In October 2011, the Advisory Committee on Immunization Practices (ACIP) recommended hepatitis B vaccination for adults with diabetes in the United States (1). Serosurvey data from the 1999–2010 National Health and Nutrition Examination Survey found that noninstitutionalized adults aged ≥18 years with diabetes have an increased seroprevalence of past or current hepatitis B virus (HBV) infection (1). Outbreaks of hepatitis B in elderly persons with diabetes in long-term care facilities have been linked to diabetes care procedures (including blood glucose monitoring), with likely vehicles for transmission including spring-loaded finger-stick devices used on multiple patients and blood glucose testing meters that were not cleaned between uses on different patients.
In the U.S., hepatitis B vaccination is routinely recommended for infants, children, and adolescents. It also is recommended for adults at increased risk of HBV infection, including persons with end-stage renal disease or chronic liver disease, health care personnel, injection-drug users, and men who have sex with men (2–4). A hepatitis B vaccination series results in protection in a high proportion (>95%) of infants, children, and young adults (5). As with other vaccines, the efficacy of the hepatitis B vaccine progressively declines with advancing age, as well as with the presence of obesity and other comorbid conditions (6–12). Results of studies of the hepatitis B vaccine among persons with diabetes generally follow these patterns (13,14).
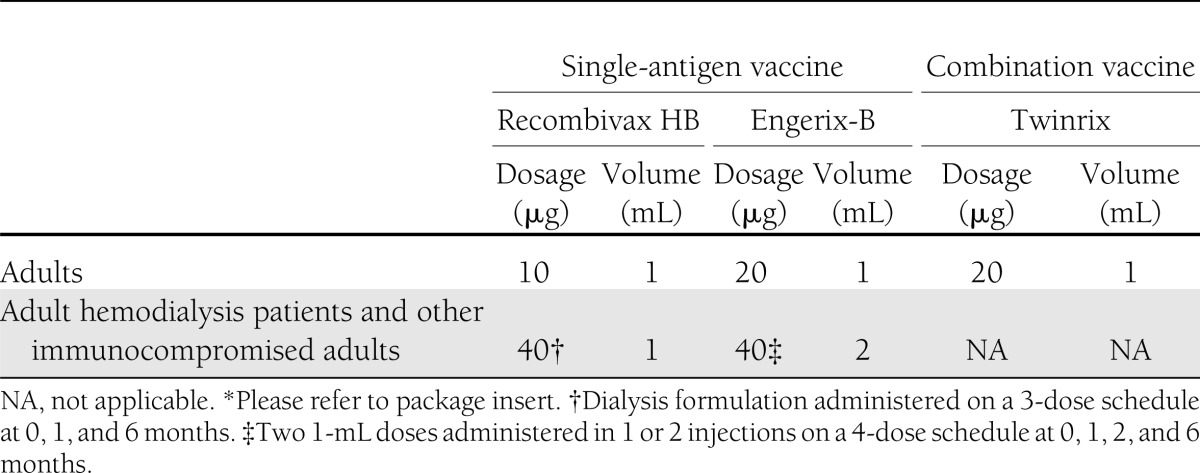
Primary hepatitis B vaccination usually consists of 3 (or 4) doses of 10 or 20 µg of recombinant hepatitis B surface antigen (HBsAg) protein administered intramuscularly into the deltoid muscle on a 0-, 1-, and 6-month schedule (Table 1). Alternative schedules are U.S.-approved for routine vaccination for specific ages and vaccine formulations, and they elicit dose-specific and final rates of seroprotection similar to those obtained on a 0-, 1-, and 6-month schedule (4). Two single-antigen recombinant vaccines, Recombivax HB (Merck & Co, Inc., Whitehouse Station, NJ) and Engerix-B (GlaxoSmithKline Biologicals, Rixensart, Belgium), and one combination hepatitis A/hepatitis B vaccine, Twinrix (GlaxoSmithKline Biologicals), are approved for use in adults in the U.S. (15). Hepatitis B vaccines are safe for all age groups. Administration of additional vaccine doses for nonresponders is not associated with an increase in adverse events (16). Vaccination is not contraindicated in persons with autoimmune or chronic diseases, or in those who are pregnant (4).
Table 1.
Recommended dosages of hepatitis B vaccine for adults aged >20 years* (4)
No review has been published of the efficacy of the hepatitis B vaccine among persons with diabetes; results of studies among persons with diabetes show some heterogeneity compared with adults without diabetes. Differences in diabetes type, management, and glycemic control, as well as vaccine, dosage, administration route, or schedule, may underlie this heterogeneity. The 2011 ACIP recommendation for hepatitis B vaccination for adults with diabetes, an increasing incidence of diabetes, and the high prevalence of diabetes among certain groups recommended for hepatitis B vaccination (e.g., persons with end-stage renal disease) suggests that a review of vaccine efficacy among persons with diabetes may be timely. We therefore performed a systematic review of the literature and summarized the evidence for seroprotection after hepatitis B vaccination among persons with diabetes.
RESEARCH DESIGN AND METHODS
Search strategy
Electronic searches of MEDLINE (via PubMed), EMBASE (via Ovid), Cochrane Library, and Web of Knowledge databases were performed. The search terms consisted of (hepatitis b vaccin* OR hbv vaccin* OR hepatitis b immuni* OR hbv immuni*) AND (immunogeni* OR immune response OR antibody) AND (diabetes). The search terms also were used as Medical Subject Heading terms (MEDLINE) or key words (EMBASE), as applicable. Where possible, limits included publication date from 1986 through 30 April 2012, English language, and study of humans. The MEDLINE and EMBASE searches were limited to items containing abstracts. Studies conducted in the U.S. and other countries were eligible for inclusion.
Inclusion criteria
Peer-reviewed, published randomized clinical trials or observational studies (all types) assessing response to hepatitis B vaccine among persons with type 1 or type 2 diabetes were included. Studies among both children and adults were included to ascertain the effect of age upon vaccine response among persons with diabetes. Reports had to specify the proportion (numerator and denominator) of subjects seroprotected by diabetes status (or data available that allowed for calculation of the proportions), or available odds ratios from a multivariate analysis, which included diabetes as a predictor variable. Studies of specific subject populations of which persons with diabetes represented a subset were included.
