
In Fig. 1, models labeled as model 1 present the age- and sex-adjusted odds of incident IGT or diabetes in Mexican Americans compared with non-Hispanic whites. In individuals with normal 2-h glucose, Mexican Americans had greater odds of future development of either IGT (OR 1.48 [95% CI 1.16–1.89]) or diabetes (2.20 [1.48–3.29]). In individuals with IGT, Mexican Americans also had greater odds of developing diabetes (1.72 [1.08–2.74]). In the second set of models adjusted for BMI and family history of diabetes (model 2), the excess risk of incident IGT and incident diabetes in Mexican Americans was partially attenuated.
Figure 1.
Ethnicity ORs of incident IGT and incident diabetes by baseline categories of 2-h glucose. ○, model 1, results adjusted for age and sex; ●, model 2, results adjusted for age, sex, BMI, and family history of diabetes. Ethnicity OR as shown indicates excess risk for Mexican Americans.
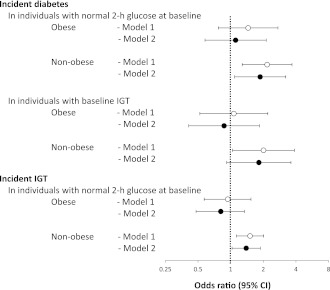
Obesity accounted for 31.8% of the total increase in the incidence of diabetes in Mexican Americans relative to non-Hispanic whites. However, there was an interaction effect of obesity on the relationship between ethnicity and progression to IGT or diabetes (P = 0.034), with Mexican Americans having a greater age- and sex-adjusted risk among the nonobese (OR 1.73 [95% CI 1.36–2.21]) and a comparable risk among the obese (1.08 [0.75–1.56]). Therefore, we analyzed the ethnic odds of progression to IGT and diabetes according to the presence or absence of IGT and obesity at baseline (Fig. 2). In models adjusted for age and sex (model 1), Mexican Americans had excess odds of incident IGT among individuals who were nonobese (1.51 [1.13–2.02]) but not among the obese (0.94 [0.57–1.54]). In individuals who had IGT at baseline, the odds of developing diabetes were twice as high for nonobese Mexican Americans as for nonobese non-Hispanic whites (2.01 [1.04–3.87]). However, the odds were similar among obese counterparts (1.07 [0.52–2.20]). In individuals who had normal 2-h glucose at baseline, nonobese Mexican Americans had greater odds of incident diabetes than nonobese non-Hispanic whites (2.17 [1.28–3.68]), but the ethnic OR was not statistically significant among obese counterparts (1.45 [0.77–2.73]). Among these obese participants who had normal 2-h glucose at baseline, Mexican Americans still had higher BMI (34.7 ± 0.2 vs. 33.9 ± 0.3 kg/m2, P = 0.039) and higher prevalence of family history of diabetes (44.2% [95% CI 39.9–48.5] vs. 23.3% [17.5–30.1], P < 0.001). The ethnic difference in diabetes incidence was reduced after adjustment for BMI and family history of diabetes (OR 1.11 [95% CI 0.58–2.13]) (model 2). In nonobese participants, BMI and family history of diabetes only explained a small proportion of the ethnic difference in either the incidence of diabetes (regardless of the 2-h glucose category) or the incidence of IGT.
Figure 2.
Ethnicity ORs of incident IGT and incident diabetes by baseline categories of 2-h glucose and BMI. ○, model 1, results adjusted for age and sex; ●, model 2, results adjusted for age, sex, BMI, and family history of diabetes. Ethnicity OR as shown indicates excess risk for Mexican Americans.
In individuals with baseline IFG, 82 of 233 (35.2%) Mexican Americans and 34 of 136 (25.0%) non-Hispanic whites progressed to diabetes. In individuals with normal fasting glucose, 91 of 999 (9.1%) non-Hispanic whites and 203 of 1,488 (13.6%) Mexican Americans developed new-onset IFG during the follow-up period. Mexican Americans had higher age- and sex-adjusted odds of developing incident IFG (OR 1.71 [95% CI 1.31–2.23]) and incident diabetes (2.74 [1.84–4.06]) than non-Hispanic whites. In individuals with IFG, Mexican Americans also had greater odds of developing diabetes (1.84 [1.13–2.98]). Participants with both IFG and IGT were relatively few (92 Mexican Americans and 46 non-Hispanic whites) and had a high baseline prevalence of obesity (52.2% in Mexican Americans and 45.7% in non-Hispanic whites) as well as a high risk of developing diabetes (56.5% in Mexican Americans and 47.8% in non-Hispanic whites). In this group of participants, the age- and sex-adjusted ethnic difference was not statistically significant (1.56 [0.76–3.20]).
