Abstract
OBJECTIVE
Heart failure is common in individuals with type 2 diabetes, and early detection of individuals at risk may offer opportunities for prevention. We aimed to explore 1) prospective associations of B-type natriuretic peptide (BNP) levels in a non–heart failure range with changes in markers of left ventricular (LV) function and 2) possible effect modification by type 2 diabetes in a population-based cohort.
RESEARCH DESIGN AND METHODS
Echocardiographic measurements were performed at baseline (2000–2001) and follow-up (2007–2009), together with standardized physical examinations and BNP measurements on 300 individuals (mean age 66 years, 32% with type 2 diabetes) of the longitudinal Hoorn Study. Multivariate linear regression analyses were performed to investigate associations of baseline BNP (<100 pg/mL) in individuals without prevalent heart failure at baseline with changes in LV mass index, LV ejection fraction, left atrial volume index, and ratio of early diastolic LV inflow velocity (E) to early diastolic lengthening velocity (e′) (E/e′).
RESULTS
In all individuals, higher BNP was associated with 8-year increases in left atrial volume index. Higher BNP was also associated with increasing LV mass index and E/e′. These associations were significantly stronger in individuals with type 2 diabetes compared with the nonsignificant associations in individuals without type 2 diabetes.
CONCLUSIONS
This 8-year follow-up study shows that higher BNP levels in a non–heart failure range were associated with an increased LV mass and deteriorated LV diastolic function, particularly in individuals with type 2 diabetes. This implies that the presence or absence of type 2 diabetes should be taken into account if BNP levels are used to assess future heart failure risk.
It is well known that individuals with type 2 diabetes are at increased risk of developing heart failure and face a worse prognosis after diagnosis than individuals without type 2 diabetes (1,2). For heart failure with a normal ejection fraction (EF), which is more common in type 2 diabetes, no effective treatment has been developed as of yet (3). Nevertheless, left ventricular (LV) diastolic dysfunction can be detected before heart failure develops, and adherence to a healthy lifestyle has been shown to effectively lower the risk of heart failure (4,5). This provides a window of opportunity for prevention, risk profiling, and early treatment of individuals at risk for developing heart failure.
B-type natriuretic peptide (BNP) is a neurohormone secreted predominantly by cardiomyocytes in the LV in response to volume expansion and pressure overload (6). BNP levels >200 pg/mL (equivalent to 57 pmol/L) indicate the potential presence of heart failure (sensitivity 90%), whereas levels >100 pg/mL (equivalent to 28 pmol/L) rule out heart failure (7,8). Prospective data showed that increased BNP levels in this non–heart failure range predict heart failure, atrial fibrillation, ischemic stroke, and cardiovascular mortality (9,10). On the basis of a cross-sectional analysis, we have previously shown that slightly elevated levels below this threshold are associated with diminished LV diastolic function (11). This may explain why slightly elevated BNP levels are associated with future cardiovascular disease and mortality. The relationship between BNP in a non–heart failure range and LV diastolic function appeared to be particularly strong in individuals with type 2 diabetes (11). It is not known whether or not BNP levels in a non–heart failure range are also associated with future changes in LV function. To our knowledge, there has never been a population-based cohort study in which the relationship between BNP and changes in LV function (assessed by echocardiography) was examined.
Therefore, the current study aimed to investigate prospective associations of BNP levels in a non–heart failure range with changes in markers of LV function and potential effect modification by type 2 diabetes.
RESEARCH DESIGN AND METHODS
Study population
The Hoorn Study is an observational population-based cohort study on glucose metabolism in the general Dutch Caucasian population (N = 2,484) initiated in 1989. The study design and population have been described in detail elsewhere (12). In 2000, random samples of participants (n = 898) were invited to participate along with all participants who were previously diagnosed with type 2 diabetes (n = 176). Of the 1,074 invited, 648 (60%) participated. In addition, 217 participants who were diagnosed with type 2 diabetes in the Hoorn Screening Study (1998–2000) were invited, of whom 188 (87%) participated. In the follow-up examinations, we excluded 40 (5%) individuals for whom no satisfactory echocardiography data were measured at baseline, 12 (1%) individuals who lacked the mental competence required to participate, and 129 (15%) individuals died. Of the remaining 655 individuals, 441 (67%) participated in echocardiographic examinations at follow-up. Participants with LV systolic (an EF <50%) or diastolic dysfunction (a left atrial [LA] volume index >40 mL/m2) at baseline (n = 46) and for whom data on BNP (n = 78) or glucose status (n = 4) were missing were excluded from the analyses. To maintain reliability of the echocardiographic data, individuals who had atrial fibrillation, wall motion abnormalities, and/or moderate/severe aortic or mitral valve insufficiencies or stenoses (n = 13) were also excluded. Thus, in total 300 individuals were included. The study complied with the Declaration of Helsinki, the local ethics committee approved the study, and written informed consent was obtained from all participants.
