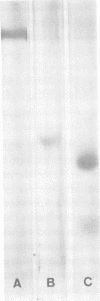
Abstract
Fibronectin (Fn) was found to bind to protein A-containing isolates of Staphylococcus aureus, but not to mutant strains devoid of this protein nor to clinical isolates of S. epidermidis. Fn was purified from human plasma by affinity chromatography on gelatin-Sepharose. After elution with 4 M urea, sodium dodecyl sulfate-polyacrylamide gel electrophoresis of purified material detected no immunoglobulin contamination. This purified Fn was radiolabeled with 125I and used in binding assays. Quantitatively, Fn binding was directly correlated with the cellular protein A content of the various strains tested. Mannitol salt broth preculture or organisms resulted in a reduction of their cellular protein A and a decrease in Fn binding by these cells. However, soluble protein A maximally inhibited the binding of radiolabeled Fn to protein A-positive strains of staphylococci by only 50%, indicating the possibility of multiple Fn binding sites. Fn's binding to protein A-containing S. aureus strains may play a role in the pathogenicity of these organisms by promoting their attachment to and subsequent invasion of host tissues.
Full text
PDF

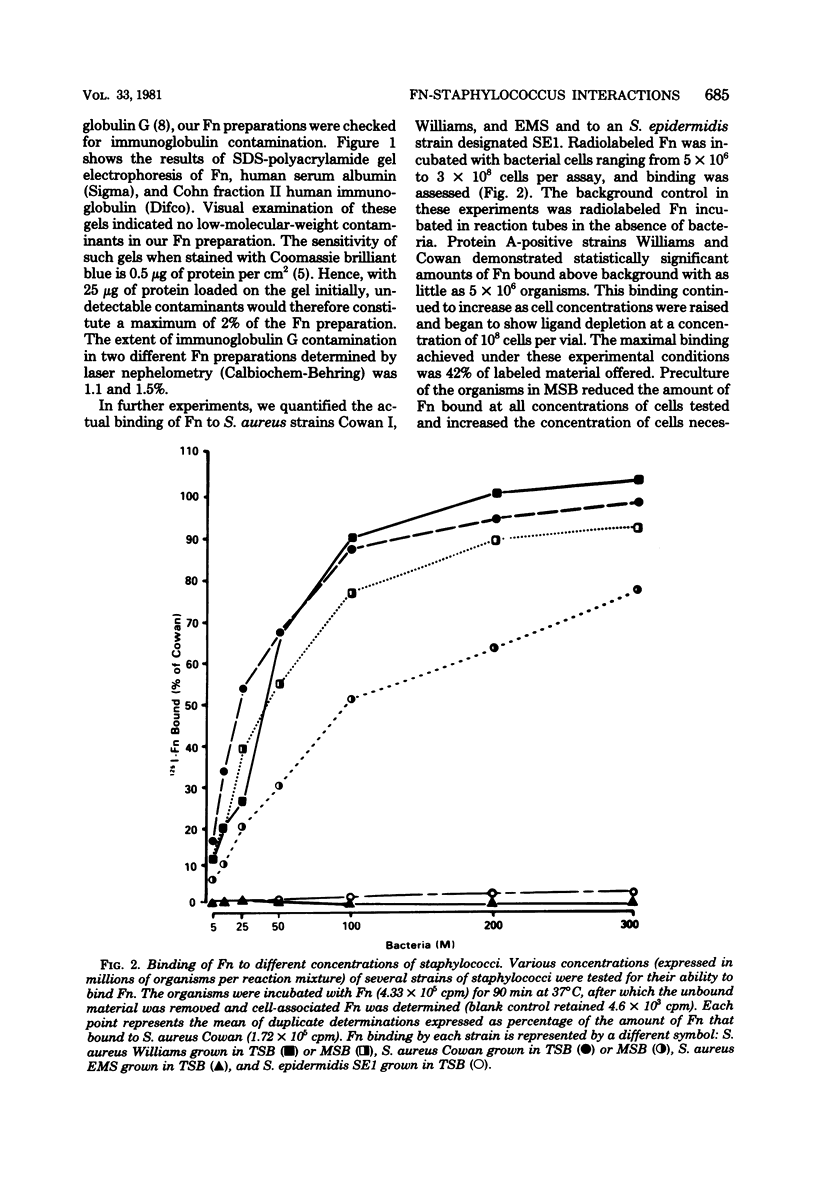

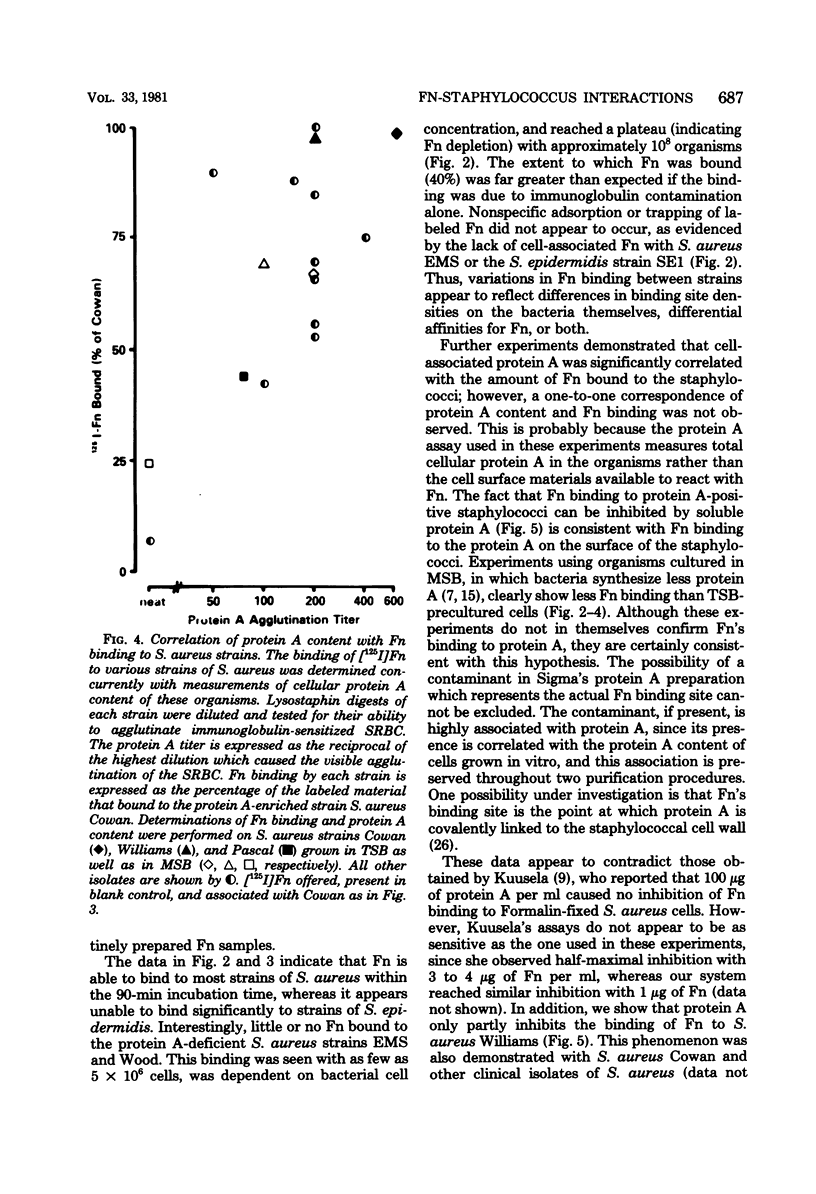
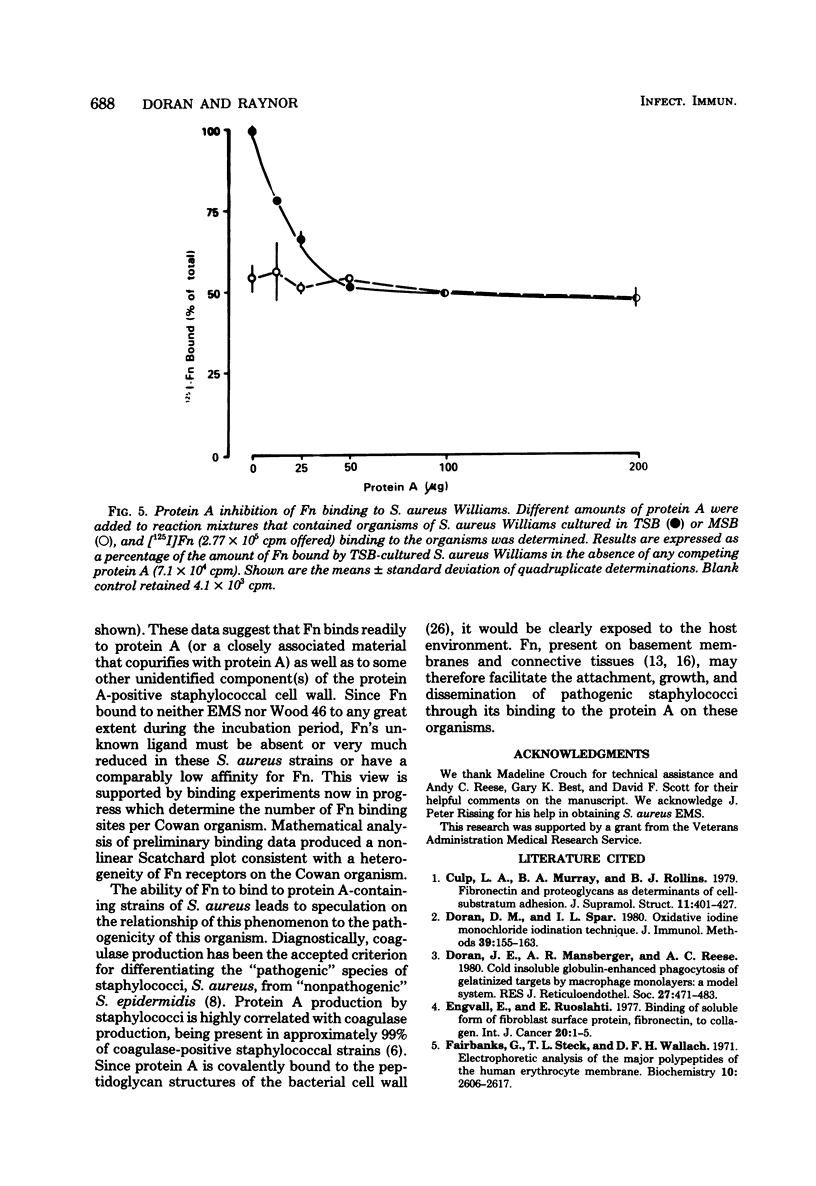

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Culp L. A., Murray B. A., Rollins B. J. Fibronectin and proteoglycans as determinants of cell-substratum adhesion. J Supramol Struct. 1979;11(3):401–427. doi: 10.1002/jss.400110314. [DOI] [PubMed] [Google Scholar]
