Abstract
OBJECTIVE
Advanced glycation end products (AGEs) evoke inflammatory reactions, contributing to the development and progression of atherosclerosis. We investigated the relationship between serum AGE level and vascular inflammation.
RESEARCH DESIGN AND METHODS
The study involved 275 outpatients at Kurume University, Japan (189 males and 86 females; mean age 61.2 ± 8.8 years) who underwent complete history and physical examinations and determinations of blood chemistry and anthropometric variables, including AGEs. Serum AGE level was examined by enzyme-linked immunosorbent assay. Vascular [18F]fluorodeoxyglucose (FDG) uptake, an index of vascular inflammation, was measured as blood-normalized standardized uptake value, known as the target-to-background ratio (TBR), by FDG–positron emission tomography (FDG-PET). Furthermore, we examined whether the changes in serum AGE level after treatment with oral hypoglycemia agents (OHAs) were correlated with those of TBR in another 18 subjects whose AGE value was >14.2 units/mL (mean ± 2 SD).
RESULTS
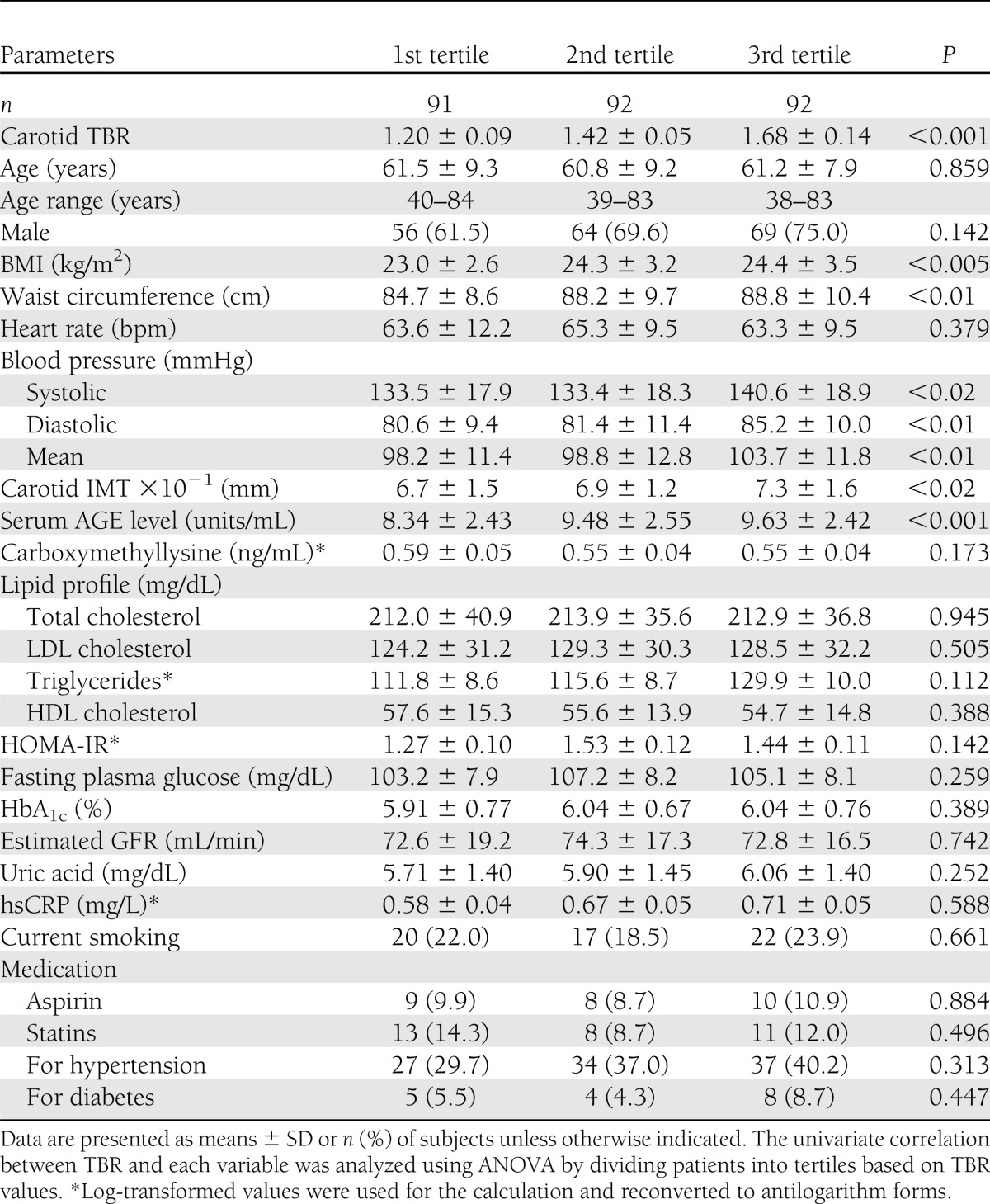
Mean serum AGE level and carotid TBR values were 9.15 ± 2.53 and 1.43 ± 0.22 units/mL, respectively. Multiple stepwise regression analysis revealed that TBR was independently correlated with AGEs (P < 0.001), carotid intima-media thickness (P < 0.01), and BMI (P < 0.02). When age- and sex-adjusted AGE values stratified by TBR tertiles were compared using ANCOVA, a significant trend was observed (P < 0.01). In addition, the changes in AGEs after OHA treatment were positively (r = 0.50, P < 0.05) correlated with those in TBR value.