BNP levels
Plasma BNP was determined in spare frozen EDTA samples that had been stored at −80°C for 4 years. BNP was determined in picomoles per liter (equivalent to 3.5 pg/mL) using an immunoradiometric assay kit (Shionoria, Osaka, Japan). Inter- and intra-assay variability coefficients were in the relevant range of <10% (13).
Markers of LV function
Echocardiography was performed at baseline, and after a mean follow-up of 8 years with an HP SONOS 5500 echocardiography system (2–4 MHz transducer; Andover, MA) according to a standardized protocol consisting of two-dimensional, M-mode, and pulsed-wave Doppler assessments as previously described (14). At follow-up, this protocol was expanded with tissue Doppler assessments of LV annular myocardial lengthening velocities.
LV mass was determined as LV mass index and LV mass index divided by the LV end diastolic volume index (LVMI/LVEDVI) as previously described (11). LV systolic function was determined by measuring the LV EF. A set of three markers of LV diastolic function was used: LA volume index and the product of LA volume and LV mass index (LAV*LVMI) were determined as described (11). The ratio of early diastolic LV inflow velocity (E) to early diastolic lengthening velocity (e′), E/e′, was determined using tissue and pulsed-wave Doppler assessments.
Additional measurements (baseline)
We assessed BMI, waist/hip ratio, heart rate, blood pressure, prior cardiovascular disease (CVD), serum total and HDL cholesterol, serum triglycerides, insulin, and HbA1c, as described elsewhere (12). The simplified Modification of Diet in Renal Disease (MDRD) equation was used to determine the estimated glomerular filtration rate (eGFR; mL/min), with serum creatinine values recalculated into values standardized to reference methods (15). All participants were classified as non–type 2 diabetes or type 2 diabetes according to the 2006 World Health Organization criteria on the basis of a standard oral glucose tolerance test (16). We obtained self-reported information on health status, medical history, current medication use, and lifestyle habits. All participants were screened for atrial fibrillation according to the Minnesota criteria code 8-3-1 using a 12-lead electrocardiogram (17).
Statistical analysis
Baseline characteristics and markers of LV function at follow-up are presented as mean ± SD or in case of a skewed distribution as median (interquartile range). The F test for ANOVA in the case of continuous variables and χ2 test in the case of proportions were performed to test for differences between tertiles of BNP. In the case of skewed distributions, tests were conducted on log-transformed data.
Linear regression was used to assess the relationship between BNP levels at baseline with markers of LV function at follow-up stratified for glucose status and adjusted for age and sex. Regression coefficients (β) and 95% CI were reported. Linearity was judged based on P-P plots and residual histograms. To investigate whether or not LV function had changed according to baseline BNP levels, we adjusted all models that assessed associations with markers of LV function for baseline values of these markers. Although E/e′ at baseline was not available, we addressed changes in E/e′ by adjusting the relevant models for baseline LA volume index instead. We addressed additional confounding or mediation by adding baseline values of BMI, waist-to-hip ratio, systolic and diastolic blood pressure, mean arterial blood pressure, use of antihypertensive, oral antidiabetic, or lipid-lowering medication, HbA1c, insulin, total and HDL cholesterol, triglycerides, eGFR, prior CVD, valvular disease, and diabetes duration one by one to the models. Variables that changed the regression coefficients >10% were deemed to be relevant confounders and were added to the final model.
The P values <0.05 were considered statistically significant. The influence of glucose status on the relationship was investigated based on the reported regression coefficients for each group. Regression coefficients of the groups were considered significantly different if they were not present in each other’s CIs. Statistical analyses were performed with SPSS for Windows (version 15.0, SPSS Inc.).
In order to investigate whether or not the associations between BNP and LV diastolic and systolic function were modified by abnormal levels of total cholesterol (≥6.5 mmol/L) and/or triglycerides (2 mmol/L), insulin (≥100 pmol/L), eGFR (MDRD ≤60 mL/min/1.73 m2) or a low heart rate (≤60 bpm), we repeated all regression analyses excluding these individuals (one factor at a time) and determined whether or not this changed the results.