- Doran D. M., Spar I. L. Oxidative iodine monochloride iodination technique. J Immunol Methods. 1980;39(1-2):155–163. doi: 10.1016/0022-1759(80)90304-x. [DOI] [PubMed] [Google Scholar]
- Doran J. E., Mansberger A. R., Reese A. C. Cold insoluble globulin-enhanced phagocytosis of gelatinized targets by macrophage monolayers: a model system. J Reticuloendothel Soc. 1980 May;27(5):471–483. [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Forsgren A. Significance of protein a production by staphylococci. Infect Immun. 1970 Nov;2(5):672–673. doi: 10.1128/iai.2.5.672-673.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukenes G. Serological typing of Staphylococcus aureus. 7. Technical aspects. Acta Pathol Microbiol Scand. 1967;70(4):590–600. doi: 10.1111/j.1699-0463.1967.tb01328.x. [DOI] [PubMed] [Google Scholar]
- Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978 Dec 14;276(5689):718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Molnar J., McLain S., Allen C., Laga H., Gara A., Gelder F. The role of an alpha2-macroglobulin of rat serum in the phagocytosis of colloidal particles. Biochim Biophys Acta. 1977 Jul 22;493(1):37–54. doi: 10.1016/0005-2795(77)90258-6. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Amrani D. L. The structure and biologic activities of plasma fibronectin. Blood. 1980 Aug;56(2):145–158. [PubMed] [Google Scholar]