Exclusion criteria
Studies were excluded when immune response using an antibody to hepatitis B surface antigen (anti-HBs) threshold of 10 mIU/mL was not reported; immune response was not measured between 0 and 6 months after the last vaccine dose (because anti-HBs titers wane over time); 10 or fewer subjects with diabetes were included; vaccine was administered intradermally; or subjects were not naïve to the vaccine or had positive serology for HBsAg, anti-HBs, or antibody to hepatitis B core antigen, indicating past or current hepatitis B infection. When two studies reported results for duplicate subjects, one study was excluded. Studies were not excluded when subjects received additional vaccine dose(s) because they did not achieve seroprotective concentrations or when inclusion of a fraction of subjects meeting an exclusion criterion was deemed unlikely to affect the findings. The determination as to inclusion or exclusion was based on the above criteria and was made irrespective of study results.
Data extraction and categorization
Data were manually extracted and electronically recorded. Studies were classified into one of three subject categories: adults (mean age >18 years), children (mean age ≤18 years), and hemodialysis/chronic kidney disease patients. Elements for extraction included study characteristics (e.g., study design, sample size, publication year, country); subject characteristics (e.g., age, diabetes type); vaccine characteristics (e.g., vaccine, dosage, route, schedule); and the proportion of subjects attaining seroprotection, including the time interval (in months) from the last dose at which immune response was measured. When vaccine or route of administration differed by study arm, extraction elements were recorded for each arm when possible.
An anti-HBs threshold of 10 mIU/mL after series completion (an accepted marker of immune protection) defined seroprotection (4). When multiple immune response measurements were reported reflecting seroprotection at different intervals during the primary vaccine series, the result after the final dose was recorded. When immune response was measured more than once after vaccination, the result from the interval closest to 1 to 2 months after the last dose (corresponding to the recommended interval for postvaccination testing for serologic response in the U.S. [4]) was recorded. When immune response was measured after a booster dose(s) or novel adjuvant administration, results were recorded separately and noted.
Odds ratios for attainment of seroprotection were recorded for studies reporting results from a multiple logistic regression model. When odds ratios and 95% CIs for the diabetes predictor variable were reported for failure to achieve seroprotection, the probability of attaining seroprotection was calculated by taking the inverse of the reported values.
RESULTS
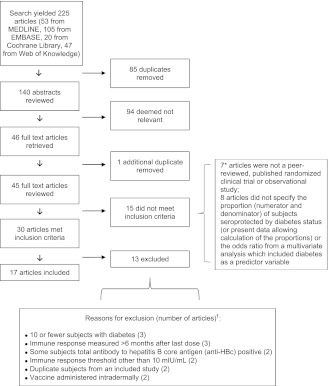
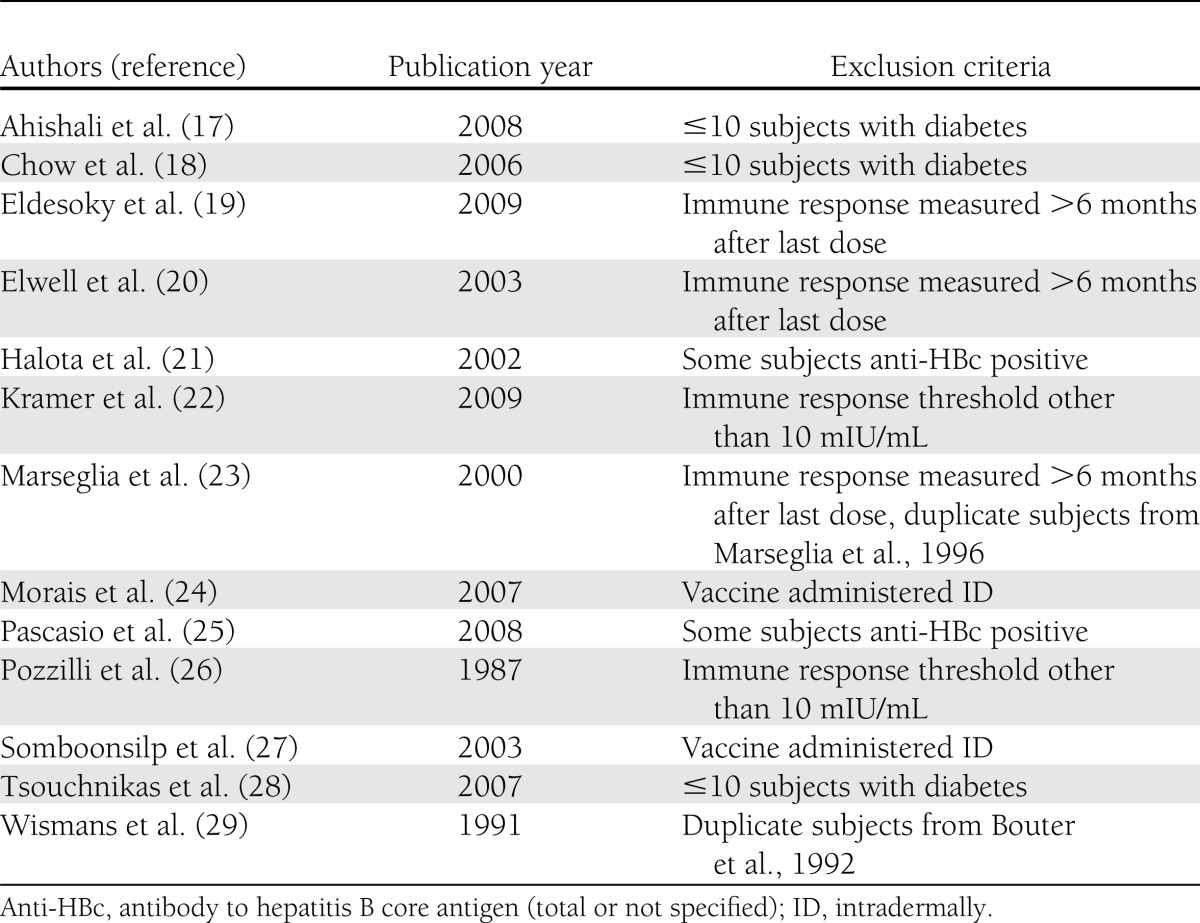
The systematic electronic search yielded 225 studies (53 from MEDLINE, 105 from EMBASE, 20 from Cochrane Library, and 47 from Web of Knowledge; a list of these studies is available upon request). Of these 225 studies, 85 duplicates were identified. Abstracts from the remaining 140 studies (62.2%) were reviewed, and 94 (67.1%) were deemed not relevant. Forty-six (32.9%) full-text studies were retrieved and reviewed, and one additional duplicate was identified (Fig. 1). Fifteen studies did not fulfill inclusion criteria, and 13 met at least one criterion for exclusion (Table 2 and Fig. 1), leaving 17 studies.