CONCLUSIONS
The current study reveals that serum AGE level is independently associated with vascular inflammation evaluated by FDG-PET, suggesting that circulating AGE value may be a biomarker that could reflect vascular inflammation within an area of atherosclerosis.
There is a growing body of evidence, ranging from the results of in vitro experiments to pathological analysis to epidemiological studies, that atherosclerosis is intrinsically an inflammatory disease (1). Proinflammatory cytokines such as tumor necrosis factor-α and interleukin-1 have been shown to cause endothelial dysfunction—an initial step of atherosclerosis (1). Furthermore, atherosclerotic plaques contain numerous inflammatory cells, particularly macrophages, which could secrete a variety of growth factors, cytokines, and enzymes and subsequently contribute to the weakening of the fibrous cap of the plaques (2). Therefore, inflammatory plaques are considered vulnerable and prone to rupture, which could lead to acute coronary syndromes (3). We, along with others, have recently found that [18F]fluorodeoxyglucose (FDG) accumulation corresponds to macrophage-rich areas of carotid plaques and that FDG–positron emission tomography (FDG-PET) is capable of identifying and quantifying vascular inflammation within an area of atherosclerosis (4,5).
Reducing sugars can react nonenzymatically with the amino groups of protein to form Amadori products. These early glycation products undergo further complex reactions such as rearrangement, dehydration, and condensation to become irreversibly cross-linked, heterogeneous fluorescent derivatives, termed advanced glycation end products (AGEs) (6). The formation and accumulation of AGEs have been shown to progress during the normal aging process and at an accelerated rate under hyperglycemic and/or inflammatory and oxidative stress conditions (6). There is a growing body of evidence to show that AGEs evoke inflammatory and thrombogenic reactions in various cell types, thus being implicated in the development and progression of atherosclerosis (7–10). However, it remains unknown whether the circulating level of AGEs is independently correlated with vascular inflammation evaluated by FDG-PET. In this study, we investigated which anthropometric and metabolic variables, including serum level of AGEs, are independently associated with vascular inflammation evaluated as target-to-background ratio (TBR) by FDG-PET in Japanese subjects. Furthermore, we examined whether the changes in serum AGE levels (ΔAGEs) after treatment with oral hypoglycemia agents (OHAs) were correlated with those of TBR values (ΔTBR) in another 18 impaired glucose tolerance or type 2 diabetic patients whose AGE value was >14.2 units/mL (mean ± 2 SD).
RESEARCH DESIGN AND METHODS
Subjects and design of study 1
Study 1 involved 275 outpatients in Kurume University Hospital (189 males and 86 females) with a mean age of 61.2 ± 8.8 years. The numbers of patients who received aspirin, statins, antihypertension drugs, and OHA were 27, 32, 98, and 17, respectively. We excluded patients with chronic inflammatory disease, recent active infection, and neoplastic disorders and those who had a radiographically documented cerebrovascular disease, angiographically documented coronary artery disease, and a history of coronary vascular events. Patients who received insulin injections for the treatment of diabetes were also excluded. All participants gave informed consent to participate in this study. The Ethics Committee for Clinical Research of Kurume University approved this study.
Data collection
The medical history and smoking status were ascertained by questionnaire. Smoking status was classified as current habitual smoking or not. Waist circumference was measured as an index of central obesity. Blood pressure was measured in the sitting position using an upright standard sphygmomanometer. Vigorous physical activity and smoking were avoided for at least 30 min before blood pressure and resting heart rate measurements.
Blood was drawn after 12-h fasting from the antecubital vein in the morning for determinations of lipids (total cholesterol, LDL cholesterol, triglycerides, and HDL cholesterol), plasma glucose, insulin, HbA1c, blood urea nitrogen, creatinine, uric acid, and high-sensitivity C-reactive protein (hsCRP). These blood chemistry variables were measured by standard methods at a commercial laboratory (The Kyodo Igaku Laboratory, Fukuoka, Japan) as described previously (11). Measurement of serum AGE level was performed by competitive enzyme-linked immunosorbent assay (ELISA) as described previously (12). In brief, 96-well microtiter plates were coated with 0.1 μg/mL AGE-BSA. Then, test samples (50 µL) were added to each well as a competitor for 50 µL polyclonal antibodies directed against AGE-BSA (1:1,000), followed by incubation for 2 h at room temperature with gentle shaking on a horizontal rotary shaker. After incubation of each well with alkaline phosphatase–conjugated anti-rabbit IgG, p-nitrophenyl phosphate was added as a colorimetric substrate. Then, the plate was read using a microplate reader. In this study, one unit corresponds to 1 μg glyceraldehyde-derived AGE-BSA standard. Intra- and interassay coefficients of variation were 6.2 and 8.8%, respectively. Serum carboxymethyllysine (CML) level was measured with a commercially available kit according to the supplier’s recommendations (CycLex, Nagano, Japan). Insulin resistance was estimated using the homeostasis model assessment of insulin resistance (HOMA-IR). HOMA-IR index was calculated from the values of FPG (milligrams per deciliter) and fasting insulin (microunits per milliliter) using the following formula: (glucose × insulin)/405. Estimated glomerular filtration rate (GFR) was calculated using the Modification of Diet in Renal Disease study equation modified with a Japanese coefficient (13).