CONCLUSIONS
Mexican Americans have an excess risk of developing diabetes—in part related to their greater adiposity and higher baseline glucose levels. Ethnic disparities between Mexican Americans and non-Hispanic whites are demonstrated both proximal to and during the IGT and IFG stages. However, the ethnic difference appears to be stronger in leaner subjects.
Insulin resistance, insulin secretion, and plasma glucose levels are involved in the disease process before and after the onset of IGT (5,13,14). These risk factors predict type 2 diabetes equally well in high- and low-risk populations (13,15). The causes of the ethnic differences in the development of diabetes are not completely known. It is likely that ethnic differences are the result of distinct interactions between genetic and environmental factors. However, in the DPP, there were no racial/ethnic disparities in the incidence of diabetes (6). Baseline characteristics could account for the high incidence of diabetes (~11.0% per year) in all racial/ethnic groups. All participants had IGT, and most had IFG and/or were obese. In contrast to the DPP, participants in the SAHS have a broad range of characteristics and a significantly lower rate of conversion to diabetes (even among those with IGT) (5,8). Direct comparison with the Hispanic subgroup of DPP is not feasible, as the San Antonio study recruited only Mexican Americans, whereas the DPP had a heterogeneous Hispanic population. (Mexican Americans account for two-thirds of the Hispanics in the U.S.) In the SAHS, ethnic disparities can be detected both in participants with and in participants without IGT. Consequently, our results do not support the hypothesis of Dagogo-Jack et al. (7).
Ethnic differences in the incidence of diabetes are also demonstrated in individuals with and without IFG. These results are similar to those derived from analyzing ethnic differences by IGT status. In contrast, our study did not detect a significant difference in the development of diabetes between Mexican Americans with combined IFG and IGT and non-Hispanic white counterparts (OR 1.56 [95% CI 0.76–3.20]). The absence of statistical significance for this category may be related to the high rates of developing diabetes, lack of effect of factors associated with racial/ethnic disparities, high prevalence of obesity, and insufficient statistical power (relatively low number of both Mexican Americans and non-Hispanic whites).
Adiposity does not fully account for the higher degree of insulin resistance in minority populations (16). This suggests that minority populations may have a different susceptibility to diabetogenic risk factors (17). Adiposity has been linked to disease progression before and after the development of IGT (5). In Pima Indians, weight gain was associated with progression from normal glucose tolerance to IGT and from IGT to diabetes (17). In the DPP, adiposity predicted future development of diabetes in overweight/obese individuals with IGT (18). Moreover, a lifestyle intervention was effective in both decreasing the rate of conversion to diabetes by 58% and reverting IGT to normal 2-h glucose in 40% of participants (6). In the SAHS, non-Hispanic whites who develop obesity lose much of the ethnic advantage in the early and later stages of the disease process. Therefore, obesity attenuates the relative risk of ethnicity, but the absolute risk is still high. Even if we examine the nonstatistical higher risk of Mexican Americans with normal 2-h glucose and obesity at baseline compared with non-Hispanic white counterparts (OR 1.45 [95% CI 0.77–2.73]), BMI and family history of diabetes still account for part of the ethnic difference (1.11 [0.58–2.13]). Obesity appears to reduce the ethnic difference in the incidence of diabetes in the SAHS, but we cannot exclude that an excess risk of diabetes may be present in obese individuals from other high-risk populations.
A particularly low insulin secretory capacity could explain an “accelerated course” of diabetes (19). Baseline insulin secretion and insulin sensitivity were significantly reduced in individuals who progressed from normal glucose tolerance to diabetes. Insulinogenic index in these participants was comparable with that in individuals with IGT who went on to develop diabetes. Mexican Americans were more prevalent among the group of participants who progressed from normal glucose tolerance to diabetes. However, Mexican Americans were also more prevalent among those who progressed at all levels of the disease process. Insulin resistance and insulin secretion in individuals who converted to IGT were not as deteriorated as in those with normal glucose tolerance who developed diabetes. A longer duration of the sequence of events may characterize the development of diabetes in individuals who had increased insulin resistance and, to a certain extent, adequate secretory capacity (appropriate compensation for the degree of insulin resistance). We have previously reported that Hispanics and Mexican Americans tend to have appropriate compensation as measured by acute insulin response or insulinogenic index (16,20). However, insulin resistance and insulin secretion may not fully explain the excess risk of diabetes in Mexican Americans (21). Further studies are needed to examine ethnic differences in terms of both longitudinal changes and duration of the conversion process to diabetes.
