Abstract
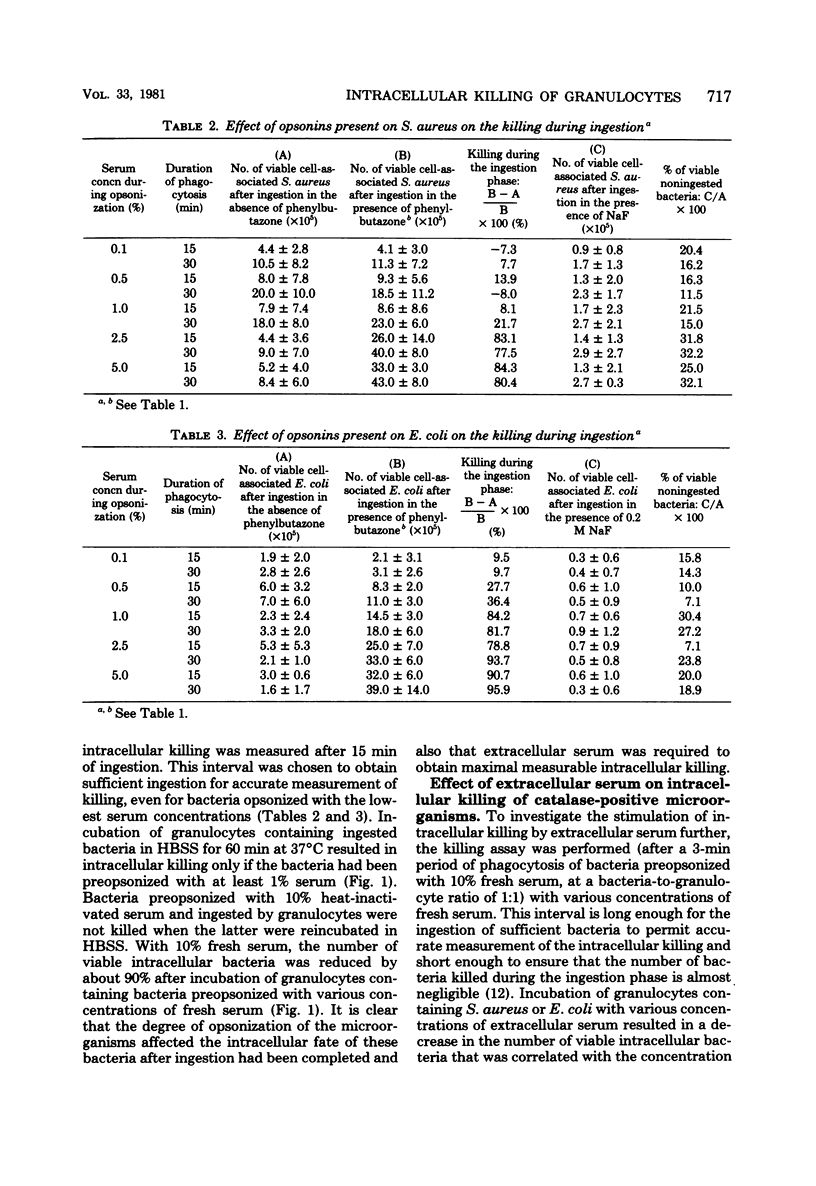
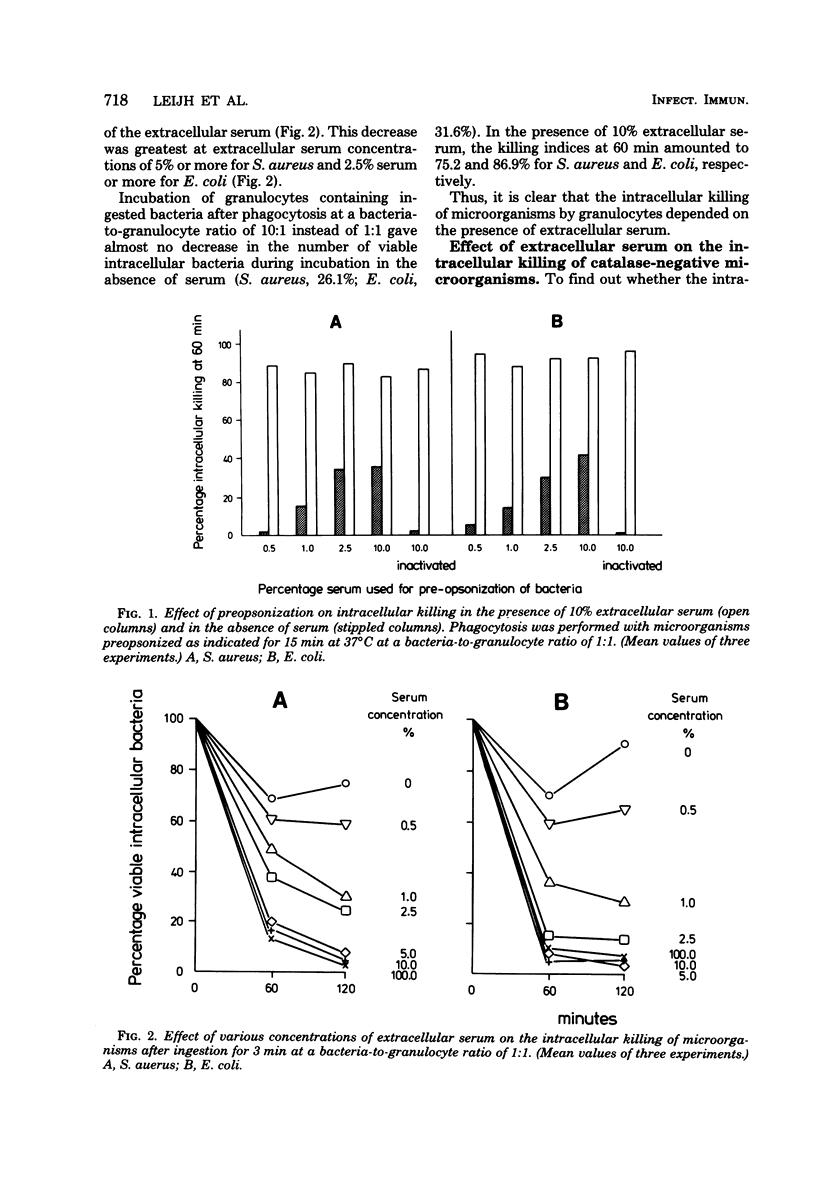
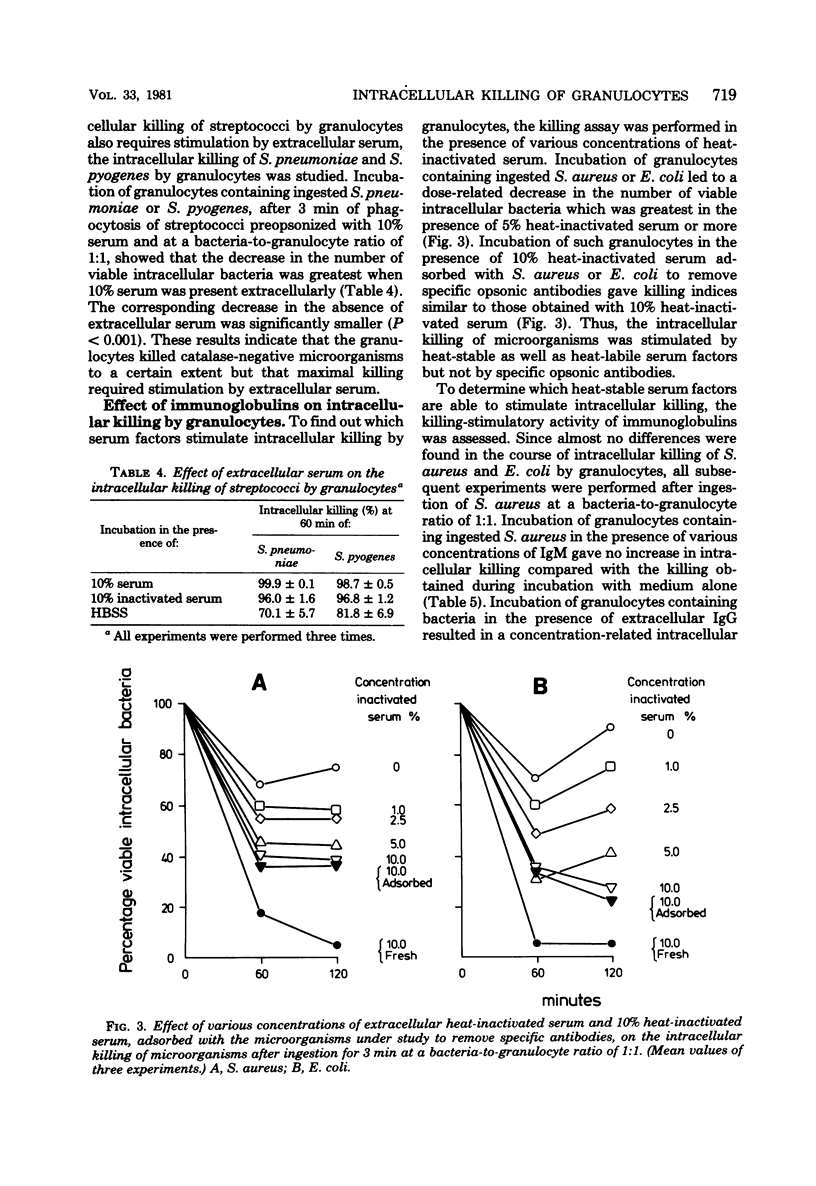
Immunoglobulins and complement components are required for optimal ingestion and optimal killing of microorganisms by granulocytes. The degree of opsonization of microorganisms necessary for their ingestion was lower than that required for the killing of these bacteria during the ingestion phase. Killing during this phase was found to depend mainly on the presence of heat-labile opsonins, probably C3b, present on the microorganisms. Extracellular immunoglobulin G (IgG) and C3b were indispensable for optimal intracellular killing after ingestion was complete. This was established with an assay permitting assessment of the course of the number of viable intracellular bacteria independent of the ingestion of new live bacteria. Maximal intracellular killing by human granulocytes of ingested catalase-positive (Staphylococcus aureus and Escherichia coli) or catalase-negative (Streptococcus pyogenes and S. pneumoniae) microorganisms was found only when fresh serum was present extracellularly. Killing was suboptimal in the absence of serum. With heat-inactivated serum, the killing index lay between