Measurement of carotid artery intima-media thickness
Carotid artery intima-media thickness (IMT) was determined as a parameter of atherosclerosis. IMT of the common carotid artery was determined using duplex ultrasonography with a 10-MHz transducer (SSA-380A; Toshiba, Tokyo, Japan) according to a method described previously (5). In brief, longitudinal B-mode images at the diastolic phase of cardiac cycles were recorded by a single trained technician who was blinded to the subjects’ background. Measurements of carotid IMT were made by the same technician using fine-slide calipers at three levels of the lateral and medial walls 1–3 cm proximal to the carotid bifurcation. The mean of these six measurements was taken as the value for the carotid IMT. The intraobserver or interobserver variability of IMT measurements was <5%.
FDG-PET imaging
FDG-PET imaging was performed according to previously reported methods (4,5,14–16). In brief, after at least 12 h of fasting, the study patients were intravenously administered FDG (4.2 MBq [0.12 mCi])/kg body wt). One hour after the FDG injection, three-dimensional whole-body PET imaging was carried out using a PET scanner (Allegro; Philips Medical Systems, Cleveland, OH). We performed attenuation correction for the PET imaging using a rotating rod of activity in the PET scanner. Contrast-enhanced computed tomography images were also taken from the skull base to the diaphragm using Light Speed Ultra 16 (GE Healthcare, Milwaukee, WI). To overcome the spatial resolution limitations of PET in this study, we carefully performed coregistration of PET and computed tomography imaging for review on a workstation (Sun Microsystems, Santa Clara, CA). The intensity of FDG uptake was quantified by measuring the standardized uptake value (SUV) corrected for body weight. The SUV was calculated using the maximum pixel activity value within the region of interest placed on the vascular wall of the transaxial PET/computed tomography image. The arterial SUV score was determined as the average of the SUVs of both the common carotid arteries obtained from 10 consecutive PET/computed tomography images, each separated by 4 mm in length with the most cranial site starting at the carotid bifurcation. Subsequently, in order to reduce the influence of difference in FDG clearance from blood of each patient, TBR was calculated as arterial SUV score divided by venous blood SUV as previously described (4,5,14–16). Two blinded radiologists measured the TBR values. The intraobserver or interobserver variability of TBR measurements was <5%.
Subjects and design of study 2
We measured AGE levels in 52 subjects whose clinical data were previously published (17). Then, 18 patients with impaired glucose tolerance or type 2 diabetes (10 males and 8 females, mean age 65.8 ± 8.2 years) who had ultrasonic evidence of atherosclerosis and increased FDG uptake and whose AGE value was >14.2 units/mL (mean ± 2 SD) (14.4–22.6 units/mL) were enrolled in study 2. Impaired glucose tolerance was diagnosed by a 75-g oral glucose tolerance test. Although 7 of 18 patients were already treated for diabetes, glimepiride or pioglitazone was further added to all the patients for 4 months, and then serum AGE levels and TBR values were reevaluated. During the study period, subjects were instructed not to change their lifestyles and to continue taking the same dose of any concomitant drugs. We examined the correlation between ΔAGEs and ΔTBR after OHA therapy. The study protocol was also approved by the ethics committee of Kurume University. All subjects provided written informed consent.
Statistical analysis
Data are described as means ± SD. Discontinuous variables were coded as dummy variables. Because of skewed distributions, natural logarithmic transformations were performed for triglycerides, HOMA-IR, carboxymethyllysine, serum creatinine, and hsCRP. Mean values (95% CI) were exponentiated and are presented as geometric means ± SD, where the SD is approximated as the difference of the exponentiated CI/3.92, which is the number of SDs in a 95% CI where data are normally distributed. We confirmed that the histogram of serum AGE levels showed a normal distribution using the Shapiro-Wilk test (Supplementary Fig. 1). The univariate correlation between TBR and each variable was analyzed using ANOVA by dividing patients into tertiles on the basis of TBR values. To determine the independent parameters related to the TBR values, we performed multiple stepwise regression analysis. Age- and sex-adjusted AGE values stratified by TBR tertiles were compared using ANCOVA. Pearson product moment correlation test was performed to determine the correlation between ΔAGEs and ΔTBR. Statistical significance was defined as P < 0.05. All statistical analyses were performed using the SPSS system (SPSS, Chicago, IL). For statistical comparisons of clinical values at baseline and after treatment, paired t test was performed.
RESULTS
Mean serum AGE levels and carotid TBR values were 9.15 ± 2.53 and 1.43 ± 0.22 units/mL, respectively. Clinical variables stratified by tertiles of TBR in subjects in study 1 are presented in Table 1. Statistical significance and a dose-response relationship were demonstrated between TBR and BMI (P < 0.005), waist circumference (P < 0.01), carotid IMT (P < 0.02), systolic blood pressure (P < 0.02), diastolic blood pressure (P < 0.01), mean blood pressure (P < 0.01), and AGEs (P < 0.001) (Table 1). Because these significant parameters could be closely correlated with each other, we performed multiple stepwise regression analysis in order to determine the independent correlates of TBR values. AGEs (P < 0.001), carotid IMT (P < 0.01), and BMI (P < 0.02) remained significant and were independently correlated with carotid TBR levels (R2 = 0.107). AGEs were independently correlated with TBR values adjusted for FPG as well (data not shown). There was no significant correlation between TBR values and carboxymethyllysine, a well-characterized and major immunologic epitope of AGEs (8) (Table 1).
Table 1.
