Abstract
OBJECTIVE
The recommended HbA1c diagnostic categories remain controversial and their utility in doubt in pediatrics. We hypothesized that alterations in the pathophysiologic mechanisms of type 2 diabetes may be evident in the American Diabetes Association recommended at-risk/prediabetes category (HbA1c 5.7 to <6.5%).
RESEARCH DESIGN AND METHODS
We compared in vivo hepatic and peripheral insulin sensitivity by [6,6-2H2] glucose and a 3-h hyperinsulinemic-euglycemic clamp and β-cell function by a 2-h hyperglycemic clamp (∼225 mg/dL) in overweight/obese (BMI ≥85th percentile) adolescents with prediabetes (HbA1c 5.7 to <6.5%) (n = 160) to those with normal HbA1c (<5.7%) (n = 44). β-Cell function was expressed relative to insulin sensitivity (i.e., the disposition index = insulin sensitivity × first-phase insulin).
RESULTS
In the prediabetes versus normal HbA1c category, fasting glucose, insulin, and oral glucose tolerance test (OGTT) area under the curve for glucose and insulin were significantly higher; hepatic and peripheral insulin sensitivity were lower; and β-cell function relative to insulin sensitivity was lower (366 ± 48 vs. 524 ± 25 mg/kg/min; P = 0.005). A total of 27% of youth in the normal HbA1c category and 41% in the prediabetes HbA1c category had dysglycemia (impaired fasting glucose and/or impaired glucose tolerance) by a 2-h OGTT.
CONCLUSIONS
Overweight/obese adolescents with HbA1c in the at-risk/prediabetes category demonstrate impaired β-cell function relative to insulin sensitivity, a metabolic marker for heightened risk of type 2 diabetes. Thus, HbA1c may be a suitable screening tool in large-scale epidemiological observational and/or interventional studies examining the progression or reversal of type 2 diabetes risk.
Glycated hemoglobin (HbA1c) is used to monitor diabetes control in diagnosed patients (1). In 2009, an international expert committee recommended that HbA1c also be used for diagnosis of diabetes and risk of diabetes (1). Subsequently, HbA1c diagnostic cutoffs were incorporated into the 2010 American Diabetes Association (ADA) guidelines for diabetes (HbA1c ≥6.5%) and prediabetes (HbA1c 5.7 to <6.5%) (2). Unlike glycemic measures (e.g., fasting glucose, oral glucose tolerance test [OGTT]), the HbA1c may be performed in the nonfasting state (2). However, adoption of these proposed criteria continues to be debated (3–8). In cross-sectional studies of adults, the HbA1c criteria had lower sensitivity for diabetes diagnosis compared with OGTT (6) or a single fasting plasma glucose (9). But, the sensitivity of the HbA1c criteria improved when compared with repeated fasting plasma glucose samples (3 years apart), and the combination of fasting glucose and HbA1c provided the greatest predictive value for 10-year diabetes risk compared with fasting glucose alone (single or repeated) (9). Furthermore, in a longitudinal study, HbA1c identified fewer cases of prediabetes at baseline, but had similar predictive value for progression to diabetes as fasting glucose (∼5-year follow-up) (10). Accordingly, recent pediatric studies indicate that HbA1c identifies fewer adolescents with diabetes/prediabetes compared with glycemic measures (4,5,11). However, similar to adults, HbA1c improved the predictive value of glycemic measures alone after a 2-year follow-up in adolescents (5). Because glycemic measures of impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) are linked to impaired insulin secretion relative to insulin sensitivity, conferring an increased risk of type 2 diabetes (12,13), we hypothesized that alterations in the pathophysiologic mechanisms of type 2 diabetes could be detected in the ADA recommended at-risk/prediabetes category (HbA1c 5.7 to <6.5%). Therefore, we aimed to evaluate in vivo insulin sensitivity and β-cell function in overweight/obese youth categorized according to the 2010 ADA HbA1c criteria (2) as normal versus prediabetes.