RESULTS
The final study population consisted of 300 individuals with BNP levels in a range of 0.5–46.1 pmol/L, a heart rate <90 beats per min, of whom 96 individuals were classified as having type 2 diabetes. In participating individuals, baseline BNP levels were significantly lower and type 2 diabetes more prevalent than in nonattendees. In addition, baseline LV mass index, LVMI/LVEDVI, LA volume index, and LAV*LVM were higher and LV EF was lower in those that did not participate in the follow-up (data not shown).
Baseline characteristics
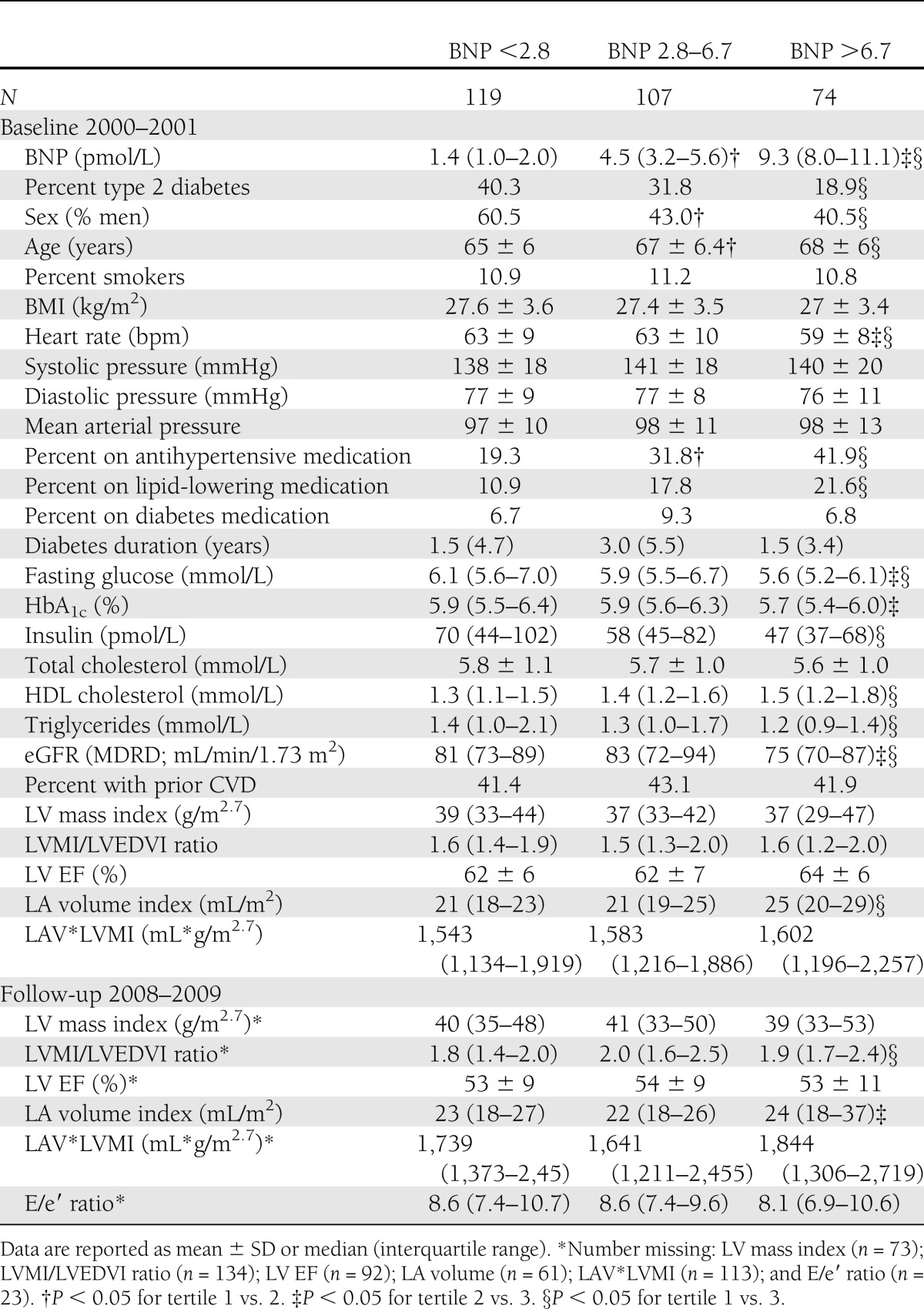
Individuals with higher BNP levels were more likely to be women and were less likely to have type 2 diabetes compared with those in the lowest tertile of BNP (Table 1). Individuals in both the highest and middle tertile were relatively older and more likely to use antihypertensive medication compared with those in the lowest tertile. Heart rate, triglycerides, and eGFR were lower with higher BNP levels. The use of lipid-lowering medication and HDL cholesterol were higher with higher BNP.