Clinical variables stratified by tertiles of TBR in subjects in study 1
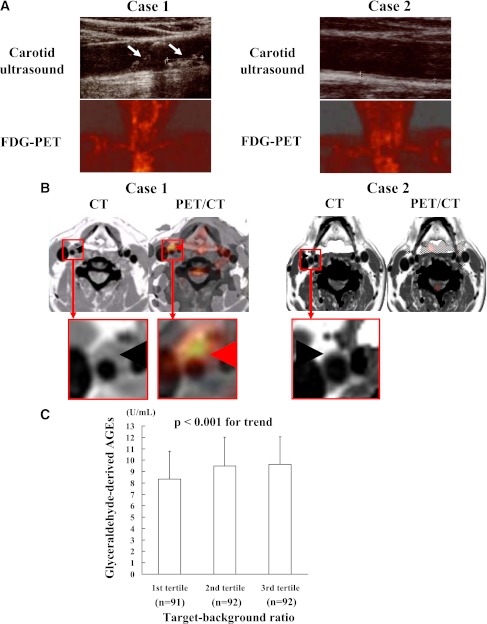
Figure 1A shows representative images of ultrasound and FDG-PET in the carotid arteries of two case subjects. One is the case subject with high serum AGE level (case 1) and the other with low AGE level (case 2).
Figure 1.
Representative coronal images of ultrasound and FDG-PET (A) and transaxial images of contrast-enhanced computed tomography (CT) and coregistration of FDG-PET and computed tomography (PET/CT) (B) in the carotid arteries of two case subjects. Age- and sex-adjusted AGE values stratified by carotid TBR tertiles (C). One is a case subject with high serum AGE levels (case 1) and the other with low serum AGE levels (case 2). A: White arrows show carotid atherosclerotic plaques, while red arrows indicate vascular FDG uptake. B: Black arrowhead denotes vessel wall or atherosclerotic plaque, while red arrowheads indicate vascular FDG uptake. C: When age- and sex-adjusted AGE values stratified by TBR tertiles were compared using ANCOVA, a significant trend was observed (P < 0.01). (A high-quality digital representation of this figure is available in the online issue.)
Coregistration of FDG-PET and computed tomography images revealed that FDG was taken up into the plaques of carotid arteries in case 1 but not in case 2 (Fig. 1B). When age- and sex-adjusted AGE values stratified by TBR tertiles were compared using ANCOVA, a significant trend was observed (P < 0.01) (Fig. 1C).
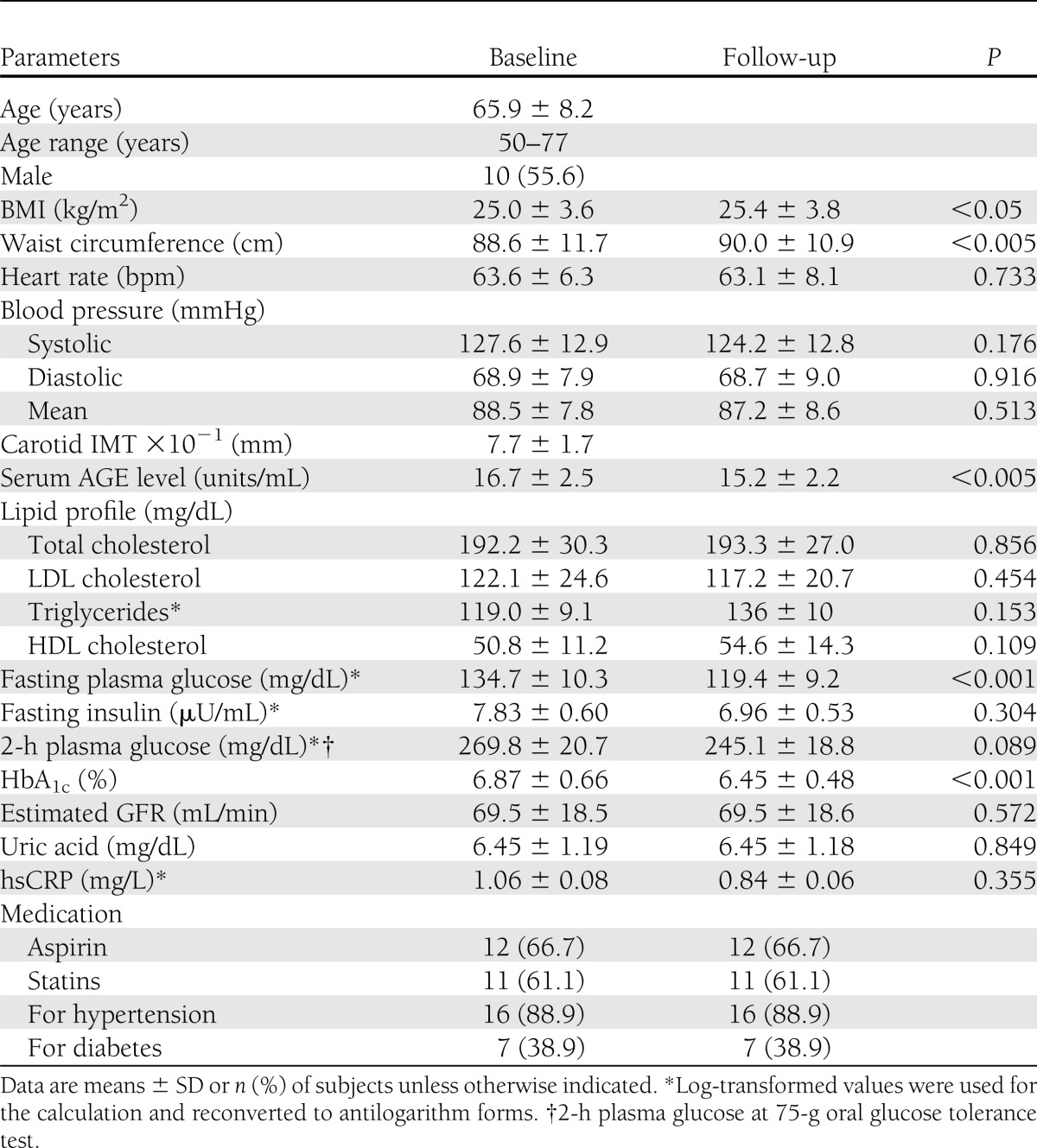
In 18 subjects with impaired glucose tolerance or type 2 diabetes whose AGE value was >14.2 units/mL (mean ± 2 SD), OHA treatment (11 pioglitazone and 7 glimepiride) for 4 months significantly (P < 0.01) decreased AGE and HbA1c levels from 16.7 ± 2.5 to 15.2 ± 2.2 units/mL and from 6.87 ± 0.66 to 6.54 ± 0.48%, respectively (Table 2). Furthermore, when the data in the two groups were analyzed separately, AGE and HbA1c values were significantly (P < 0.01) reduced by the treatment with pioglitazone but not glimepiride. AGE and HbA1c levels in the pioglitazone group were decreased from 17.5 ± 2.8 to 15.4 ± 2.6 units/mL and from 6.73 ± 0.66 to 6.29 ± 0.48%, respectively, while those in the glimepiride group were decreased from 15.6 ± 1.2 to 15.0 ± 1.4 units/mL and from 7.10 ± 0.60 to 6.70 ± 0.36%. ΔAGEs obtained by OHA treatment were positively (r = 0.50, P < 0.05) correlated with ΔTBR (Fig. 2).
Table 2.
Characteristics of the subjects in study 2
Figure 2.
Correlation of ΔAGEs obtained by OHA treatment with ΔTBR (r = 0.50, P < 0.05). Pearson product moment correlation test was performed to determine the relationships between ΔAGEs obtained by OHA treatment and ΔTBR.
CONCLUSIONS