- Mosher D. F., Proctor R. A. Binding and factor XIIIa-mediated cross-linking of a 27-kilodalton fragment of fibronectin to Staphylococcus aureus. Science. 1980 Aug 22;209(4459):927–929. doi: 10.1126/science.7403857. [DOI] [PubMed] [Google Scholar]
- Nickerson D. S., White J. G., Kronvali G., Williams R. C., Jr, Quie P. G. Indirect visualization of Staphylococcus aureus protein A. J Exp Med. 1970 May 1;131(5):1039–1047. doi: 10.1084/jem.131.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein E., Gold L. I., Garcia-Pardo A. Fibronectin: a review of its structure and biological activity. Mol Cell Biochem. 1980 Feb 8;29(2):103–128. doi: 10.1007/BF00220304. [DOI] [PubMed] [Google Scholar]
- Plow E. F., Birdwell C., Ginsberg M. H. Identification and quantitation of platelet-associated fibronectin antigen. J Clin Invest. 1979 Mar;63(3):540–543. doi: 10.1172/JCI109334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. B., Doran J. E., Reese A. C., Mansberger A. R., Jr Cold insoluble globulin levels in operative trauma: serum depletion, wound sequestration, and biological activity: an experimental and clinical study. Am Surg. 1980 Dec;46(12):663–672. [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E. Immunochemical and collagen-binding properties of fibronectin. Ann N Y Acad Sci. 1978 Jun 20;312:178–191. doi: 10.1111/j.1749-6632.1978.tb16802.x. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Vaheri A. Interaction of soluble fibroblast surface antigen with fribrinogen and fibrin. J Exp Med. 1975 Feb 1;141(2):497–501. doi: 10.1084/jem.141.