Table 1.
Characteristics of the study population for the total population and according to tertiles of BNP
BNP and changes in LV mass and markers of LV systolic and diastolic function
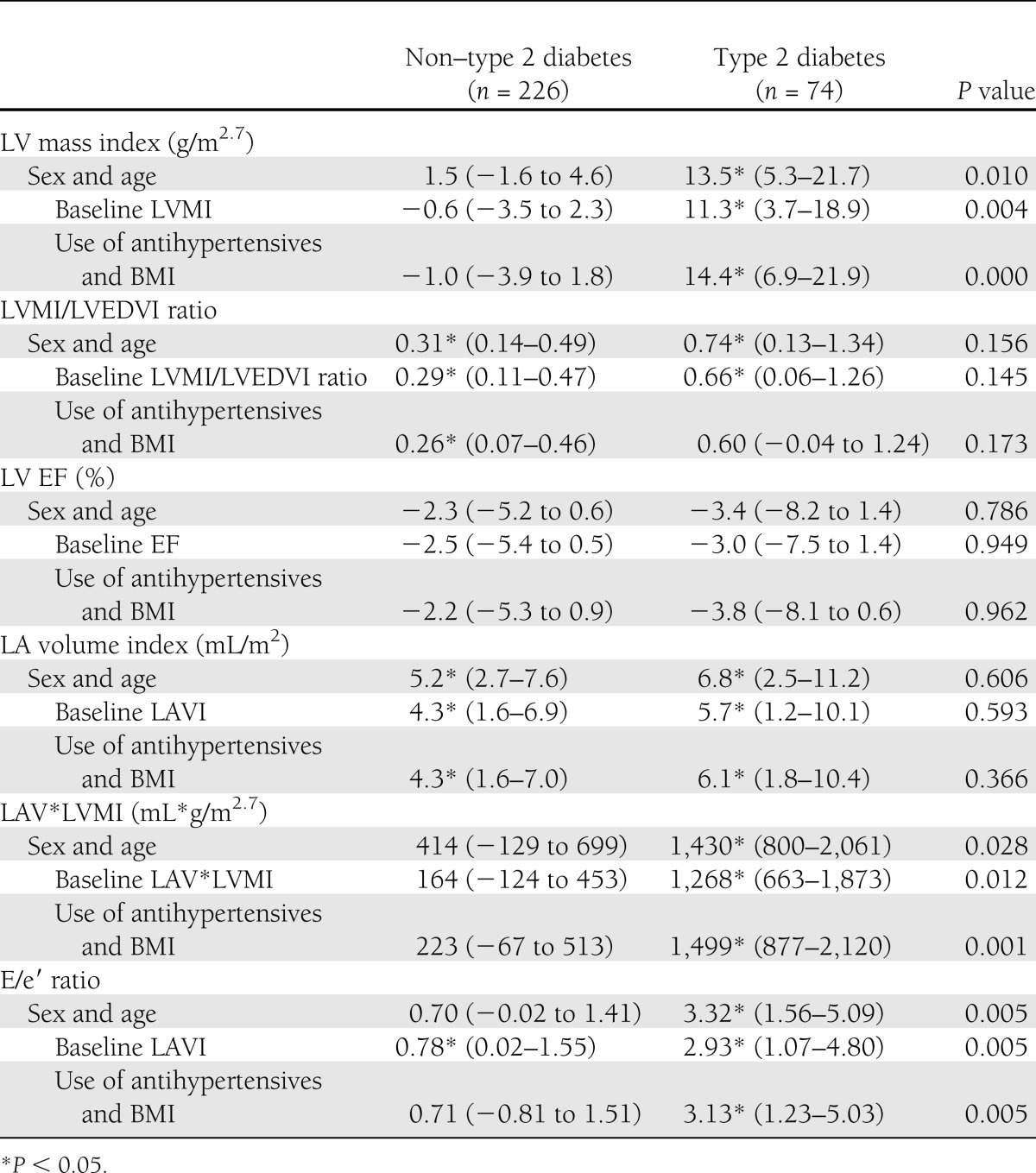
After adjustment for age and sex, a 10 pmol/L higher baseline BNP was significantly associated with a 13.5 g/m2.7 higher LV mass index after 8 years of follow-up in individuals with type 2 diabetes (Table 2). This association was largely independent of baseline LV mass index, the use of antihypertensive medication, and BMI. After these adjustments, a 10 pmol/L higher baseline BNP was associated with a 0.31 higher LVMI/LVEDVI ratio in individuals without type 2 diabetes.