There is accumulating evidence that vascular inflammation plays a central role in the development and progression of atherosclerosis (1). We demonstrated here for the first time that AGE level was most strongly correlated with vascular inflammation evaluated by FDG-PET, which was independent of BMI, IMT, and metabolic parameters. Furthermore, ΔAGEs after OHA treatment were positively correlated with ΔTBR. These findings suggest that although carotid artery IMT determined by high-resolution ultrasonography is one of the best indicators for atherosclerosis and may be associated with vascular inflammation (18) and that the significance between ΔAGEs and ΔTBR disappeared after taking out the obvious outliner (Fig. 2), circulating levels of AGEs could also be a novel biomarker that reflects vascular inflammation in humans.
The current study has extended the previous clinical observations showing that 1) AGEs were detected within an area of atherosclerosis in humans (19,20) and 2) serum level of AGEs is positively associated with inflammatory and thrombogenic biomarkers, endothelial dysfunction, and the presence of coronary artery disease in patients with diabetes or nondiabetic subjects (21–24). In addition, Kilhovd et al. (25,26) recently reported that increased serum level of AGEs predicted coronary heart disease mortality in both nondiabetic and diabetic women. These observations suggest that circulating AGE level could partly reflect local AGE burden within the vessels and may also be a biomarker for predicting future cardiovascular events in humans. Stitt et al. (20) reported that serum and arterial tissue accumulation levels of AGEs were correlated with each other, suggesting the clinical relevance of measuring serum level of AGEs in evaluating AGE burden within an area of atherosclerosis. In this study, we did not find any association of TBR with carboxymethyllysine, FPG, HbA1c, or HOMA-IR (Table 1). This lack of association may be caused by a different turnover in AGEs and these parameters.
In the current study, for the quantitative analysis of vascular inflammation we measured TBR values in the carotid arteries by FDG-PET because TBR value is a quantitative parameter of glucose metabolic rate within the vessels, and thus high TBR values could indicate vascular inflammation (4,5,14–16). Furthermore, Rudd et al. (27) reported that FDG accumulation was histologically located in the atherosclerotic plaques of eight human specimens. These findings suggest that TBR value evaluated by FDG-PET is a reliable marker for vascular inflammation. Yang et al. (15) reported that TBR values were inversely associated with soluble levels of RAGE, a decoy receptor for AGEs in type 2 diabetes, thus supporting the active participation of AGEs in vascular inflammation. It should be noted here that hsCRP, one of the best-characterized biomarkers for systemic low-grade inflammation in patients with coronary artery disease (28), was not correlated with the vascular inflammation evaluated by FDG-PET in our subjects. This finding was consistent with a previous observation showing that FDG uptake was not correlated with systemic inflammatory biomarkers such as hsCRP and interleukin-18 (29). Therefore, although TBR values had the positive correlation with hsCRP in healthy individuals (16), FDG-PET may be more sensitive than hsCRP for the detection of local vascular inflammation, and circulating AGE levels may reflect the vascular inflammation more sensitively than hsCRP.
