Abstract
OBJECTIVE
Alterations of pancreatic β-cell cholesterol content may contribute to β-cell dysfunction. Two important determinants of intracellular cholesterol content are the ATP-binding cassette (ABC) transporters A1 (ABCA1) and -G1 (ABCG1). Whether genetic variation in ABCA1 and ABCG1 predicts risk of type 2 diabetes in the general population is unknown.
RESEARCH DESIGN AND METHODS
We tested whether genetic variation in the promoter and coding regions of ABCA1 and ABCG1 predicted risk of type 2 diabetes in the general population. Twenty-seven variants, identified by previous resequencing of both genes, were genotyped in the Copenhagen City Heart Study (CCHS) (n = 10,185). Two loss-of-function mutations (ABCA1 N1800H and ABCG1 g.-376C>T) (n = 322) and a common variant (ABCG1 g.-530A>G) were further genotyped in the Copenhagen General Population Study (CGPS) (n = 30,415).
RESULTS
Only one of the variants examined, ABCG1 g.-530A>G, predicted a decreased risk of type 2 diabetes in the CCHS (P for trend = 0.05). Furthermore, when validated in the CGPS or in the CCHS and CGPS combined (n = 40,600), neither the two loss-of-function mutations (ABCA1 N1800H, ABCG1 g.-376C>T) nor ABCG1 g.-530A>G were associated with type 2 diabetes (P values >0.57 and >0.30, respectively).
CONCLUSIONS
Genetic variations in ABCA1 and ABCG1 were not associated with increased risk of type 2 diabetes in the general population. These data were obtained in general population samples harboring the largest number of heterozygotes for loss-of-function mutations in ABCA1 and ABCG1.
The cellular mechanisms underlying pancreatic β-cell dysfunction in type 2 diabetes are incompletely understood. Recent evidence suggests that alterations of β-cell cholesterol content may contribute to islet dysfunction and loss of insulin secretion (1). Two important determinants of intracellular cholesterol content are the ATP-binding cassette (ABC) transporters A1 (ABCA1) and -G1 (ABCG1), which have been suggested to regulate cholesterol homeostasis and insulin secretion in β cells (2,3). The question of whether genetic variation in ABCA1 and ABCG1 predicts risk of type 2 diabetes in the general population remains unanswered.
Homozygosity for mutations in ABCA1 causes a rare HDL-deficiency syndrome, Tangier disease, a disorder characterized by accumulation of cholesterol esters in peripheral tissues (4–7). Deficiency of ABCA1 in β cells results in altered intracellular cholesterol homeostasis and impaired insulin secretion in mice (2). In humans, family studies and small case-control studies have suggested that loss-of-function mutations in ABCA1 associate with decreased insulin secretion (8) or type 2 diabetes (9). Further, macrophages from diabetic mice (10) and humans (11) display decreased ABCG1 expression. Finally, we recently reported that two functional variants, ABCA1 N1800H and ABCG1 g.-376C>T, were associated with, respectively, substantial reductions in levels of HDL cholesterol or reduced ABCG1 mRNA expression levels and increased risk of ischemic heart disease and myocardial infarction (12,13)—risk factors and disease entities closely related to diabetes. Collectively, these data suggest that decreased ABCA1 and ABCG1 function may influence normal β-cell action and susceptibility to type 2 diabetes. For clarification of whether genetic variation in ABCA1 and ABCG1 contributes to risk of type 2 diabetes in humans, large studies of the general population are needed.
We tested the following hypotheses: 1) genetic variation in ABCA1 and ABCG1 associates with measures of glucose metabolism, markers of inflammation, and lipid and lipoprotein levels in the general population and 2) genetic variation in ABCA1 and ABCG1 is associated with risk of type 2 diabetes in the general population. These hypotheses were tested in two studies of the general population, the Copenhagen City Heart Study (CCHS) (n = 10,185) and the Copenhagen General Population Study (CGPS) (n = 30,415), harboring the largest known number of heterozygotes for loss-of-function mutations in ABCA1 and ABCG1 (n = 322).