RESEARCH DESIGN AND METHODS
Approval by the Institutional Review Board of the University of Pittsburgh, parental consent and child assent were obtained prior to any research procedure. A total of 204 overweight/obese youth (according to age- and sex-specific BMI percentiles [14]) (89 African Americans and 115 Caucasians; 88 males and 116 females; ages 9 to <20 years old; Tanner stage II–V) (15) who had complete hyperinsulinemic-euglycemic and hyperglycemic clamp data obtained while participating in our ongoing studies of National Institutes of Health grants “Childhood Metabolic Markers of Adult Morbidity in Blacks” and “Childhood Insulin Resistance” were included. Some of these participants’ data have been reported along with details of our recruitment and screening procedures (13,16–18). Tests were conducted at the Pediatric Clinical and Translational Research Center of the Children’s Hospital of Pittsburgh.
Experimental procedures
A 3-h hyperinsulinemic (80 μU/m2/min)-euglycemic (100 mg/dL) clamp was performed after a 10–12-h overnight fast (13,19). Fasting endogenous/hepatic glucose production (HGP) was measured in 164 participants using a primed (2.2 µmol/kg)–constant rate infusion of [6,6-2H2] glucose (Isotech, Miamisburg, OH) at 0.22 µmol/kg/min for 2 h (−120 to 0 min) (20,21). Four baseline blood samples were collected (−30 to 0 min) for determination of glucose, insulin, and isotopic enrichment of glucose prior to the initiation (0 min) of the clamp. On a separate occasion, 1 to 3 weeks apart, and in random order, a 2-h hyperglycemic clamp (∼225 mg/dL) was performed (12,13,22). Either the day preceding one of the clamp procedures or on a separate visit within a 1- to 3-week period, a 2-h OGTT (1.75 g/kg glucola, maximum 75 g) was performed in 142 participants (23,24). Normal versus impaired glycemia was defined according to standards for fasting or 2-h OGTT glucose (2).
Percent body fat was measured with dual energy X-ray absorptiometry and abdominal visceral adipose tissue (VAT) with computed tomography in 154 subjects and magnetic resonance imaging in 50 subjects (17,21).
Biochemical analyses
Plasma glucose was measured by the glucose oxidase method (Yellow Springs Instrument Co., Yellow Springs, OH), and insulin by a commercial radioimmunoassay (catalog number 1011; LINCO Research, St. Charles, MO) (13). HbA1c was measured by high-performance liquid chromatography (Tosoh Medics), and “normal” was defined as HbA1c <5.7% and “prediabetes” as HbA1c ≥5.7% to <6.5% according to ADA criteria (2). Deuterium enrichment of glucose in the plasma was determined on a Hewlett-Packard 5971 mass spectrometer coupled to a 5890 series II gas chromatograph (Hewlett-Packard) (20,21).
Calculations
Fasting HGP was measured during the last 30 min of the 2-h baseline isotope infusion and hepatic insulin sensitivity (HIS) was calculated as 1,000/(HGP × fasting insulin) (12,20). Peripheral insulin sensitivity (mg/kg/min per µU/mL) was calculated during the last 30 min (150–180 min) of the hyperinsulinemic-euglycemic clamp (12,13,19,22). First-phase insulin (µU/mL) was calculated as the mean insulin concentration at times 2.5, 5, 7.5, 10, and 12.5 min during the hyperglycemic clamp (21,25). β-Cell function relative to insulin sensitivity, the disposition index (DI; mg/kg/min), was calculated as the product of insulin sensitivity and first-phase insulin (13,16). In the subset of 142 participants with OGTT, area under the curve (AUC) for glucose and insulin was calculated by the trapezoidal rule and the insulinogenic index (ΔI30/ΔG30) and the oral DI (oDI) as before (24–26).
Statistical analyses
Differences in categorical variables were determined by χ2 analysis, and differences in continuous variables were determined by two-tailed t test or by ANCOVA adjusting for race. Differences in insulin sensitivity, first-phase insulin, and DI across HbA1c categories (normal versus prediabetes) were determined by two-tailed t test and also by ANCOVA adjusting for race and adiposity (BMI, percent body fat, or VAT). Overall differences across subgroups of NGT versus dysglycemia by OGTT and normal versus prediabetes by HbA1c were determined using ANCOVA, adjusting for race and VAT in SPSS (PASW 18; SPSS Inc., Chicago, IL). Data are presented as mean ± SE. A P value of ≤0.05 was considered statistically significant, and P ≤ 0.10 was considered a trend.