Study 1 in this work had a cross-sectional design; therefore, it did not enable elucidation of the causal relationships between serum levels of AGEs and vascular inflammation in humans. However, in study 2, reduction of serum AGE level after OHA treatment was positively associated with the decrease in vascular inflammation evaluated by TBR in impaired glucose tolerance or type 2 diabetic subjects. Furthermore, there have been several studies showing the pathological role of AGEs in atherosclerosis (7–10). The finding that ΔAGEs after OHA treatment are positively correlated with ΔTBR further suggests that circulating AGEs are not just a biomarker but might be a mediator of vascular inflammation within an area of atherosclerosis.
We have very recently found in a randomized control trial that pioglitazone, but not glimepiride, decreases TBR values in common carotid arteries and ascending aorta of the aortic arch in patients with impaired glucose tolerance or diabetes, although both treatments reduced HbA1c values comparably (17). These findings suggest that vascular inflammation evaluated by TBR could not be regulated in a glucose lowering–dependent manner. In addition, in this study there was a significant positive association between ΔAGEs and ΔTBR (Fig. 2) but not between ΔHbA1c and ΔTBR (Supplementary Fig. 2A). These observations indicate that AGEs could have a greater influence on vascular inflammation than HBA1c. AGEs are one of the inflammatory biomarkers and could be generated under oxidative stress and inflammatory conditions as well (8,9,30). Furthermore, the turnover rate may differ between HbA1c and AGEs, and there was no significant correlation between ΔHbA1c and ΔAGEs (Supplementary Fig. 2B). Therefore, although study 2 had a small number of subjects, given the present findings that AGE level was significantly (P < 0.01) reduced by pioglitazone, but not glimepiride, attenuation of vascular inflammation by pioglitazone observed in our recent randomized control trial (17) may be ascribed in part to its AGE- but not HbA1c-lowering property.
There are multiple types of immunologically distinct and structurally identified AGEs such as pyrraline and pentosidine in humans (31). However, they constitute a small percentage of circulating AGEs in vivo and their biological relevance in vascular inflammation has remained unclear (31). In addition, lack of a standardized method for quantifying AGEs has made it difficult to determine which types of AGEs are clinically relevant to vascular injury in vivo. In this study, to examine the relationship between serum level of AGEs and vascular inflammation we used an ELISA system that specially recognized glyceraldehyde-derived AGEs because we have recently found that 1) glyceraldehyde-derived AGE levels are increased under inflammatory and/or hyperglycemic conditions (30,32,33), 2) glyceraldehyde-derived AGE levels are inversely associated with adiponectin, an adipocytokine with anti-inflammatory properties in humans (34), and 3) this type of AGE mimics the biological effects of AGE-rich serum fractions purified from diabetic patients on endothelial cells (35). Furthermore, because of the stronger binding affinity to RAGE (36), they were more toxic than glucose-derived AGEs. Therefore, although glyceraldehyde, which could be derived from glucose metabolism, is not a major sugar in vivo and its incubation with proteins will generate a large number of AGEs and although there is some criticism that measurement of AGEs using liquid chromatography–tandem mass spectrometry technique may produce results different from those acquired using ELISA (37), our present study suggests that glyceraldehyde-derived AGEs could contribute to vascular inflammation in humans. Antiglyceraldehyde-derived AGE antibodies used in the ELISA did not cross-react with several structurally identified AGEs such as carboxymethyllysine-BSA, carboxyethyllysine-BSA, pyrraline-BSA, pentosidine-BSA, argpyrimidine-BSA, 3-deoxyglucosone imidazolone–BSA, glyoxal-lysine dimer, methylglyoxal-lysine dimer, and glyceraldehyde-derived pyridinium (Supplementary Fig. 3). Therefore, our ELISA system could not quantitatively evaluate these structurally identified AGEs. Thus, although carboxymethyllysine was not correlated with vascular inflammation here, it would be interesting to examine further whether serum levels of other structurally identified AGEs such as pyrraline and pentosidine are associated with TBR.
Limitations
In this study, we enrolled patients without overt cardiovascular diseases. In addition, most of the subjects in study 1 were nondiabetic. Furthermore, although the correlations shown between AGEs and TBR showed a statistically significant trend to increase with TBR tertile (study 1) and that ΔAGEs obtained by OHA treatment were positively associated with ΔTBR (study 2), the clinical significance was still unclear, and whether serum levels of AGEs were correlated with vascular inflammation in patients with diabetes or cardiovascular disease remained unknown. Furthermore, the positive association between ΔAGEs and ΔTBR may be driven by one outliner (Fig. 2). Therefore, further longitudinal intervention studies are needed to clarify the clinical utility of measuring glyceraldehyde-derived AGEs for evaluating the vascular inflammation in these patients.
In the current study, although univariate analysis revealed no significant correlation between various medications and vascular inflammation, we cannot totally exclude the possibility that medication could affect the present results because both vascular inflammation and serum levels of AGEs can be influenced by OHA, antihypertension drugs, or statins (8–10). However, during the study period, subjects were instructed to continue taking the same dose of any concomitant drugs. Therefore, it is unlikely that statin therapy could affect the present results of study 2.
Partial volume effects and recovery coefficient may affect maximum TBR and SUV values. The recovery coefficients of the tomograph and reconstruction method in relation to the carotid arteries and venous vessels were 20 and 93%, respectively. However, we measured the TBR and SUV values with FDG-PET according to the gold standard method previously published in several articles (4,5,14–16). Furthermore, even if there are some errors associated with partial volume effects, recovery coefficient, and SUV maximum pixel intensity, they could equally affect the TBR and SUV values of each patient. So, it is unlikely that they could confound the present findings. Average diameters of carotid arteries and venous vessel for targeting regions measured by PET/computed tomography imaging were 7.40 ± 0.75 and 20.15 ± 3.07 mm, respectively. Since the spatial resolution of our PET scanner had 4.8 mm full width at half maximum at the center, it can adequately evaluate vascular inflammation.