Figure 1.
Search results. *Articles that were not a peer-reviewed, published randomized clinical trial or observational study were not double-counted (among articles that did not specify the proportion [numerator and denominator] of subjects seroprotected by diabetes status [or data available to allow the calculation of the proportions] or the odds ratio from a multivariate analysis that included diabetes as a predictor variable) so that the total would equal 15. †The number of articles does not total 13 because one article met two inclusion criteria.
Table 2.
Exclusion criteria as applicable for 13 studies
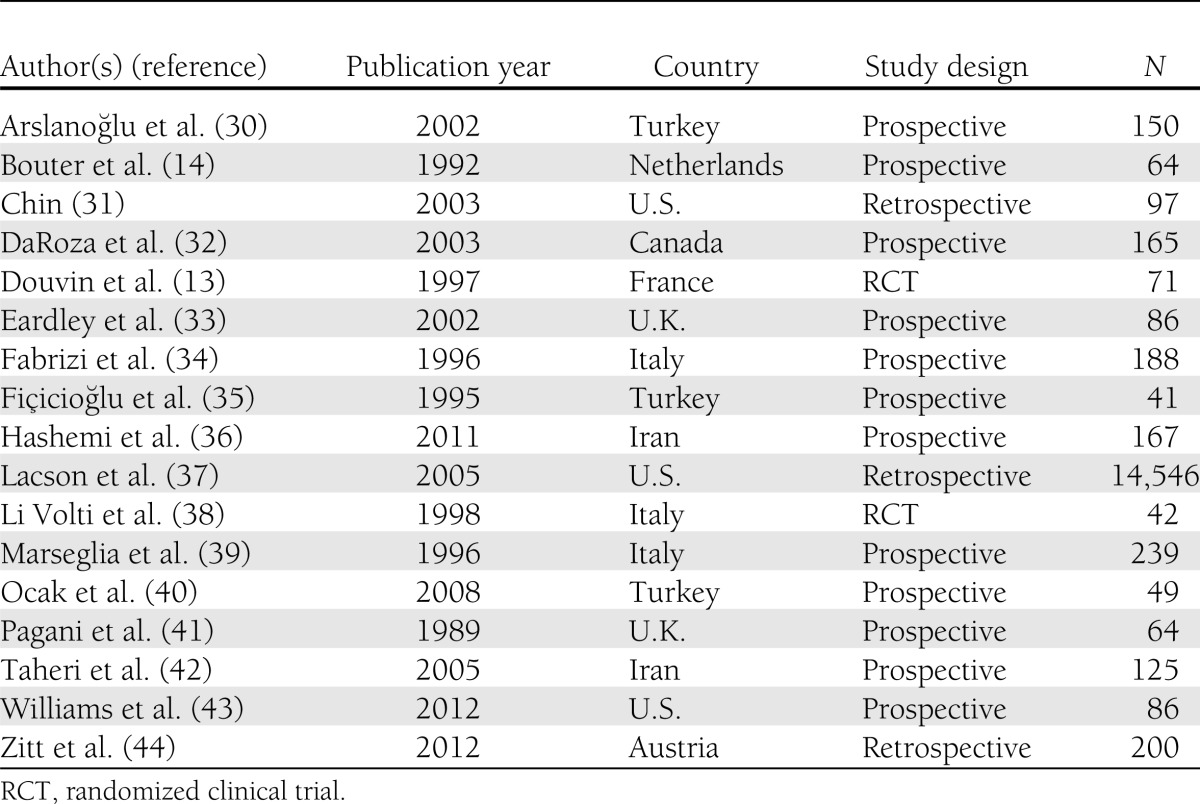
The 17 studies included 2 randomized clinical trials, 12 prospective, and 3 retrospective studies published between 1989 and 2012 (Table 3). The 17 studies included 16,310 unique subjects, of which approximately 9,286 had diabetes. The subject categories consisted of adults for 4 studies (285 subjects, of whom 152 had diabetes); children for 4 studies (472 subjects, of whom 206 had diabetes); and hemodialysis/chronic kidney disease patients for 9 studies (15,553 subjects, of whom approximately 8,928 had diabetes). Subjects in the studies among adults and children generally lacked comorbidities (including kidney disease) other than diabetes. One study among hemodialysis/chronic kidney disease patients included only subjects with chronic kidney disease (i.e., no hemodialysis patients) (32), and three studies included both hemodialysis and chronic kidney disease patients (36,42,44), one of which also included peritoneal dialysis patients (44). The remainder were comprised entirely of hemodialysis patients. Seroprotection against hepatitis B virus infection was assessed in subjects by diabetes status in 16 studies (1,764 subjects, 633 with diabetes). Five studies included diabetes in a multivariate model (31,32,37,42,44). No serious vaccine-related adverse event was reported in any study.
Table 3.