RESULTS
Subject characteristics
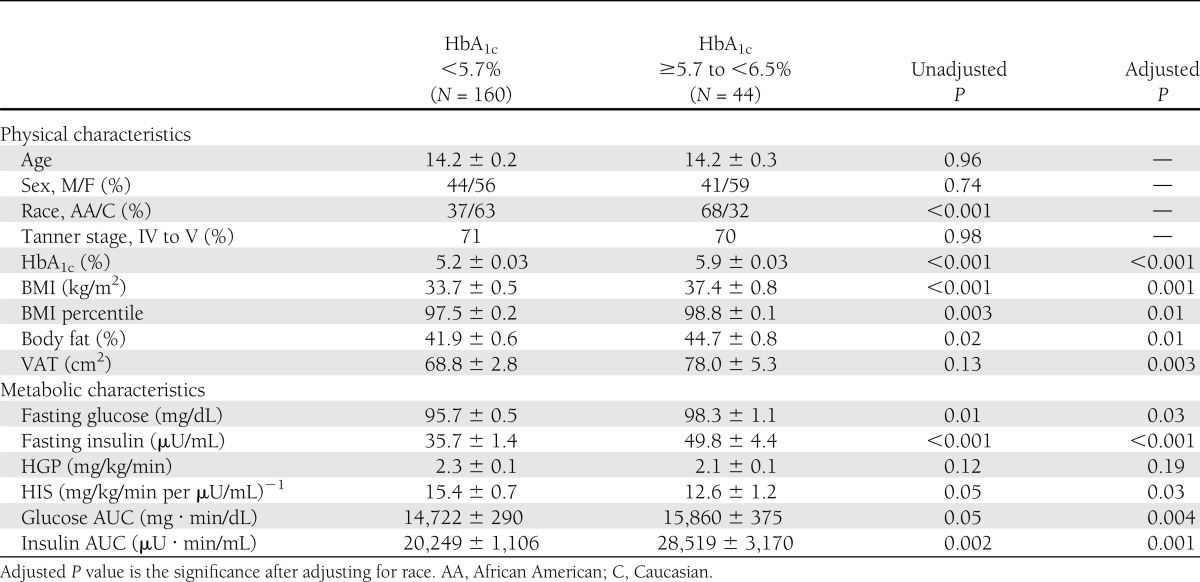
Age, sex, and Tanner stage distributions were not different between participants divided according to HbA1c category (normal versus prediabetes) (Table 1). There were more Caucasians in the normal HbA1c category and more African Americans in the prediabetes category. Adiposity measures (BMI, BMI percentile, percent body fat, and VAT) were significantly higher in the prediabetes HbA1c category than normal HbA1c, before and after adjustment for race.
Table 1.
Subjects’ physical and metabolic characteristics according to HbA1c category (normal, HbA1c <5.7% and prediabetes, HbA1c ≥5.7 to <6.5%)
Metabolic characteristics by HbA1c categories
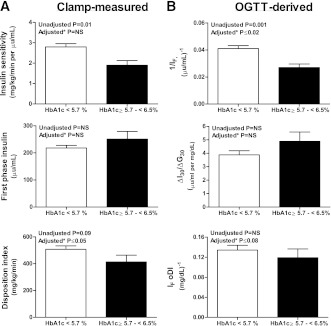
Fasting glucose, insulin, OGTT glucose AUC, and insulin AUC were higher, and HIS was lower, in the prediabetes versus normal HbA1c category (Table 1). These differences persisted after adjusting for race and percent body fat. Peripheral insulin sensitivity was lower in the prediabetes versus normal HbA1c category before and after adjustment for race (P = 0.004; data not shown), but was not different after additional adjustment for adiposity (Fig. 1A). First-phase insulin was not different between the two groups; however, DI, β-cell function relative to insulin sensitivity, was significantly lower in the prediabetes versus normal HbA1c category after adjusting for race and adiposity (BMI, percent body fat, or VAT; Fig. 1A). In the subset of participants with OGTT-derived indices (Fig. 1B), results of insulin sensitivity (1/IF) and oDI mirrored the results observed with the clamps.
Figure 1.
Clamp-measured insulin sensitivity, first-phase insulin, and DI (A) and OGTT-derived measures of insulin sensitivity, insulinogenic index (ΔI30/ΔG30), and oDI (B) in normal (<5.7%) versus prediabetes (5.7 to <6.5%) HbA1c categories. Adjusted P is the significance after adjusting for race and adiposity (BMI, percent body fat, or VAT).