RESEARCH DESIGN AND METHODS
Studies were approved by institutional review boards and Danish ethics committees (nos. KF-100.2039/91, KF-01-144/01, and H-KF-01-144/01) and conducted according to the Declaration of Helsinki. Informed consent was obtained from all participants. All participants were white and of Danish descent. No participants appeared in more than one of the two studies, permitting independent confirmation of the findings in each group.
CCHS.
This was a prospective study of the general population initiated in 1976–1978 with follow-up examinations in 1981–1983, 1991–1994, and 2001–2003. Individuals were selected based on the National Danish Civil Registration System to reflect the adult Danish population aged 20 to ≥80 years. Data were obtained from a questionnaire, a physical examination, and blood samples. Blood samples for DNA extraction were available on 10,185 participants attending the 1991–1994 and/or 2001–2003 examinations.
CGPS.
This is a prospective study of the general population initiated in 2003 with ongoing enrollment. For the current study, we used the CGPS cross-sectionally in order to include all available events occurring from the establishment of the National Danish Patient Registry and National Danish Causes of Death Registry in 1976 to the end of follow-up in 2010. Participants were recruited from the general population and examined as in the CCHS. At the time of genotyping, 30,415 had been included.
Genotyping
The ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) was used to genotype the CCHS for all variants in the core promoter and nonsynonymous variants identified by previous resequencing of the ABCG1 and ABCA1 genes (12–14). Two loss-of-function mutations (ABCA1 N1800H and ABCG1 g.-376C>T), as well as a common variant associated with reduced risk of type 2 diabetes in the CCHS (ABCG1 g.-530A>G), were further genotyped in the CGPS. TaqMan-based assays were used.
Measures of glucose metabolism, markers of inflammation, and lipid and lipoprotein levels
Glucose concentrations were measured using a standard hexokinase/glucose-6-phosphate-dehydrogenease assay (15), and HbA1c was measured on a Kone analyzer (Kone-Laboratory, Helsinki, Finland). Soluble CD163, a marker of low-grade inflammation and a strong predictor of type 2 diabetes (16), was measured using an in-house sandwich enzyme-linked immunosorbent assay on a BEP-2000 ELISA analyzer as previously described (16,17). High-sensitivity C-reactive protein (hsCRP) was measured by standard nephelometric or turbidimetric assays (18). Colorimetric assays were used to measure HDL cholesterol and triglycerides (Boehringer Mannheim, Mannheim, Germany), and LDL cholesterol was calculated using the Friedewald equation (19), when plasma triglycerides were <4.00 mmol/L (<354.00 mg/dL), and otherwise was measured directly (Thermo Fisher Scientific, Waltham, MA).
Events
Information on diagnoses of type 2 diabetes (World Health Organization; ICD-8, 250; and ICD-10, E11, E13, E14) was collected from the National Danish Patient Registry (94% of end points) and the National Danish Causes of Death Registry (6% of end points). All diagnoses in these registries are given by a hospital-employed physician on the day of discharge or death of the patient and are thus based on international criteria, medical records, and pharmaceutical therapy. Follow-up ended in August 2010 and was 100% complete; i.e., no individual was lost to follow-up in either study.
Other covariates
BMI was measured as weight (kilograms) divided by the square of measured height in meters. Use of lipid-lowering therapy was self-reported. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg and/or use of antihypertensive drugs. Smoking was defined as current smoking. Physical inactivity was defined as <2–4 h per week of light physical activity at leisure time.
Statistical analysis
Data were analyzed using Stata/SE statistical software (version 12.0; StataCorp, College Station, TX). Two-sided probability values <0.05 were considered significant. Mann-Whitney U test and Kruskal-Wallis one-way ANOVA evaluated two- and three-group comparisons for continuous variables, and Pearson χ2 or Fischer exact tests were used for categorical variables. For trend tests, groups of individuals were classified by ABCA1 or ABCG1 genotypes and ranked 0, 1, and 2, with 0 (noncarriers) as the reference group. Pairwise linkage disequilibria and haplotype association analyses for all 23 common variants in ABCA1 and ABCG1 were estimated using the software Haploview 4.0 (available at www.broad.mit.edu/mpg/haploview/download.php).