Characteristics of included studies
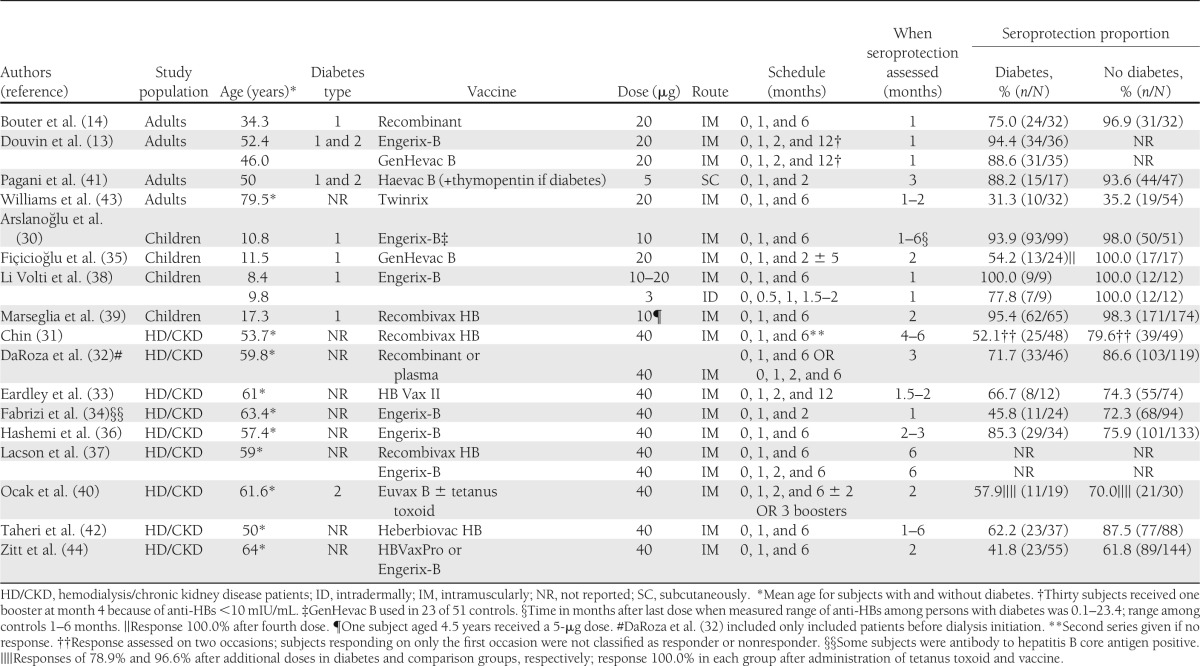
The average age of subjects by study ranged from 8.4 to 79.5 years. Diabetes type was type 1 in five studies, type 2 in one study, and type 1 and type 2 in two studies. Diabetes type was not specified in eight of the nine studies among hemodialysis/chronic kidney disease patients; persons with type 2 diabetes constitute more than half of persons with diabetes starting dialysis in the U.S. (45). The vaccine type was exclusively recombinant in 15 studies, one of which used a combination hepatitis A/hepatitis B vaccine (43), and was exclusively plasma derived in one study; both recombinant and plasma-derived vaccines were used in one study. A novel adjuvant was administered to subjects in two studies (40,41). Vaccine dosage ranged from 3 to 40 µg. Route of administration was exclusively intramuscular in 15 studies and exclusively subcutaneous in one study; one study used intramuscular or intradermal administration routes in separate study arms. The standard 0-, 1-, and 6-month schedule was used exclusively or partially in 11 studies. Others were a 0-, 1-, 2-, and 12-month schedule and a 0-, 1-, 2-, and 6-month schedule (both U.S.-approved alternatives), as well as a 0-, 1-, and 2-month schedule (not approved in the U.S.). The sex distribution was generally equivalent for most studies, although men predominated in the studies among hemodialysis/chronic kidney disease patients. The type of vaccine, dosage, route of administration, schedule, and the interval after the last dose when seroprotection was assessed were equivalent for the diabetes and comparison groups except as noted in Table 4.
Table 4.
Studies included in review: demographic data, vaccination schedule, and seroprotection (SP) proportion (anti-HBs cutoff of 10 mIU/mL)
Seroprotection proportions ranged from 31.3–100.0% (median, 73.4%) among persons with diabetes and 35.2–100.0% (median, 87.1%) for those without diabetes. The proportion protected was generally greatest among children, ranging from 54.2–100.0% (median, 93.9%) among children with diabetes and 98.0–100.0% (median 100.0%) among those without diabetes. Among adults, seroprotection proportions ranged from 31.3–94.4% (median, 88.2%) for those with diabetes compared with 35.2–96.9% (median, 93.6%) for those without diabetes. Seroprotection proportions were lowest for hemodialysis/chronic kidney disease patients, ranging from 41.8–85.3% (median, 60.1%) for those with diabetes and 61.8–87.5% (median, 75.1%) for those without diabetes.
Seroprotection proportions among subjects with diabetes were generally lower when vaccine was administered on a 0-, 1-, and 2-month schedule (not approved in the U.S.) and greater when vaccine was administered on a 0-, 1-, 2-, and 12-month schedule (U.S.-approved alternative schedule) compared with the standard 0-, 1-, and 6-month schedule. Two studies using a 0-, 1-, and 2-month schedule reported relatively low seroprotection proportions for persons with diabetes in their corresponding subject categories (54.2% among children [35] and 45.8% among hemodialysis/chronic kidney disease patients [34]), and two studies using a 0-, 1-, 2-, and 12-month schedule reported relatively high seroprotection proportions among subjects with diabetes in their corresponding subject categories (88.6% and 94.4% [13] among adults and 66.7% among hemodialysis/chronic kidney disease patients [33]). When a fourth dose was administered to children with diabetes after a primary series on a 0-, 1-, and 2-month schedule, seroprotection increased from 54.2 to 100.0% in one study (35). Another study, however, achieved an 88.2% seroprotection proportion when vaccinating adults with diabetes subcutaneously using a 0-, 1-, and 2-month schedule with thymopentin as an adjuvant (41). Thymopentin is a synthetic pentapeptide with activity characteristic of the thymic hormone thymopoietin and is not approved for use in the U.S. It has been used to enhance interleukin 2 production and function of macrophages (46).
Administration of additional hepatitis B vaccine doses may improve immune response among adults with diabetes. Douvin et al. (13) reported seroprotective anti-HBs levels in 91.5% of adult subjects with diabetes compared with 75.0% as reported by Bouter et al. (14). Subjects in both studies received 20-μg dosages of recombinant hepatitis B vaccine intramuscularly. Subjects in the study by Douvin et al. were, on average, older than those in the study by Bouter et al. (mean age, 49.2 vs. 34.3 years). It is therefore reasonable that subjects from the study by Douvin et al. would have responded more poorly than those from the study by Bouter et al. because older age (6–9,31,47) is associated with a decline in response. However, differences in the number of doses administered likely contributed to higher seroprotective proportions in the Douvin et al. study, in which 30 subjects (42.3%) receiving vaccine on a 0-, 1-, 2-, and 12-month schedule also received a booster dose at month 4 (for an anti-HBs titer <10 mIU/mL, according to the study protocol). Therefore these subjects received five total doses, compared with three total doses on a 0-, 1-, and 6-month schedule reported by Bouter et al.