HbA1c categories and glycemic measures from OGTT
In the normal HbA1c category, 27% had dysglycemia, and in the prediabetes HbA1c category, 41% had dysglycemia (P = 0.09). Within each HbA1c category, DI was lower by ∼35% in dysglycemia versus NGT groups. After adjustment for race and VAT, there were significant differences in DI among the four groups (P < 0.001), with the highest value in NGT in the normal HbA1c category (562 ± 33 mg/kg/min) and the lowest value in the dysglycemia group in the prediabetes HbA1c category (308 ± 58 mg/kg/min) (Fig. 2).
Figure 2.
Flow chart illustrating the distribution of NGT vs. dysglycemia (IFG and/or IGT) within each HbA1c category and their DI (mg/kg/min). *P < 0.05: NGT vs. dysglycemia within each HbA1c category analyzed by one-tailed t test based on previous findings of lower DI in dysglycemia compared with NGT (12,13).
CONCLUSIONS
The current study demonstrates that overweight/obese adolescents meeting the ADA HbA1c diagnostic criteria (2) for prediabetes have evidence of impaired β-cell function relative to insulin sensitivity, a metabolic signal for heightened risk of type 2 diabetes (27). This finding persisted after adjustment for race and for the greater adiposity in the youth with elevated HbA1c. These data support our hypothesis that alterations in the pathophysiologic mechanisms of type 2 diabetes are evident in the ADA-recommended at-risk/prediabetes category (HbA1c 5.7 to <6.5%).
Since the release of the HbA1c-based diagnostic criteria for prediabetes/diabetes by the ADA in 2010 (2), reports of adults and some in pediatrics have concluded that HbA1c is inferior to glycemic measures for diagnostic purposes (4–6,11). These studies evaluated the diagnostic sensitivity and specificity of HbA1c compared with glycemic measures, treating the latter as the gold standard. One such study determined that the inferior sensitivity of the recommended HbA1c cutoff compared with OGTT was worse for adolescents than for adults (4). However, although these studies evaluated equivalence of the new HbA1c criteria compared with glycemic criteria, they did not evaluate metabolic characteristics linked to future diabetes risk. Moreover, as noted in a Comment published in Diabetes Care, “it is a fallacy that the OGTT is a gold standard… if you define one test as a gold standard, all comparators will be inferior” (28). Indeed, in longitudinal studies, HbA1c criteria predicted a similar rate of progression to diabetes as did IFG, and the combination of fasting glucose and HbA1c provided the greatest predictive value of diabetes incidence (9,10).
The DI, which expresses β-cell function relative to insulin sensitivity, is an established metabolic predictor of progression to diabetes (12,13,29–31). Numerous longitudinal studies in adults have demonstrated the predictive value of DI (including oral DI) for the future development of diabetes (27,29,32,33). In addition, we (12,13) and others (30,31) have reported progressively declining DI across the spectrum of glycemia from normal to IFG and/or IGT to type 2 diabetes in youth. Therefore, even though the ADA HbA1c diagnostic criteria have a lower sensitivity for diagnosis of prediabetes when compared with glycemic measures, we show in this study that these criteria are consistent with greater metabolic risk (lower clamp DI, oral DI, and insulinogenic index) using sensitive metabolic assessments.
Evaluating HbA1c and glycemia together, youth with normal HbA1c combined with normoglycemia had the highest DI compared with the other groups, and DI seemed to be lowest overall in the group with both elevated HbA1c and dysglycemia (Fig. 2). Moreover, rates of dysglycemia were higher in the prediabetes (41%) versus normal HbA1c category (27%) (Fig. 2). Overall, these data support the use of HbA1c to identify youth with lower β-cell function for epidemiological observational and/or interventional studies of overweight/obese youth and progression to type 2 diabetes. However, the independent use of HbA1c for clinical diagnostic purposes may be premature. Collectively, these observations indicate that the combination of HbA1c and a glycemic measure may best pinpoint subjects at lowest or greatest risk of eventual diabetes (9,10). Longitudinal studies will be necessary to determine whether there are differences in rates of the development of diabetes between youth with normal HbA1c and dysglycemia versus those with elevated HbA1c and dysglycemia.