To test our first hypothesis that genetic variation in ABCA1 and ABCG1 associates with measures of glucose metabolism, inflammatory markers, and levels of lipids and lipoproteins in the general population, we used nonparametric Mann-Whitney U and trend tests across genotypes with two and three groups, respectively. To test our second hypothesis, that genetic variation in ABCA1 and ABCG1 is associated with risk of type 2 diabetes in the general population, we used Cox proportional hazards regression models, with age as time scale and use of left truncation (delayed entry), to estimate the hazard ratios of type 2 diabetes in the prospective CCHS; individuals diagnosed with an end point before entry were excluded from Cox regression analyses, and those dying during follow-up were censored at their death date. In the CGPS and in the CCHS and CGPS combined, logistic regression analysis was used to estimate odds ratios. Multifactorial adjustment was for age, sex, BMI, hypertension, smoking, and physical inactivity, which are the most important risk factors for type 2 diabetes (20). For the end point prediabetes (BMI ≥25 kg/m2, HbA1c ≥5.7%, physical inactivity, and hypertension [20]), logistic regression analysis adjusted for age in 10-year age-groups and sex was used to estimate odds ratios in the CCHS. Missing values were imputed. Burden testing for rare variants (21) was performed in two ways: 1) collapsing rare variants with minor allele frequencies <1% and 2) collapsing rare variants that were experimentally verified to be functional. Fischer exact test evaluated whether collapsed rare variant frequencies differed between type 2 diabetes case subjects and participants without type 2 diabetes.
RESULTS
Characteristics of participants and resequencing of ABCA1 and ABCG1
Characteristics of subjects in the CCHS and in the CGPS with or without type 2 diabetes are shown in Supplementary Table 1. Individuals with type 2 diabetes were older and more often men, had lower HDL cholesterol and apolipoprotein (apo)A-I levels and higher HbA1c and triglyceride levels, were more obese, were more often on lipid-lowering therapy, more often had hypertension, and were less physically active compared with control subjects.
Resequencing of the core promoter and coding regions of ABCA1 and ABCG1 in individuals from the CCHS with the 1–2% lowest (n = 95–180) and 1–2% highest (n = 95–180) plasma HDL cholesterol levels identified 27 promoter and nonsynonymous variants with a minor allele frequency >0.02% (13,14) (Supplementary Table 2). Except for three very rare ABCG1 variants (g.-686G>A, g.-269G>A, and S630 L), all variants identified in the current study were also reported in the publicly available 1000 Genomes Project (Supplementary Table 2). In the 1000 Genomes Project, 31 additional very rare variants, not identified in the CCHS, were reported. (For detailed information, see the legend to Supplementary Table 2.) All 27 variants identified by resequencing extreme HDL cholesterol groups in the CCHS were further genotyped in the entire CCHS cohort. Four variants were rare (allele frequency 0.02–0.07%), and 23 were more common (minor allele frequency 0.13–34%). The linkage disequilibrium patterns for these 23 common variants are shown for each gene in Supplementary Figs. 1 and 2. Two loss-of-function mutations, as well as a common variant with effect on risk of type 2 diabetes in the CCHS, were further genotyped in the CGPS and displayed minor allele frequencies similar to those in the CCHS: 0.1, 0.2, and 6.7% for ABCA1 N1800H, ABCG1 g.-376C>T, and ABCG1 g.-530A>G, respectively. Genotype frequencies did not differ from those predicted by Hardy-Weinberg equilibrium (P values 0.11–0.98), except for ABCA1 E1172D (P = 0.03), in the CCHS. However, sequencing 299 base pairs spanning this variant in all heterozygotes and homozygotes (>500 individuals) showed 100% concordance with Taqman results.