Supplementary Material
Acknowledgments
This study was supported in part by a grant from Mitsui Life Social Welfare Foundation (to N.T. and S.-i.Y.); by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan (to N.T., S.-i.Y., and M.T.); and by grants of Collaboration with Venture Companies Project from the Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan (to S.-i.Y.).
No potential conflicts of interest relevant to this article were reported.
N.T. and S.-i.Y. conceptualized and designed the study; acquired, analyzed, and interpreted data; and drafted the manuscript. M.T. analyzed and interpreted data. A.H., A.T., Y.N., N.K., and M.M., acquired, analyzed, and interpreted data. H.K., M.I., and N.H. acquired, analyzed, and interpreted data and critically revised the manuscript for important intellectual content. T.M. acquired, analyzed, and interpreted data. T.I. conceptualized and designed the study; acquired, analyzed, and interpreted data; and drafted and critically revised the manuscript for important intellectual content. N.T. and S.-i.Y. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Kazumi Hirakawa and Naoko Tanaka, PET Center, Kurume University School of Medicine; Yuri Nishino, Department of Pathophysiology and Therapeutics of Diabetic Vascular Complications, Kurume University School of Medicine; and Miho Kogure, Miyuki Nishikata, Makiko Kiyohiro, and Kimiko Kimura, Department of Medicine, Division of Cardio-Vascular Medicine, Kurume University School of Medicine, for their excellent technical assistance.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0087/-/DC1.
References
- 1.Lamon BD, Hajjar DP. Inflammation at the molecular interface of atherogenesis: an anthropological journey. Am J Pathol 2008;173:1253–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newby AC. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol 2008;28:2108–2114 [DOI] [PubMed] [Google Scholar]
- 3.Takenaka K, Yamagishi S, Matsui T, et al. Pigment epithelium-derived factor (PEDF) administration inhibits occlusive thrombus formation in rats: a possible participation of reduced intraplatelet PEDF in thrombosis of acute coronary syndromes. Atherosclerosis 2008;197:25–33 [DOI] [PubMed] [Google Scholar]
- 4.Tawakol A, Migrino RQ, Bashian GG, et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 2006;48:1818–1824 [DOI] [PubMed] [Google Scholar]
- 5.Tahara N, Kai H, Yamagishi S, et al. Vascular inflammation evaluated by [18F]-fluorodeoxyglucose positron emission tomography is associated with the metabolic syndrome. J Am Coll Cardiol 2007;49:1533–1539 [DOI] [PubMed] [Google Scholar]
- 6.Vlassara H, Bucala R, Striker L. Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest 1994;70:138–151 [PubMed] [Google Scholar]
- 7.Schmidt AM, Yan SD, Wautier JL, Stern D. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res 1999;84:489–497 [DOI] [PubMed] [Google Scholar]
- 8.Yamagishi S, Imaizumi T. Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des 2005;11:2279–2299 [DOI] [PubMed] [Google Scholar]
- 9.Yamagishi S, Nakamura K, Matsui T, Ueda S, Noda Y, Imaizumi T. Inhibitors of advanced glycation end products (AGEs): potential utility for the treatment of cardiovascular disease. Cardiovasc Ther 2008;26:50–58 [DOI] [PubMed] [Google Scholar]
- 10.Jandeleit-Dahm K, Cooper ME. The role of AGEs in cardiovascular disease. Curr Pharm Des 2008;14:979–986 [DOI] [PubMed] [Google Scholar]
- 11.Yamagishi S, Adachi H, Abe A, et al. Elevated serum levels of pigment epithelium-derived factor in the metabolic syndrome. J Clin Endocrinol Metab 2006;91:2447–2450 [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi M, Makita Z, Bucala R, Suzuki T, Koike T, Kameda Y. Immunological evidence that non-carboxymethyllysine advanced glycation end-products are produced from short chain sugars and dicarbonyl compounds in vivo. Mol Med 2000;6:114–125 [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuo S, Imai E, Horio M, et al. Collaborators developing the Japanese equation for estimated GFR Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009;53:982–992 [DOI] [PubMed] [Google Scholar]
- 14.Fayad ZA, Mani V, Woodward M, et al. dal-PLAQUE Investigators Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet 2011;378:1547–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang SJ, Kim S, Hwang SY, et al. Association between sRAGE, esRAGE levels and vascular inflammation: analysis with (18)F-fluorodeoxyglucose positron emission tomography. Atherosclerosis 2012;220:402–406 [DOI] [PubMed] [Google Scholar]
- 16.Yoo HJ, Kim S, Park MS, et al. Vascular inflammation stratified by C-reactive protein and low-density lipoprotein cholesterol levels: analysis with 18F-FDG PET. J Nucl Med 2011;52:10–17 [DOI] [PubMed] [Google Scholar]
- 17.Mizoguchi M, Tahara N, Tahara A, et al. Pioglitazone attenuates atherosclerotic plaque inflammation in patients with impaired glucose tolerance or diabetes a prospective, randomized, comparator-controlled study using serial FDG PET/CT imaging study of carotid artery and ascending aorta. JACC Cardiovasc Imaging 2011;4:1110–1118 [DOI] [PubMed] [Google Scholar]