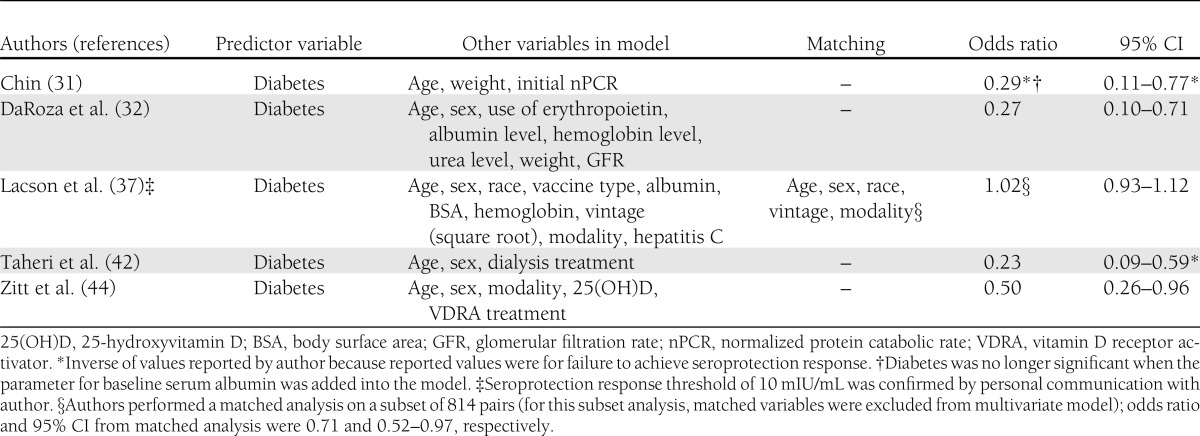
Five studies among hemodialysis/chronic kidney disease patients included diabetes in a multivariate model (31,32,37,42,44) (Table 5). All studies controlled for age, and three controlled for obesity. Four studies, with a total of 587 subjects (186 with diabetes), reported that diabetes was independently associated with failure to achieve seroprotection (odds ratio [OR] 0.23–0.50 [95% CI 0.09–0.96]) (31,32,42,44). In their study of 14,546 predominantly hemodialysis patients, Lacson et al. (37) reported no decrease in seroprotection associated with diabetes (OR 1.02 [95% CI 0.93–1.12]), although a subanalysis of 814 pairs (including subjects with and without diabetes) matched for age, sex, race, dialysis vintage, and dialysis modality reported a decrease in seroprotection associated with diabetes (OR 0.71 [95% CI 0.52–0.97]).
Table 5.
Multiple logistic regression model results for attainment of seroprotective response
A lower seroprotection proportion was achieved among children with diabetes who received the vaccine using intradermal administration, which is not a U.S.-approved route of administration. Li Volti et al. (38) reported 77.8% seroprotection with intradermal administration compared with 100.0% seroprotection with intramuscular administration. Among children without diabetes, 100.0% in both intradermal and intramuscular arms achieved seroprotection (38). Other studies have found improved results with intradermal administration of the hepatitis B vaccine in dialysis patients (48).
Thymopentin or priming with tetanus toxoid (TT) was used in two studies to enhance the immune response to the hepatitis B vaccine. Thymopentin was administered to adults with diabetes three times per week for a week before hepatitis B vaccination and for 3 weeks afterward. The results were compared with hepatitis B vaccination among normal control subjects (41). TT was administered 2 days before an additional dose of hepatitis B vaccine to hemodialysis/chronic kidney disease patients who were nonresponders after 6 or 7 doses of hepatitis B vaccine that had been administered during the previous 18 months (40). Although differences in route of administration and number of booster doses administered prevent comparison with other studies, seroprotection was reported in 88.2% of adults with diabetes who received the thymopentin adjuvant and in 100.0% of previously nonresponding hemodialysis/chronic kidney disease patients with diabetes who had received TT before vaccination. Subsequent meta-analysis of studies using thymopentin adjuvant with hepatitis B vaccine in end-stage renal disease patients (with and without diabetes) found limited benefit from the addition of thymopentin to hepatitis B schedules (46).
CONCLUSIONS
When hepatitis B vaccination is performed in accordance with standard administration procedures, children and young adults with diabetes generally have responses similar to persons of comparable age without diabetes. Older adults have a reduced response, and older adults with diabetes seem to have further impairment in vaccine response, particularly those with coexisting kidney conditions.
There are several hypotheses about the biological basis for potentially impaired response to vaccination among persons with diabetes. Although persons with diabetes have appropriate humoral immune responses to vaccination (49), impaired cellular response may account for less robust antibody production after hepatitis B vaccination (14). Proposed explanations include a reduction in the number of circulating helper T cells, the CD4-to-CD8 lymphocyte ratio, and lymphocyte blastogenesis (23) and defects with antigen presentation (39). Impaired vaccine response also has been linked to the presence of DR3, DR7, and DQ2 human leukocyte antigen alleles among persons with diabetes (13). However, no association between seroprotection and glycemic control (13,14,30,39), duration of diabetes (13,30,39), insulin requirement (30,39), or microangiopathic complications (39) was demonstrated in studies included in this review.
Obesity is a major risk factor for type 2 diabetes; 53% of adults with diabetes are obese (50). Studies have demonstrated an association between obesity and a reduced response to hepatitis B vaccine in adults (51). A needle length that is inadequate to penetrate the deltoid fat pad and reach the muscle mass may account for the reduced immune response among persons with obesity (4,52). The less abundant blood supply in adipose tissue may delay antigen presentation to the B and T cells responsible for the immune response (52). However, the needle should not be so long that it involves the underlying bone (4).
In addition to obesity (52), older age (6–9,47), comorbid conditions (11), and medication use (12) have been associated with impaired vaccine response and may confound the relationship between diabetes and immune response. Four multivariate analyses among hemodialysis/chronic kidney disease patients included in this review, all of which controlled for age and three of which controlled for obesity, found diabetes to be an independent factor for impaired vaccine response (31,32,42,44). Lacson et al. (37), however, reported a null association with diabetes and seroprotection among hemodialysis/chronic kidney disease patients (N = 14,546; OR 1.02 [95% CI 0.93–1.12]) in their principal multivariate analysis.