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Vaheri A., Kuusela P., Linder E. Fibroblast surface antigen: a new serum protein. Biochim Biophys Acta. 1973 Oct 18;322(2):352–358. doi: 10.1016/0005-2795(73)90310-3. [DOI] [PubMed] [Google Scholar]
- Saba T. M., Blumenstock F. A., Scovill W. A., Bernard H. Cryoprecipitate reversal of opsonic alpha2-surface binding glycoprotein deficiency in septic surgical and trauma patients. Science. 1978 Aug 18;201(4356):622–624. doi: 10.1126/science.675246. [DOI] [PubMed] [Google Scholar]
- Scovill W. A., Saba T. M., Blumenstock F. A., Bernard H., Powers S. R., Jr Opsonic alpha2 surface binding glycoprotein therapy during sepsis. Ann Surg. 1978 Oct;188(4):521–529. doi: 10.1097/00000658-197810000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöquist J., Meloun B., Hjelm H. Protein A isolated from Staphylococcus aureus after digestion with lysostaphin. Eur J Biochem. 1972 Sep 25;29(3):572–578. doi: 10.1111/j.1432-1033.1972.tb02023.x. [DOI] [PubMed] [Google Scholar]
- Sjöquist J., Movitz J., Johansson I. B., Hjelm H. Localization of protein A in the bacteria. Eur J Biochem. 1972 Oct 17;30(1):190–194. doi: 10.1111/j.1432-1033.1972.tb02086.x. [DOI] [PubMed] [Google Scholar]
- Winblad S., Ericson C. Sensitized sheep red cells as a reactant for Staphylococcus aureus protein A. Methodology and epidemiology with special reference to weakly reacting methicillin-resistant strains. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Feb;81(1):150–156. [PubMed] [Google Scholar]