Table 2.
Regression coefficients per 10 pmol/L increase of baseline BNP for LV mass and LV systolic and LV diastolic function, stratified for glucose status, and P values for interaction tests
The association between BNP and LVMI/LVEDVI in individuals with type 2 diabetes was not significantly different from those without type 2 diabetes, but lost statistical significance. Baseline BNP was not significantly associated with LV systolic function.
After adjustment for age and sex, a 10 pmol/L increase of baseline BNP was associated with 5.2 and 6.8 mL/m2 higher LA volume index in individuals without and with type 2 diabetes, respectively. In individuals without type 2 diabetes, there was a nonsignificant trend toward higher LAV*LVMI and E/e′ ratio with higher BNP. In individuals with type 2 diabetes, a 10 pmol/L higher baseline BNP was significantly associated with a 1,430 mL*g/m2.7 higher LAV*LVMI and a 3.32 higher E/e′ ratio. These associations remained significant after adjustment for baseline values and other confounders. The relationship between BNP and the other markers of LV diastolic function were stronger for individuals with type 2 diabetes compared with those without type 2 diabetes.
The nondiabetic versus diabetic groups were not significantly different with regard to LVMI/LVEDVI ratio, LV EF, and LA volume index. Pooled results (adjusted for sex, age, and baseline heart function) show that a 10 pmol/L higher baseline BNP was significantly associated with a 0.33 (95% CI 0.14–0.52) higher LVMI/LVEDVI ratio, a 2.7 (−5.1 to −0.3) lower LV EF, and a 4.2 (1.9–6.3) mL/m2 higher LA volume index.
All associations were similar for both sexes (P > 0.10 for interaction), with the exception of the associations of BNP with LAV*LVMI and E/e′ in the same subgroup of individuals with type 2 diabetes. These were stronger in women with type 2 diabetes compared with men with type 2 diabetes. Exclusion of subgroups with high pulse pressure, high lipid levels, high insulin levels, low heart rate, and/or low eGFR did not alter any of the adjusted associations (data not shown). Associations were similar when analyzing subjects with all of the measurements available (no missing echocardiographic values).
CONCLUSIONS
The present population-based study with an 8-year follow-up addressing the prospective associations between BNP and markers of LV function had two main findings. First, we observed strong associations between elevated BNP levels in a non–heart failure range and future changes in LV mass and markers of LV diastolic function at 8-year follow-up in individuals with type 2 diabetes. BNP was weakly associated with changes in LV mass and markers of LV diastolic dysfunction in individuals without type 2 diabetes.
The current study extends previously reported cross-sectional associations of BNP levels in a non–heart failure range with markers of LV diastolic function (11,18,19). To our knowledge, this is the first study that shows that BNP levels also predict future changes in LV dimensions and function. Other prospective studies showed that higher BNP levels in a non–heart failure range predicted heart failure, cardiovascular events, atrial fibrillation, stroke, transient ischemic attack, and increased mortality (10,20). Our results indicate that these slightly elevated BNP levels might reflect ongoing deterioration of LV diastolic function. The relationship between BNP and 8-year deterioration of LV systolic function was not significant and was similar for individuals with and without type 2 diabetes.
There was an overall trend toward more deterioration of LV systolic and diastolic function in individuals without type 2 diabetes with higher BNP levels, but this was only statistically significant for LA volume index and LVMI/LVEDVI. Previous studies have shown that BNP levels in a non–heart failure range were associated with markers of LV function in individuals without type 2 diabetes, and our results indicate a similar trend. This lack of significance in our study might be due to the fact that the relationships were weaker in individuals without type 2 diabetes and that the number of individuals involved in this study was smaller than in previous studies.