A potential challenge in the application of the HbA1c is the higher HbA1c in African Americans than Caucasians, present in our study and reported by others (5,34). Racial differences in HbA1c result in more African Americans and fewer Caucasians being identified as having prediabetes using HbA1c compared with glycemic criteria (6,7). Potentially, the ADA-recommended cut points may not be appropriate for all racial groups, and exploration of cut points customized for race/ethnicity in larger scale studies may be warranted. Regardless, in this study, we report impaired β-cell function as well as lower peripheral and hepatic insulin sensitivity in youth with elevated versus normal HbA1c, independent of the effects of race.
Strengths of the current investigation include the simultaneous assessment of HbA1c, clamp-measured in vivo insulin sensitivity and β-cell function, and an OGTT in a large group of adolescents. A limitation of this report is that we included only overweight/obese adolescents, and our population does not represent other minority groups (e.g., Hispanics) in which the utility of HbA1c may vary (34). Lastly, the number of youth with elevated HbA1c and dysglycemia is somewhat limited.
In conclusion, the recently recommended HbA1c criteria coincide with lower peripheral and hepatic insulin sensitivity and lower β-cell function relative to insulin sensitivity in overweight/obese adolescents. Larger and longitudinal studies are required to more thoroughly investigate the interplay of race within categories of dysglycemia and HbA1c in relation to metabolic risk factors for diabetes (e.g., DI). Although controversy is likely to continue surrounding the adoption of HbA1c-based criteria for the diagnosis of prediabetes and diabetes in youth, these data support that the ADA HbA1c criteria for prediabetes correspond to impaired β-cell function in overweight/obese youth, a metabolic marker of heightened type 2 diabetes risk.
Acknowledgments
This project was supported by U.S. Public Health Service grants R01 HD-27503 (to S.A.A.) and K24 HD-01357 (to S.A.A.), Richard L. Day Endowed Chair (to S.A.A.), the Department of Defense (to L.A.S., S.L., H.T., F.B., and S.A.A.), and the National Institutes of Health through grants M01-RR-00084, UL1-RR-024153, and UL1-TR-000005.
No potential conflicts of interest relevant to this article were reported.
L.A.S. analyzed the data and first-authored the manuscript. S.F.M. provided technical support for data analysis, editing, and review of the manuscript. S.L., H.T., and F.B. contributed research participants and data. L.F. managed the data entry and database maintenance. S.A.A. provided the study concept and design; acquired data; obtained funding; provided administrative, technical, and material support; supervised the study; and critically reviewed and edited the manuscript. S.A.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented orally at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
These studies would not have been possible without the nursing staff of the Pediatric Clinical and Translational Research Center, the devotion of the research team (Nancy Guerra, CRNP; Kristin Porter, RN; Sally Foster, RN, CDE; and Lori Bednarz, RN, CDE), the laboratory expertise of Resa Stauffer, and, most importantly, the commitment of the study participants and their parents.
References
- 1.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fonseca V, Inzucchi SE, Ferrannini E. Redefining the diagnosis of diabetes using glycated hemoglobin. Diabetes Care 2009;32:1344–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JM, Wu EL, Tarini B, Herman WH, Yoon E. Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr 2011;158:947–952, e1–e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nowicka P, Santoro N, Liu HB, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 2011;34:1306–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson DE, Rhee MK, Herrick K, Ziemer DC, Twombly JG, Phillips LS. Screening for diabetes and pre-diabetes with proposed A1C-based diagnostic criteria. Diabetes Care 2010;33:2184–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malkani S, Mordes JP. Implications of using hemoglobin A1C for diagnosing diabetes mellitus. Am J Med 2011;124:395–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misra A, Garg S. HbA1c and blood glucose for the diagnosis of diabetes. Lancet 2011;378:104–106 [DOI] [PubMed] [Google Scholar]
- 9.Selvin E, Steffes MW, Gregg E, Brancati FL, Coresh J. Performance of A1C for the classification and prediction of diabetes. Diabetes Care 2011;34:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heianza Y, Hara S, Arase Y, et al. HbA1c 5.7-6.4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): a longitudinal cohort study. Lancet 2011;378:147–155 [DOI] [PubMed] [Google Scholar]