Genetic variation in ABCA1 and ABCG1 and biochemical phenotypes
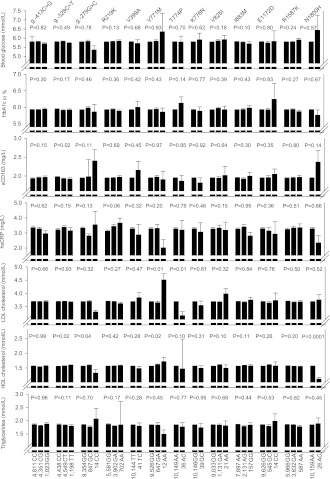
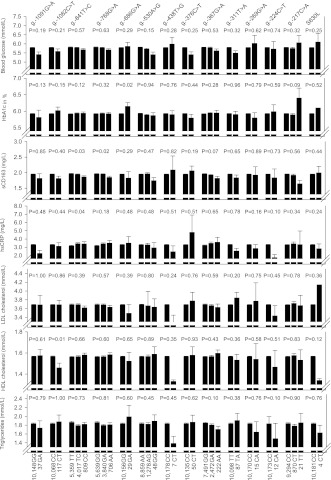
Measures of glucose metabolism, markers of inflammation, and lipid and lipoprotein levels are presented for 13 ABCA1 variants and 14 ABCG1 variants in Figs. 1 and 2, respectively. After application of a Bonferroni-corrected P value of 0.0002 (0.05/8 biochemical markers multiplied by 27 genetic variants), only one association remained significant: plasma levels of HDL cholesterol were reduced by 0.45 mmol/L in heterozygotes for ABCA1 N1800H compared with noncarriers (P = 0.0001) (Fig. 1); the corresponding reduction for apoAI was 27.6 mg/dL. Similar findings for HDL cholesterol and apoAI levels were seen in the CGPS (P values = 0.0001) (data not shown).
Figure 1.
Measures of glucose metabolism, inflammatory markers, and levels of lipids and lipoproteins as a function of 13 ABCA1 variants. Values shown are means ± SEM. P values by Kruskal-Wallis one-way ANOVA or Mann-Whitney U test. Parametric ANOVA and Student t tests gave similar results. All calculations were performed for 10,185 individuals in CCHS except for HbA1c, soluble CD163 (sCD163), and hsCRP, for which measurements were available for 5,661, 8,191, and 9,752 individuals, respectively.
Figure 2.
Measures of glucose metabolism, inflammatory markers, and levels of lipids and lipoproteins as a function of 14 ABCG1 variants. Values shown are means ± SEM. P values by Kruskal-Wallis one-way ANOVA or Mann-Whitney U test. Parametric ANOVA and Student t tests gave similar results. All calculations were performed for 10,185 individuals in CCHS except for HbA1c, soluble CD163 (sCD163), and hsCRP, for which measurements were available for 5,661, 8,191, and 9,752 individuals, respectively.
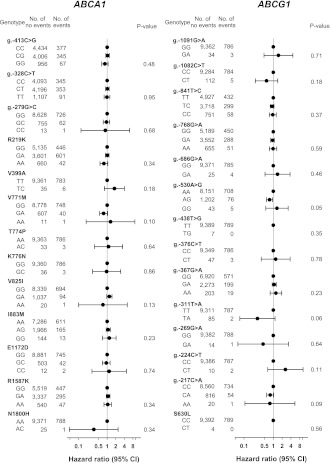
Genetic variation in ABCA1 and ABCG1 and risk of type 2 diabetes
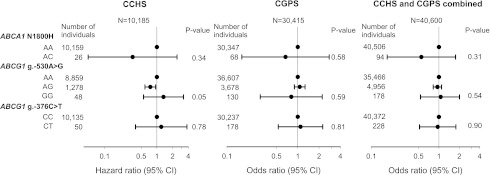
Risk of type 2 diabetes for all 27 genetic variants in ABCA1 and ABCG1 is presented in Fig. 3. The g.-530A>G ABCG1 variant associated with decreased risk of type 2 diabetes in the CCHS (P for trend = 0.05). The hazard ratios for type 2 diabetes (multifactorially adjusted) did not differ significantly from 1.0 for any of the 26 remaining genetic variants in the CCHS (Fig. 3). Sixteen ABCA1 and five ABCG1 haplotypes with frequencies >1% were estimated on the basis of 13 common ABCA1 variants and 10 common ABCG1 variants, respectively. None of the estimated haplotypes associated with type 2 diabetes in the CCHS (Supplementary Table 3) (all P values >0.27 after 1,000 permutations). Neither the two loss-of-function mutations nor the ABCG1 g.-530A>G associated with type 2 diabetes in the CGPS (ABCA1 N1800H AC vs. AA odds ratio 0.7 [95% CI 0.2–2.8], ABCG1 g.-376C>T CT vs. CC 1.1 [0.5–2.3], ABCG1 g.-530A>G AG vs. AA 1.1 [0.9–1.3], and GG vs. AA 0.8 [0.3–2.2]) or in CCHS and CGPS combined (N1800H AC vs. AA 0.6 [0.2–1.8], g.