The influence of glucose status on BNP
An important finding of this study is that the associations between BNP and markers of LV function were strengthened by the presence of type 2 diabetes. Similar BNP levels predicted a steeper increase of LV mass index and a faster deterioration of LV diastolic function in individuals with type 2 diabetes compared with those without type 2 diabetes. In addition, individuals with type 2 diabetes had lower levels of BNP in the present population. This has also been found in other population-based studies (21,22). This may be partially due in part to the high prevalence of obesity in type 2 diabetes. There is a hypothesis to explain this phenomenon: lower BNP levels in obese individuals are observed due to a higher clearance of natriuretic peptides as receptors for clearance of natriuretic peptides are present in adipose tissue (21). In the Dallas Heart Study, it was found that lean mass and not fat mass was responsible for the association between higher BMI and lower natriuretic peptide levels (22). One of the explanations given was that a substance produced in the lean mass suppresses either synthesis or release of natriuretic peptides from cardiomyocytes. Although the mechanism behind this process remains to be identified, part of its explanation may lie in these differences in the observed BNP levels of individuals with type 2 diabetes.
Strengths and limitations
Strengths of the current study include its population-based prospective design, echocardiography at baseline and follow-up, and the detailed characterization of all individuals who participated in the study. There were also some limitations. As usual in cohort studies, the study population was subject to selective nonresponse. The participants that underwent echocardiography at baseline but not at follow-up were more likely to have type 2 diabetes and had higher BNP levels, higher LVMI, and worse LV systolic and LV diastolic function. Most likely, this selection bias has led to an underestimation of reported relationships particularly in our analyses of individuals with type 2 diabetes. BNP values are known to be influenced by age, sex, other cardiac diseases such as myocardial infarction and valvular heart disease, renal insufficiency, medication use, and BMI, as mentioned above (23). We have adjusted for these factors in our analyses in order to take these influences into account. Individuals with moderate or severe aortic or mitral valve insufficiencies were excluded a priori.
Another limitation is the high percentage of missing values for some echocardiographic measurements. Nonetheless, secondary analyses proved that results were not altered after exclusion of individuals who had missing values for any of the echocardiographic measures.
In conclusion, our results demonstrate that higher BNP levels in a non–heart failure range were independently associated with increased LV mass and deteriorated LV diastolic function, but not LV systolic function over time, particularly in individuals with type 2 diabetes. Therefore, slightly elevated BNP levels should be interpreted differently in individuals with and without type 2 diabetes.
Acknowledgments
This work was supported by the Dutch Diabetes Research Foundation (grant 2005.00.010), the Netherlands Heart Foundation (grant 98154), and funding from Novartis Pharma BV, the Netherlands.
M.D. disclosed advisory board membership for Abbott, Eli Lilly & Co, Merck Sharp & Dohme, and Novo Nordisk; is a consultant for AstraZeneca/BMS, Eli Lilly & Co, Merck Sharp & Dohme, Novo Nordisk, and sanofi-aventis; and is on the speaker’s bureau for Eli Lilly & Co, Merck Sharp & Dohme, and Novo Nordisk. Through M.D., the Diabetes Center, VU University Medical Center, receives all fees related to the activities mentioned above, as well as research support from Amylin Pharmaceuticals Inc., Eli Lilly & Co, Merck Sharp & Dohme, Novartis, Novo Nordisk, and Takeda. W.J.P. is the recipient and coordinator of a European Commission, Research Directorate General, FP7-Health-2010 grant on diastolic heart failure (261409–MEDIA). No other potential conflicts of interest relevant to this article were reported.
M.H.K. researched data and wrote the manuscript. K.v.d.H. and M.A. researched data and reviewed and edited the manuscript. O.K., C.D.A.S., G.N., and J.M.D. conceived and designed the study and reviewed and edited the manuscript. R.M.A.H. researched data and reviewed and edited the manuscript. M.D., F.B., and W.J.P. reviewed and edited the manuscript. M.H.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 46th Annual Meeting of the European Association for the Study of Diabetes, Stockholm, Sweden, 20–24 September 2010 (Diabetologia 2010;53:112).
The authors thank Y. de Groot of the VU University Medical Center Amsterdam for providing excellent training and supervision of echocardiographic assessments at follow-up. The authors also thank M.R.F.T. van Eijck-Weel of the VU University Medical Center Amsterdam for organizing and performing baseline echocardiography and for sharing her experience and the employees of the Diabetes Research Center in Hoorn for skilled performance in the organization and fulfillment of data collection at follow-up.