- 11.Lee JM, Gebremariam A, Wu EL, LaRose J, Gurney JG. Evaluation of nonfasting tests to screen for childhood and adolescent dysglycemia. Diabetes Care 2011;34:2597–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bacha F, Gungor N, Lee S, Arslanian SA. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care 2009;32:100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacha F, Lee S, Gungor N, Arslanian SA. From pre-diabetes to type 2 diabetes in obese youth: pathophysiological characteristics along the spectrum of glucose dysregulation. Diabetes Care 2010;33:2225–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosner B, Prineas R, Loggie J, Daniels SR. Percentiles for body mass index in U.S. children 5 to 17 years of age. J Pediatr 1998;132:211–222 [DOI] [PubMed] [Google Scholar]
- 15.Tanner JM. Growth and endocrinology in the adolescent. In Endocrine and Genetic Diseases of Childhood. 1st ed Philadelphia, W.B. Saunders, 1969, p. 19–60 [Google Scholar]
- 16.Tfayli H, Bacha F, Gungor N, Arslanian S. Phenotypic type 2 diabetes in obese youth: insulin sensitivity and secretion in islet cell antibody-negative versus -positive patients. Diabetes 2009;58:738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee S, Guerra N, Arslanian S. Skeletal muscle lipid content and insulin sensitivity in black versus white obese adolescents: is there a race differential? J Clin Endocrinol Metab 2010;95:2426–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George L, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Surrogate estimates of insulin sensitivity in obese youth along the spectrum of glucose tolerance from normal to prediabetes to diabetes. J Clin Endocrinol Metab 2011;96:2136–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burns SF, Lee S, Arslanian SA. In vivo insulin sensitivity and lipoprotein particle size and concentration in black and white children. Diabetes Care 2009;32:2087–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacha F, Saad R, Gungor N, Arslanian SA. Adiponectin in youth: relationship to visceral adiposity, insulin sensitivity, and beta-cell function. Diabetes Care 2004;27:547–552 [DOI] [PubMed] [Google Scholar]
- 21.Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in African-American children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes 2002;51:3014–3019 [DOI] [PubMed] [Google Scholar]
- 22.Arslanian SA. Clamp techniques in paediatrics: what have we learned? Horm Res 2005;64(Suppl. 3):16–24 [DOI] [PubMed] [Google Scholar]
- 23.Tfayli H, Bacha F, Gungor N, Arslanian S. Islet cell antibody-positive versus -negative phenotypic type 2 diabetes in youth: does the oral glucose tolerance test distinguish between the two? Diabetes Care 2010;33:632–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab 2008;93:4231–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bacha F, Gungor N, Arslanian SA. Measures of beta-cell function during the oral glucose tolerance test, liquid mixed-meal test, and hyperglycemic clamp test. J Pediatr 2008;152:618–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjaarda LG, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Oral disposition index in obese youth from normal to prediabetes to diabetes: relationship to clamp disposition index. J Pediatr 2012;161:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cnop M, Vidal J, Hull RL, et al. Progressive loss of beta-cell function leads to worsening glucose tolerance in first-degree relatives of subjects with type 2 diabetes. Diabetes Care 2007;30:677–682 [DOI] [PubMed] [Google Scholar]
- 28.Buse JB. Screening for diabetes and prediabetes with proposed A1C-based diagnostic criteria: comment on Olson et al. Diabetes Care 2010;33:e174–; author reply e175. [DOI] [PubMed] [Google Scholar]
- 29.Utzschneider KM, Prigeon RL, Faulenbach MV, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009;32:335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Adamo E, Caprio S. Type 2 diabetes in youth: epidemiology and pathophysiology. Diabetes Care 2011;34(Suppl. 2):S161–S165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cali AMG, Man CD, Cobelli C, et al. Primary defects in beta-cell function further exacerbated by worsening of insulin resistance mark the development of impaired glucose tolerance in obese adolescents. Diabetes Care 2009;32:456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyssenko V, Almgren P, Anevski D, et al. Botnia study group Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes 2005;54:166–174 [DOI] [PubMed] [Google Scholar]
- 33.Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M. What is the best predictor of future type 2 diabetes? Diabetes Care 2007;30:1544–1548 [DOI] [PubMed] [Google Scholar]
- 34.Herman WH, Ma Y, Uwaifo G, et al. Diabetes Prevention Program Research Group Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care 2007;30:2453–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]