-376C>T CT vs. CC 1.0 [0.5–1.8], g.-530A>G AG vs. AA 1.0 [0.8–1.1], and GG vs. AA 1.1 [0.5–2.0]) (Fig. 4). Finally, when burden testing of rare variants was performed, neither collapsed genotypes including all rare variants with minor allele frequencies <1% (CCHS: P = 0.34) nor collapsed genotypes including rare variants that were experimentally verified to be functional (ABCA1 N1800H and ABCG1 g.-376C>T) associated with type 2 diabetes (CCHS and CGPS combined: P = 0.70).
Figure 3.
Risk of type 2 diabetes as a function of ABCA1 and ABCG1 genotype in CCHS. Hazard ratios were multifactorially adjusted for age, sex, BMI, hypertension, smoking, and physical inactivity. P values from Cox regression or Cox regression trend test.
Figure 4.
Risk of type 2 diabetes as a function of ABCA1 and ABCG1 genotype in CCHS, CGPS, and CCHS and CGPS combined. Hazard ratios and odds ratios were multifactorially adjusted for age, sex, BMI, hypertension, smoking, and physical inactivity. P values from Cox and logistic regression or from Cox and logistic regression trend test.
Genetic variation in ABCA1 and ABCG1 and risk of prediabetes
Risk of prediabetes for all 27 genetic variants in ABCA1 and ABCG1 is presented in Supplementary Fig. 3. No test for trends fulfilled a Bonferroni-corrected P value of 0.002 (0.05/27 genetic variants) (Supplementary Fig. 3).
CONCLUSIONS
The principal findings of the current study are that genetic variants in ABCA1 and ABCG1 are not associated with increased risk of type 2 diabetes or related disease entities in the general population. These findings were observed in two large studies of the general population comprising >40,000 individuals by applying a series of statistical analyses including Cox regression, logistic regression, haplotype association testing, and burden testing of rare variants.
This is the largest study to date of genetic variation in ABCA1 and ABCG1 and risk of type 2 diabetes and, in particular, is the largest study of loss-of-function mutations, including 94 ABCA1 N1800H heterozygotes and 228 ABCG1 g.-376C>T heterozygotes. A previous Dutch study suggested that ABCA1 influences β-cell function in humans (8). Compared with family control subjects, ABCA1 heterozygotes had mild hyperglycemia but no difference in insulin response after an oral glucose challenge compared with family control subjects. With use of hyperglycemic clamps, ABCA1 heterozygotes had reduced first-phase insulin response to hyperglycemia and normal insulin sensitivity. Collectively, these findings were suggested to point to a defect in β-cell function in ABCA1 heterozygotes but without development of type 2 diabetes (8). In a case-control study of 244 patients with type 2 diabetes versus 202 nondiabetic control subjects, ABCA1 R230C was reported to associate with type 2 diabetes in a Mexican-Mestizo population (9). In the same study, these results were validated in a second sample of similar size and ethnicity.