Administration of additional doses of hepatitis B vaccine improves the proportion of persons responding to it. Additional doses have not caused unusual adverse reactions (16). It is recommended that health care personnel at continuing risk of hepatitis B exposure and infants with perinatal hepatitis B exposure repeat a primary series of hepatitis B vaccine if they are found to be nonresponsive to the initial series (4,5). Among health care personnel who did not respond after a primary series, 67.6% mounted a protective response up to three additional doses (11). Data exclusively on revaccination of nonresponding persons with diabetes are sparse. Although postvaccination serology to determine responses among adults with diabetes is not cost effective (Centers for Disease Control and Prevention, 2011, unpublished data) and not recommended, using schedules requiring four rather than three doses or administering additional doses of hepatitis B vaccine remains an option.
A longer interval between the final two doses of the primary hepatitis B vaccine series has been associated with higher final anti-HBs levels in general (4). Findings from this review suggest that a longer interval between the final two doses also is associated with increased seroprotection proportions among persons with diabetes. Excessively long intervals have the drawback of increasing the risk for acquisition of HBV infection among those with an incomplete series (4).
Other strategies that may augment the response to hepatitis B vaccine in persons with diabetes include the use of novel adjuvants, higher dosages, or combination vaccines. No hepatitis B vaccine with a novel adjuvant is currently licensed for use in the U.S. Recent prelicensure trials using a two-dose schedule with a hepatitis B vaccine containing a toll-like receptor 9 (HBsAg-1018 ISS) agonist as the adjuvant seems promising (53). Other strategies that could be explored in clinical trials include the use of the combined hepatitis A virus and HBV vaccine Twinrix (GlaxoSmithKline Biologicals) (54,55) or hemodialysis dosage of the hepatitis B vaccine (56). Because response declines with advancing age and comorbidities (11), vaccination soon after diabetes diagnosis likely would constitute the most practical way to optimize seroprotection.
This review has several limitations. It is possible that all relevant studies may not have been identified in the literature search, especially those studies in which persons with diabetes represented a subset of the population. There is the potential for publication bias. Studies with significant results are more likely to be published than those with null results, and this review consisted only of published studies. A null result for some studies in this review would consist of an equivalent vaccine response in persons with and without diabetes. It is therefore unlikely that publication bias would lead to a reported overestimate of vaccine response among persons with diabetes. Only 17 of the identified studies were included in this review (and only 5 multivariate analyses), and data pertaining to adults with diabetes but without hemodialysis/chronic kidney disease were sparse. Observational studies were included, and the results of these studies may not be as robust as randomized clinical trials. The matched comparison groups and objective outcome measure (i.e., anti-HBs levels) strengthen the data quality. Outcomes consisted of serological correlates of protection (anti-HBs levels), which may not correlate with maintaining full vaccine effectiveness over time. Immunocompetent persons found to have anti-HBs levels of ≥10 IU/L after the primary series, consistent with the threshold used by studies included in this review, are considered to be protected (4,5,57). Duration of protection was not assessed in this review, although other evidence suggests protection lasts for two decades in healthy primary vaccine responders. However, data on the duration of immune memory in immunocompromised persons are limited (4). Although general conclusions were drawn from a synthesis of the results, no attempt was made to test for statistically significant differences. A meta-regression was not conducted because colinearity likely exists among sources of heterogeneity (e.g., children most likely have type 1 diabetes and are treated with insulin). Heterogeneity between studies by diabetes type, management, glycemic control, and vaccine type, dosage, administration route, and schedule limit synthesis of results.
High levels of seroprotection from recombinant hepatitis B vaccine are achieved safely in children with diabetes. Data from studies reviewed here suggest that adults with diabetes have a reduced response to vaccination. Seroprotection is decreased among older adults and persons on hemodialysis in general, including those with diabetes. Administration of schedules using an extended interval to the final dose, four vs. three doses, or additional vaccine doses may achieve seroprotection in a greater proportion of adults with diabetes, including those who do not respond to an initial series. Other strategies to increase response among older adults with diabetes, including those with other comorbid conditions, should be explored.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
S.F.S. performed the literature review, abstracted data, and wrote the manuscript. P.R.S. critically reviewed and edited the manuscript. T.V.M. suggested the topic and critically reviewed and edited the manuscript. S.F.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
A portion of the data from five included studies was presented at a Meeting of the Advisory Committee on Immunization Practices, Atlanta, Georgia, 25–26 October 2011.
The authors thank Jack (Rick) Colbert, MLIS, Reference Librarian, CDC Public Health Library and Information Center, Atlanta Georgia, for his assistance with the literature search.
Footnotes
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Centers for Disease Control and Prevention (CDC) Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011;60:1709–1711 [PubMed] [Google Scholar]