References
- 1.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 1974;34:29–34 [DOI] [PubMed] [Google Scholar]
- 2.Liew D, Schneider H, D’Agostino J, Shaw J, Krum H. Utility of B-type natriuretic peptide as screen for ventricular dysfunction in patients with diabetes: response to Epshteyn et al. Diabetes Care 2004;27:848–849; author reply 848–849 [DOI] [PubMed] [Google Scholar]
- 3.Greenberg B. Pre-clinical diastolic dysfunction in diabetic patients: where do we go from here? J Am Coll Cardiol 2010;55:306–308 [DOI] [PubMed] [Google Scholar]
- 4.Diamant M, Lamb HJ, Groeneveld Y, et al. Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. J Am Coll Cardiol 2003;42:328–335 [DOI] [PubMed] [Google Scholar]
- 5.Djoussé L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA 2009;302:394–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilkins MR, Redondo J, Brown LA. The natriuretic-peptide family. Lancet 1997;349:1307–1310 [DOI] [PubMed] [Google Scholar]
- 7.Maisel AS, McCord J, Nowak RM, et al. Breathing Not Properly Multinational Study Investigators Bedside B-Type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. Results from the Breathing Not Properly Multinational Study. J Am Coll Cardiol 2003;41:2010–2017 [DOI] [PubMed] [Google Scholar]
- 8.Paulus WJ, Tschöpe C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007;28:2539–2550 [DOI] [PubMed] [Google Scholar]
- 9.Takahashi T, Nakamura M, Onoda T, et al. Predictive value of plasma B-type natriuretic peptide for ischemic stroke: a community-based longitudinal study. Atherosclerosis 2009;207:298–303 [DOI] [PubMed] [Google Scholar]
- 10.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004;350:655–663 [DOI] [PubMed] [Google Scholar]
- 11.van den Hurk K, Alssema M, Kamp O, et al. Slightly elevated B-type natriuretic peptide levels in a non-heart failure range indicate a worse left ventricular diastolic function in individuals with, as compared with individuals without, type 2 diabetes: the Hoorn Study. Eur J Heart Fail 2010;12:958–965 [DOI] [PubMed] [Google Scholar]
- 12.Henry RM, Kostense PJ, Spijkerman AM, et al. Hoorn Study Arterial stiffness increases with deteriorating glucose tolerance status: the Hoorn Study. Circulation 2003;107:2089–2095 [DOI] [PubMed] [Google Scholar]
- 13.Boomsma F, Deinum J, van den Meiracker AH. Relationship between natriuretic peptide concentrations in plasma and posture during blood sampling. Clin Chem 2001;47:963–965 [PubMed] [Google Scholar]
- 14.Henry RM, Kamp O, Kostense PJ, et al. Hoorn study Left ventricular mass increases with deteriorating glucose tolerance, especially in women: independence of increased arterial stiffness or decreased flow-mediated dilation: the Hoorn study. Diabetes Care 2004;27:522–529 [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Coresh J, Greene T, et al. Chronic Kidney Disease Epidemiology Collaboration Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007;53:766–772 [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization and International Diabetes Federation Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. Geneva, Switzerland, WHO Document Production Services, 2006 [Google Scholar]
- 17.Prineas RJ, Crow RS, Blackburn H. Cardiovascular survey methods. WHO Monograph Series No 56. Geneva, World Health Organization, 1968 [PubMed] [Google Scholar]
- 18.Goetze JP, Mogelvang R, Maage L, et al. Plasma pro-B-type natriuretic peptide in the general population: screening for left ventricular hypertrophy and systolic dysfunction. Eur Heart J 2006;27:3004–3010 [DOI] [PubMed] [Google Scholar]
- 19.Epshteyn V, Morrison K, Krishnaswamy P, et al. Utility of B-type natriuretic peptide (BNP) as a screen for left ventricular dysfunction in patients with diabetes. Diabetes Care 2003;26:2081–2087 [DOI] [PubMed] [Google Scholar]
- 20.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA 2005;293:1609–1616 [DOI] [PubMed] [Google Scholar]
- 21.Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004;109:594–600 [DOI] [PubMed] [Google Scholar]
- 22.Das SR, Drazner MH, Dries DL, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation 2005;112:2163–2168 [DOI] [PubMed] [Google Scholar]
- 23.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol 2007;50:2357–2368 [DOI] [PubMed] [Google Scholar]