The negative findings between genetic variation in ABCA1 and ABCG1 and risk of type 2 diabetes in the current study are in accordance with recent genome-wide association studies (22–24). In these large datasets, 22 genes were associated with β-cell dysfunction, and ABCA1 and ABCG1 or other ABC transporter genes were not among them. Because the chromosomal region spanning ABCA1 and ABCG1 (ABCA1, chromosome 9: 107543283–107690527; ABCG1, chromosome 21: 43619799–43717354) is well tagged on commercial arrays (170 single nucleotide polymorphisms [SNPs] on the Affymetrix 500 K chip and 585 SNPs on the 6.0 chip [www.Affymetrix.com, assessed November 2011]), it is highly unlikely that common variants in ABCA1 and ABCG1 would add to risk prediction of type 2 diabetes. For rare variants with minor allele frequencies <5%, the genome-wide association study approach does not appropriately determine risk (25) because capture requires that the variant is either on the array or tagged by common SNPs on the array, which is unlikely for rare variants (25). By identifying low-frequency loss-of-function variants by detailed resequencing and subsequent large-scale genotyping (12–14), we suggest that rare functional variants in ABCA1 and ABCG1 also are not associated with risk of type 2 diabetes in the general population.
Even though this study is performed in large, well-characterized cohorts of the general population, our study has limitations that need to be addressed. As the variants selected for genotyping were obtained after resequencing participants with extremes of HDL cholesterol levels, they may not be representative of the general population and may thus be limited when they are tested against another condition—in this case, type 2 diabetes. A comparison between the presently identified variation with that obtained in public databases indicates that variants with minor allele frequencies >0.2–0.3% (21 of 27 variants in the current study), are representative of the general population, whereas variants below this frequency are more likely to be population specific. Further, because we do not have a direct measure of β-cell function, we cannot exclude that genetic variation in ABCA1 and ABCG1 impacts pancreatic β-cell function as observed previously in a family study (8). We can, however, exclude that potential effects on β-cell function of the present 27 genetic variants translate into altered levels of nonfasting glucose, HbA1c, or inflammatory markers or into increased risk of type 2 diabetes or a prediabetes state. Finally, because we studied whites only, our results may not necessarily apply to other ethnic groups.
In conclusion, genetic variation in ABCA1 and ABCG1 is not associated with increased risk of type 2 diabetes in the general population. These data were obtained in general population samples harboring the largest number of heterozygotes for loss-of-function mutations in ABCA1 and ABCG1.
Supplementary Material
Acknowledgments
This work was supported by a Specific Targeted Research Project grant from the European Union, Sixth Framework Programme Priority (FP-2005-LIFESCIHEALTH-6) contract no. 037631, the Danish Medical Research Council (Copenhagen, Denmark), the Research Fund at Rigshospitalet, Copenhagen University Hospital (Copenhagen, Denmark), Chief Physician Johan Boserup and Lise Boserup’s Fund (Copenhagen, Denmark), Ingeborg and Leo Dannin’s Grant (Copenhagen, Denmark), and Henry Hansen and Wife’s Grant (Copenhagen, Denmark). The funding sources did not direct the subject matter of research.
No potential conflicts of interest relevant to this article were reported.
J.S. and A.T.-H. researched data and wrote the manuscript. H.J.M. contributed to biochemical measurements and reviewed and edited the manuscript. B.G.N. contributed to the discussion and reviewed and edited the manuscript. R.F.-S. researched data and wrote the manuscript. R.F.-S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Mette Refstrup (Department of Clinical Biochemistry, Rigshospitalet), and Christina Dam (Department of Clinical Biochemistry, Rigshospitalet) for their persistent attention to the details of the large-scale genotyping. The authors are indebted to the staff and participants of the CCHS and the CGPS for their important contributions.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0082/-/DC1.