- 2.André FE. Summary of safety and efficacy data on a yeast-derived hepatitis B vaccine. Am J Med 1989;87(3A):14S–20S [DOI] [PubMed] [Google Scholar]
- 3.Zajac BA, West DJ, McAleer WJ, Scolnick EM. Overview of clinical studies with hepatitis B vaccine made by recombinant DNA. J Infect 1986;13(Suppl A):39–45 [DOI] [PubMed] [Google Scholar]
- 4.Mast, EE, Weinbaum CM, Fiore AE, et al.; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep 2006;55(RR-16):1–33; quiz CE1–4. [PubMed]
- 5.Mast EE, Margolis HS, Fiore AE, et al. Advisory Committee on Immunization Practices (ACIP) A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep 2005;54(RR-16):1–31 [PubMed] [Google Scholar]
- 6.Halsey NA, Moulton LH, O’Donovan JC, et al. Hepatitis B vaccine administered to children and adolescents at yearly intervals. Pediatrics 1999;103:1243–1247 [DOI] [PubMed] [Google Scholar]
- 7.Clemens R, Sänger R, Kruppenbacher J, et al. Booster immunization of low- and non-responders after a standard three dose hepatitis B vaccine schedule—results of a post-marketing surveillance. Vaccine 1997;15:349–352 [DOI] [PubMed] [Google Scholar]
- 8.Hussain Z, Ali SS, Husain SA, Raish M, Sharma DR, Kar P. Evaluation of immunogenicity and reactogenicity of recombinant DNA hepatitis B vaccine produced in India. World J Gastroenterol 2005;11:7165–7168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honorati MC, Mariani E, Dolzani P, Facchini A. Biological parameters influencing the immunological response to plasma derived and recombinant hepatitis B vaccines. Ann Ist Super Sanita 1996;32:369–374 [PubMed] [Google Scholar]
- 10.Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G. Meta-analysis: the effect of age on immunological response to hepatitis B vaccine in end-stage renal disease. Aliment Pharmacol Ther 2004;20:1053–1062 [DOI] [PubMed] [Google Scholar]
- 11.Averhoff F, Mahoney F, Coleman P, Schatz G, Hurwitz E, Margolis H. Immunogenicity of hepatitis B Vaccines. Implications for persons at occupational risk of hepatitis B virus infection. Am J Prev Med 1998;15:1–8 [DOI] [PubMed] [Google Scholar]
- 12.de Rave S, Heijtink RA, Bakker-Bendik M, Boot J, Schalm SW. Immunogenicity of standard and low dose vaccination using yeast-derived recombinant hepatitis B surface antigen in elderly volunteers. Vaccine 1994;12:532–534 [DOI] [PubMed] [Google Scholar]
- 13.Douvin C, Simon D, Charles MA, et al. Hepatitis B vaccination in diabetic patients. Randomized trial comparing recombinant vaccines containing and not containing pre-S2 antigen. Diabetes Care 1997;20:148–151 [DOI] [PubMed] [Google Scholar]
- 14.Bouter KP, Diepersloot RJ, Wismans PJ, et al. Humoral immune response to a yeast-derived hepatitis B vaccine in patients with type 1 diabetes mellitus. Diabet Med 1992;9:66–69 [DOI] [PubMed] [Google Scholar]
- 15.Historic dates and events related to vaccines and immunization. Available from: http://www.immunize.org/timeline/ Accessed 5 January 2012
- 16.Hadler SC, Margolis HS. Hepatitis B immunization: vaccine types, efficacy, and indications for immunization. Curr Clin Top Infect Dis 1992;12:282–308 [PubMed] [Google Scholar]
- 17.Ahishali E, Boztas G, Akyuz F, et al. Response to hepatitis B vaccination in patients with celiac disease. Dig Dis Sci 2008;53:2156–2159 [DOI] [PubMed] [Google Scholar]
- 18.Chow KM, Law MC, Leung CB, Szeto CC, Li PK. Antibody response to hepatitis B vaccine in end-stage renal disease patients. Nephron Clin Pract 2006;103:c89–c93 [DOI] [PubMed] [Google Scholar]
- 19.Eldesoky A, et al. Protective immunity after hepatitis B vaccination. Arab J Gastroenterol 2009;10:68–71 [Google Scholar]
- 20.Elwell RJ, Neumann M, Bailie GR. Factors associated with long-term antibody production induced by hepatitis B vaccine in patients undergoing hemodialysis: a retrospective cohort study. Pharmacotherapy 2003;23:1558–1563 [DOI] [PubMed] [Google Scholar]
- 21.Halota W, Muszyńska M, Pawłowska M. Hepatitis B virus serologic markers and anti-hepatitis B vaccination in patients with diabetes. Med Sci Monit 2002;8:CR516–CR519 [PubMed] [Google Scholar]
- 22.Kramer ES, Hofmann C, Smith PG, Shiffman ML, Sterling RK. Response to hepatitis A and B vaccine alone or in combination in patients with chronic hepatitis C virus and advanced fibrosis. Dig Dis Sci 2009;54:2016–2025 [DOI] [PubMed] [Google Scholar]
- 23.Marseglia G, Alibrandi A, d’Annunzio G, et al. Long term persistence of anti-HBs protective levels in young patients with type 1 diabetes after recombinant hepatitis B vaccine. Vaccine 2000;19:680–683 [DOI] [PubMed] [Google Scholar]
- 24.Morais EO, Resende MR, Oliveira AM, et al. Intradermal hepatitis B vaccination in patients with advanced chronic renal failure: immunogenicity and follow-up. Aliment Pharmacol Ther 2007;25:849–855 [DOI] [PubMed] [Google Scholar]
- 25.Pascasio JM, Aoufi S, Gash A, et al. Response to a vaccination schedule with 4 doses of 40 microg against hepatitis B virus in cirrhotic patients evaluated for liver transplantation. Transplant Proc 2008;40:2943–2945 [DOI] [PubMed] [Google Scholar]
- 26.Pozzilli P, Arduini P, Visalli N, et al. Reduced protection against hepatitis B virus following vaccination in patients with type 1 (insulin-dependent) diabetes. Diabetologia 1987;30:817–819 [DOI] [PubMed] [Google Scholar]
- 27.Somboonsilp W, Eiam-Ong S, Tungsanga K, Tirawatanapong T. Immune response of intradermal hepatitis B vaccination at lower dose versus intramuscular vaccination at double standard dose in predialytic chronic renal failure patients. J Med Assoc Thai 2003;86:1122–1127 [PubMed] [Google Scholar]
- 28.Tsouchnikas I, Dounousi E, Xanthopoulou K, Papakonstantinou S, Thomoglou V, Tsakiris D. Loss of hepatitis B immunity in hemodialysis patients acquired either naturally or after vaccination. Clin Nephrol 2007;68:228–234 [DOI] [PubMed] [Google Scholar]
- 29.Wismans PJ, van Hattum J, de Gast GC, et al. A prospective study of in vitro anti-HBs producing B cells (spot-ELISA) following primary and supplementary vaccination with a recombinant hepatitis B vaccine in insulin dependent diabetic patients and matched controls. J Med Virol 1991;35:216–222 [DOI] [PubMed] [Google Scholar]