- 18.Coll B, Feinstein SB. Carotid intima-media thickness measurements: techniques and clinical relevance. Curr Atheroscler Rep 2008;10:444–450 [DOI] [PubMed] [Google Scholar]
- 19.Nakamura Y, Horii Y, Nishino T, et al. Immunohistochemical localization of advanced glycosylation end products in coronary atheroma and cardiac tissue in diabetes mellitus. Am J Pathol 1993;143:1649–1656 [PMC free article] [PubMed] [Google Scholar]
- 20.Stitt AW, He C, Friedman S, et al. Elevated AGE-modified ApoB in sera of euglycemic, normolipidemic patients with atherosclerosis: relationship to tissue AGEs. Mol Med 1997;3:617–627 [PMC free article] [PubMed] [Google Scholar]
- 21.Yamagishi S, Adachi H, Takeuchi M, et al. Serum level of advanced glycation end-products (AGEs) is an independent determinant of plasminogen activator inhibitor-1 (PAI-1) in nondiabetic general population. Horm Metab Res 2007;39:845–848 [DOI] [PubMed] [Google Scholar]
- 22.Nakamura K, Yamagishi S, Adachi H, et al. Circulating advanced glycation end products (AGEs) and soluble form of receptor for AGEs (sRAGE) are independent determinants of serum monocyte chemoattractant protein-1 (MCP-1) levels in patients with type 2 diabetes. Diabetes Metab Res Rev 2008;24:109–114 [DOI] [PubMed] [Google Scholar]
- 23.Tan KC, Chow WS, Ai VH, Metz C, Bucala R, Lam KS. Advanced glycation end products and endothelial dysfunction in type 2 diabetes. Diabetes Care 2002;25:1055–1059 [DOI] [PubMed] [Google Scholar]
- 24.Kanauchi M, Tsujimoto N, Hashimoto T. Advanced glycation end products in nondiabetic patients with coronary artery disease. Diabetes Care 2001;24:1620–1623 [DOI] [PubMed] [Google Scholar]
- 25.Kilhovd BK, Juutilainen A, Lehto S, et al. High serum levels of advanced glycation end products predict increased coronary heart disease mortality in nondiabetic women but not in nondiabetic men: a population-based 18-year follow-up study. Arterioscler Thromb Vasc Biol 2005;25:815–820 [DOI] [PubMed] [Google Scholar]
- 26.Kilhovd BK, Juutilainen A, Lehto S, et al. Increased serum levels of advanced glycation endproducts predict total, cardiovascular and coronary mortality in women with type 2 diabetes: a population-based 18 year follow-up study. Diabetologia 2007;50:1409–1417 [DOI] [PubMed] [Google Scholar]
- 27.Rudd JH, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 2002;105:2708–2711 [DOI] [PubMed] [Google Scholar]
- 28.Verma S, Szmitko PE, Ridker PM. C-reactive protein comes of age. Nat Clin Pract Cardiovasc Med 2005;2:29–36; quiz 58 [DOI] [PubMed] [Google Scholar]
- 29.Rudd JH, Myers KS, Bansilal S, et al. Relationships among regional arterial inflammation, calcification, risk factors, and biomarkers: a prospective fluorodeoxyglucose positron-emission tomography/computed tomography imaging study. Circ Cardiovasc Imaging 2009;2:107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura T, Sato E, Fujiwara N, et al. Circulating levels of advanced glycation end products (AGE) and interleukin-6 (IL-6) are independent determinants of serum asymmetric dimethylarginine (ADMA) levels in patients with septic shock. Pharmacol Res 2009;60:515–518 [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi M, Yamagishi S. Possible involvement of advanced glycation end-products (AGEs) in the pathogenesis of Alzheimer’s disease. Curr Pharm Des 2008;14:973–978 [DOI] [PubMed] [Google Scholar]
- 32.Nakamura T, Sato E, Fujiwara N, et al. Positive association of serum levels of advanced glycation end products and high mobility group box-1 with asymmetric dimethylarginine in nondiabetic chronic kidney disease patients. Metabolism 2009;58:1624–1628 [DOI] [PubMed] [Google Scholar]
- 33.Tahara N, Yamagishi S, Matsui T, et al. Serum levels of advanced glycation end products (AGEs) are independent correlates of insulin resistance in nondiabetic subjects. Cardiovasc Ther 2012;30:42–48 [DOI] [PubMed] [Google Scholar]
- 34.Hyogo H, Yamagishi S, Iwamoto K, et al. Elevated levels of serum advanced glycation end products in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol 2007;22:1112–1119 [DOI] [PubMed] [Google Scholar]
- 35.Yamagishi S, Nakamura K, Matsui T, et al. Pigment epithelium-derived factor inhibits advanced glycation end product-induced retinal vascular hyperpermeability by blocking reactive oxygen species-mediated vascular endothelial growth factor expression. J Biol Chem 2006;281:20213–20220 [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto Y, Yonekura H, Watanabe T, et al. Short-chain aldehyde-derived ligands for RAGE and their actions on endothelial cells. Diabetes Res Clin Pract 2007;77(Suppl. 1):S30–S40 [DOI] [PubMed] [Google Scholar]
- 37.Ahmed N, Mirshekar-Syahkal B, Kennish L, Karachalias N, Babaei-Jadidi R, Thornalley PJ. Assay of advanced glycation endproducts in selected beverages and food by liquid chromatography with tandem mass spectrometric detection. Mol Nutr Food Res 2005;49:691–699 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.