References
- 1.Brunham LR, Kruit JK, Verchere CB, Hayden MR. Cholesterol in islet dysfunction and type 2 diabetes. J Clin Invest 2008;118:403–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunham LR, Kruit JK, Pape TD, et al. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med 2007;13:340–347 [DOI] [PubMed] [Google Scholar]
- 3.Sturek JM, Castle JD, Trace AP, et al. An intracellular role for ABCG1-mediated cholesterol transport in the regulated secretory pathway of mouse pancreatic beta cells. J Clin Invest 2010;120:2575–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assmann G, von Eckardstein A, Brewer HB., Jr Familial analphalipoproteinemia: Tangier disease. In The Metabolic and Molecular Bases of Inherited Disease. 8th ed. Scriver CR, Beaudet AL, Valle D, Sly WS, Eds. New York, NY, McGraw-Hill, 2001, p. 2937–2960 [Google Scholar]
- 5.Bodzioch M, Orsó E, Klucken J, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet 1999;22:347–351 [DOI] [PubMed] [Google Scholar]
- 6.Rust S, Rosier M, Funke H, et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet 1999;22:352–355 [DOI] [PubMed] [Google Scholar]
- 7.Brooks-Wilson A, Marcil M, Clee SM, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet 1999;22:336–345 [DOI] [PubMed] [Google Scholar]
- 8.Vergeer M, Brunham LR, Koetsveld J, et al. Carriers of loss-of-function mutations in ABCA1 display pancreatic beta-cell dysfunction. Diabetes Care 2010;33:869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villarreal-Molina MT, Flores-Dorantes MT, Arellano-Campos O, et al. Metabolic Study Group Association of the ATP-binding cassette transporter A1 R230C variant with early-onset type 2 diabetes in a Mexican population. Diabetes 2008;57:509–513 [DOI] [PubMed] [Google Scholar]
- 10.Mauldin JP, Srinivasan S, Mulya A, et al. Reduction in ABCG1 in type 2 diabetic mice increases macrophage foam cell formation. J Biol Chem 2006;281:21216–21224 [DOI] [PubMed] [Google Scholar]
- 11.Mauldin JP, Nagelin MH, Wojcik AJ, et al. Reduced expression of ATP-binding cassette transporter G1 increases cholesterol accumulation in macrophages of patients with type 2 diabetes mellitus. Circulation 2008;117:2785–2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frikke-Schmidt R, Nordestgaard BG, Stene MC, et al. Association of loss-of-function mutations in the ABCA1 gene with high-density lipoprotein cholesterol levels and risk of ischemic heart disease. JAMA 2008;299:2524–2532 [DOI] [PubMed] [Google Scholar]
- 13.Schou J, Frikke-Schmidt R, Kardassis D, et al. Genetic variation in ABCG1 and risk of myocardial infarction and ischemic heart disease. Arterioscler Thromb Vasc Biol 2012;32:506–515 [DOI] [PubMed] [Google Scholar]
- 14.Frikke-Schmidt R, Nordestgaard BG, Jensen GB, Tybjaerg-Hansen A. Genetic variation in ABC transporter A1 contributes to HDL cholesterol in the general population. J Clin Invest 2004;114:1343–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnohr P, Jensen G, Lange P, Scharling H, Appleyard M. The Copenhagen City Heart Study. Eur Heart J Suppl 2001;3:H1–H83 [Google Scholar]
- 16.Møller HJ, Frikke-Schmidt R, Moestrup SK, Nordestgaard BG, Tybjærg-Hansen A. Serum soluble CD163 predicts risk of type 2 diabetes in the general population. Clin Chem 2011;57:291–297 [DOI] [PubMed] [Google Scholar]
- 17.Møller HJ, Hald K, Moestrup SK. Characterization of an enzyme-linked immunosorbent assay for soluble CD163. Scand J Clin Lab Invest 2002;62:293–299 [DOI] [PubMed] [Google Scholar]
- 18.Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med 2008;359:1897–1908 [DOI] [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502 [PubMed] [Google Scholar]
- 20.American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li B, Leal SM. Methods for detecting associations with rare variants for common diseases: application to analysis of sequence data. Am J Hum Genet 2008;83:311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voight BF, Scott LJ, Steinthorsdottir V, et al. MAGIC investigators. GIANT Consortium Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet 2010;42:579–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dupuis J, Langenberg C, Prokopenko I, et al. DIAGRAM Consortium. GIANT Consortium. Global BPgen Consortium. Anders Hamsten on behalf of Procardis Consortium. MAGIC investigators New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 2010;42:105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saxena R, Hivert MF, Langenberg C, et al. GIANT consortium. MAGIC investigators Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet 2010;42:142–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manolio TA. Genomewide association studies and assessment of the risk of disease. N Engl J Med 2010;363:166–176 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.