- 30.Arslanoğlu I, Cetin B, Işgüven P, Karavuş M. Anti-HBs response to standard hepatitis B vaccination in children and adolescents with diabetes mellitus. J Pediatr Endocrinol Metab 2002;15:389–395 [DOI] [PubMed] [Google Scholar]
- 31.Chin AI. Hepatitis B virus vaccine response in hemodialysis: baseline patient characteristics. Hemodial Int 2003;7:296–303 [DOI] [PubMed] [Google Scholar]
- 32.DaRoza G, Loewen A, Djurdjev O, et al. Stage of chronic kidney disease predicts seroconversion after hepatitis B immunization: earlier is better. Am J Kidney Dis 2003;42:1184–1192 [DOI] [PubMed] [Google Scholar]
- 33.Eardley KS, Jones HE, Osman H, Smith SA. Efficacy of the accelerated hepatitis B vaccination schedule used in haemodialysis patients post-exposure to virus: a single-centre experience. Nephrol Dial Transplant 2002;17:1982–1987 [DOI] [PubMed] [Google Scholar]
- 34.Fabrizi F, Di Filippo S, Marcelli D, et al. Recombinant hepatitis B vaccine use in chronic hemodialysis patients. Long-term evaluation and cost-effectiveness analysis. Nephron 1996;72:536–543 [DOI] [PubMed] [Google Scholar]
- 35.Fiçicioğlu C, Mikla S, Midilli K, Aydin A, Cam H, Erğin S. Reduced immune response to hepatitis B vaccine in children with insulin dependent diabetes. Acta Paediatr Jpn 1995;37:687–690 [DOI] [PubMed] [Google Scholar]
- 36.Hashemi B, Mahdavi-Mazdeh M, Abbasi M, Hosseini-Moghaddam SM, Zinat NH, Ahmadi F. Efficacy of HBV vaccination in various stages of chronic kidney disease: is earlier better? Hepat Mon 2011;11:816–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacson E, Teng M, Ong J, Vienneau L, Ofsthun N, Lazarus JM. Antibody response to Engerix-B and Recombivax-HB hepatitis B vaccination in end-stage renal disease. Hemodial Int 2005;9:367–375 [DOI] [PubMed] [Google Scholar]
- 38.Li Volti S, Caruso-Nicoletti M, Biazzo F, et al. Hyporesponsiveness to intradermal administration of hepatitis B vaccine in insulin dependent diabetes mellitus. Arch Dis Child 1998;78:54–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marseglia GL, Scaramuzza A, d’Annunzio G, Comolli G, Gatti M, Lorini R. Successful immune response to a recombinant hepatitis B vaccine in young patients with insulin-dependent diabetes mellitus. Diabet Med 1996;13:630–633 [DOI] [PubMed] [Google Scholar]
- 40.Ocak S, Eskiocak AF. The evaluation of immune responses to hepatitis B vaccination in diabetic and non-diabetic haemodialysis patients and the use of tetanus toxoid. Nephrology (Carlton) 2008;13:487–491 [DOI] [PubMed] [Google Scholar]
- 41.Pagani S, Cruciani L, Chianelli M, Procaccini E, Pozzilli P. Thymopentin administration and increase of sero-conversion after B-hepatitis vaccine in diabetic patients. Diabetes Res 1989;12:199–201 [PubMed] [Google Scholar]
- 42.Taheri Sh, et al. Response rate to hepatitis B vaccination in patients with chronic renal failure and end-stage-renal-disease: influence of diabetes mellitus. J Res Med Sci 2005;10:384–390 [Google Scholar]
- 43.Williams RE, Sena AC, Moorman AC, et al. Hepatitis B vaccination of susceptible elderly residents of long term care facilities during a hepatitis B outbreak. Vaccine 2012;30:3147–3150 [DOI] [PubMed] [Google Scholar]
- 44.Zitt E, Sprenger-Mähr H, Knoll F, Neyer U, Lhotta K. Vitamin D deficiency is associated with poor response to active hepatitis B immunisation in patients with chronic kidney disease. Vaccine 2012;30:931–935 [DOI] [PubMed] [Google Scholar]
- 45.Molitch ME, DeFronzo RA, Franz MJ, et al. American Diabetes Association Nephropathy in diabetes. Diabetes Care 2004;27(Suppl 1):S79–S83 [DOI] [PubMed] [Google Scholar]
- 46.Fabrizi F, Dixit V, Martin P. Meta-analysis: the adjuvant role of thymopentin on immunological response to hepatitis B virus vaccine in end-stage renal disease. Aliment Pharmacol Ther 2006;23:1559–1566 [DOI] [PubMed] [Google Scholar]
- 47.Wolters B, Junge U, Dziuba S, Roggendorf M. Immunogenicity of combined hepatitis A and B vaccine in elderly persons. Vaccine 2003;21:3623–3628 [DOI] [PubMed] [Google Scholar]
- 48.Fabrizi F, Dixit V, Messa P, Martin P. Intradermal vs intramuscular vaccine against hepatitis B infection in dialysis patients: a meta-analysis of randomized trials. J Viral Hepat 2011;18:730–737 [DOI] [PubMed] [Google Scholar]
- 49.Smith SA, Poland GA, American Diabetes Association Influenza and pneumococcal immunization in diabetes. Diabetes Care 2004;27(Suppl 1):S111–S113 [DOI] [PubMed] [Google Scholar]
- 50.Age-adjusted percentage of obesity for adults with diabetes, United States, 1994–2007. Available from: http://www.cdc.gov/diabetes/statistics/comp/fig7_obesity.htmAccessed 6 February 2012
- 51.Roome AJ, Walsh SJ, Cartter ML, Hadler JL. Hepatitis B vaccine responsiveness in Connecticut public safety personnel. JAMA 1993;270:2931–2934 [PubMed] [Google Scholar]
- 52.Middleman AB, Anding R, Tung C. Effect of needle length when immunizing obese adolescents with hepatitis B vaccine. Pediatrics 2010;125:e508–e512 [DOI] [PubMed] [Google Scholar]
- 53.Barry M, Cooper C. Review of hepatitis B surface antigen-1018 ISS adjuvant-containing vaccine safety and efficacy. Expert Opin Biol Ther 2007;7:1731–1737 [DOI] [PubMed] [Google Scholar]
- 54.Nyström J, Cardell K, Björnsdottir TB, Fryden A, Hultgren C, Sällberg M. Improved cell mediated immune responses after successful re-vaccination of non-responders to the hepatitis B virus surface antigen (HBsAg) vaccine using the combined hepatitis A and B vaccine. Vaccine 2008;26:5967–5972 [DOI] [PubMed] [Google Scholar]
- 55.Murdoch DL, Goa K, Figgitt DP. Combined hepatitis A and B vaccines: a review of their immunogenicity and tolerability. Drugs 2003;63:2625–2649 [DOI] [PubMed] [Google Scholar]
- 56.Greub G, Genton B, Safary A, Thoelen S, Frei PC. Comparison of the reactogenicity and immunogenicity of a two injection combined high-dose hepatitis A and hepatitis B vaccine to those of Twinrix. Vaccine 2000;19:1113–1117 [DOI] [PubMed] [Google Scholar]
- 57.Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis 1999;179:489–492 [DOI] [PubMed] [Google Scholar]