In conclusion, ethnic differences can be detected at both the early and later stages of the diabetes disease process. Previous studies have demonstrated that adiposity influences disease progression before and after the development of IGT. However, non-Hispanic whites lose much of the ethnic advantage once they have developed obesity. Consequently, physicians need to emphasize lifestyle changes in both Mexican Americans and non-Hispanic whites, although perhaps earlier in Mexican Americans.
Acknowledgments
This work was supported by grants from the National Heart, Lung, and Blood Institute (RO1-HL24799 and RO1-HL36820).
No potential conflicts of interest relevant to this article were reported.
C.L. contributed to the study concept and design, wrote the manuscript, contributed to discussion, and reviewed and edited the manuscript. R.L. contributed to writing the manuscript and reviewed and edited the manuscript. S.M.H. researched data, contributed to the study concept and design, contributed to discussion, and reviewed and edited the manuscript. C.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
Footnotes
Retired from practice, Shavano Park, Texas.
References
- 1.Haffner SM, Hazuda HP, Mitchell BD, Patterson JK, Stern MP. Increased incidence of type II diabetes mellitus in Mexican Americans. Diabetes Care 1991;14:102–108 [DOI] [PubMed] [Google Scholar]
- 2.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA 2000;283:2253–2259 [DOI] [PubMed] [Google Scholar]
- 3.Ramachandran A, Snehalatha C, Kapur A, et al. Diabetes Epidemiology Study Group in India (DESI) High prevalence of diabetes and impaired glucose tolerance in India: National Urban Diabetes Survey. Diabetologia 2001;44:1094–1101 [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Bennett PH, Hamman RF, Miller M. Diabetes incidence and prevalence in Pima Indians: a 19-fold greater incidence than in Rochester, Minnesota. Am J Epidemiol 1978;108:497–505 [DOI] [PubMed] [Google Scholar]
- 5.Edelstein SL, Knowler WC, Bain RP, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes 1997;46:701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagogo-Jack S, Edeoga C, Nyenwe E, Chapp-Jumbo E, Wan J. Pathobiology of Prediabetes in a Biracial Cohort (POP-ABC): design and methods. Ethn Dis 2011;21:33–39 [PMC free article] [PubMed] [Google Scholar]
- 8.Burke JP, Williams K, Gaskill SP, Hazuda HP, Haffner SM, Stern MP. Rapid rise in the incidence of type 2 diabetes from 1987 to 1996: results from the San Antonio Heart Study. Arch Intern Med 1999;159:1450–1456 [DOI] [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 10.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 11.Stancáková A, Javorský M, Kuulasmaa T, Haffner SM, Kuusisto J, Laakso M. Changes in insulin sensitivity and insulin release in relation to glycemia and glucose tolerance in 6,414 Finnish men. Diabetes 2009;58:1212–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wareham NJ, Phillips DI, Byrne CD, Hales CN. The 30 minute insulin incremental response in an oral glucose tolerance test as a measure of insulin secretion. Diabet Med 1995;12:931. [DOI] [PubMed] [Google Scholar]
- 13.Lorenzo C, Wagenknecht LE, D’Agostino RB, Jr, Rewers MJ, Karter AJ, Haffner SM. Insulin resistance, β-cell dysfunction, and conversion to type 2 diabetes in a multiethnic population: the Insulin Resistance Atherosclerosis Study. Diabetes Care 2010;33:67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitabchi AE, Temprosa M, Knowler WC, et al. Diabetes Prevention Program Research Group Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes 2005;54:2404–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haffner SM, Miettinen H, Stern MP. Are risk factors for conversion to NIDDM similar in high and low risk populations? Diabetologia 1997;40:62–66 [DOI] [PubMed] [Google Scholar]
- 16.Haffner SM, D’Agostino R, Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes 1996;45:742–748 [DOI] [PubMed] [Google Scholar]
- 17.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999;104:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Diabetes Prevention Program Research Group Relationship of body size and shape to the development of diabetes in the diabetes prevention program. Obesity (Silver Spring) 2006;14:2107–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–1672 [DOI] [PubMed] [Google Scholar]
- 20.Haffner SM, Miettinen H, Stern MP. Nondiabetic Mexican-Americans do not have reduced insulin responses relative to nondiabetic non-Hispanic whites. Diabetes Care 1996;19:67–69 [DOI] [PubMed] [Google Scholar]
- 21.Lorenzo C, Hazuda HP, Haffner SM. Insulin resistance and excess risk of diabetes in Mexican-Americans: the San Antonio Heart Study. J Clin Endocrinol Metab 2012;97:793–799 [DOI] [PMC free article] [PubMed] [Google Scholar]