the indices obtained in the presence and absence of fresh serum. The stimulatory activity of heat-inactivated serum was most probably due to the interaction of IgG with the Fc receptor on the granulocyte membrane, since IgG subclasses IgG1 and IgG3 as well as pFc fragments of IgG stimulated the intracellular killing to the same degree as heat-inactivated serum did. In addition, (Fab1)2 fragments of IgG did not stimulate killing, and reduced killing was observed in the presence of heat-inactivated serum after reduction of the number of Fc receptors. The extra stimulation of the killing process in the presence of fresh serum compared with heat-inactivated serum was due to the interaction between membrane receptors and complement--most probably C3b generated by both the classical and the alternative pathways of complement activation. This conclusion is based on results obtained with sera in which one or both complement pathways were blocked, on the restoration of the killing-stimulatory activity of C3-deficient serum after addition of fresh C3, and on the reduced killing observed in the presence of fresh serum after reduction of the number of C3 receptors by the use of pronase or antigranulocyte serum.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berto R., Menzel J. The interaction of opsonins with human polymorphonuclear leucocytes (PMN). I. The influence of human complement (C) and IgG on ingestion and digestion of C-resistant E. coli. Z Immunitatsforsch Immunobiol. 1979 Feb;155(3):189–199. [PubMed] [Google Scholar]
- Brutel de la Rivière A., Verhoef-Karssen P. R., Bosma A., Kr vd Borne A. E., Engelfriet P. Specific antisera against human blood cells applicable in the indirect immunofluorescence technique. Scand J Immunol. 1976;5(9):1065–1074. doi: 10.1111/j.1365-3083.1976.tb03058.x. [DOI] [PubMed] [Google Scholar]
- Craig C. P., Suter E. Extracellular factors influencing staphylocidal capacity of human polymorphonuclear leukocytes. J Immunol. 1966 Aug;97(2):287–296. [PubMed] [Google Scholar]
- Crofton R. W., Diesselhoff-den Dulk M. M., van Furth R. The origin, kinetics, and characteristics of the Kupffer cells in the normal steady state. J Exp Med. 1978 Jul 1;148(1):1–17. doi: 10.1084/jem.148.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Cerqueira M., Lind S., Kaplan H. B. Evidence that the superoxide-generating system of human leukocytes is associated with the cell surface. J Clin Invest. 1977 Feb;59(2):249–254. doi: 10.1172/JCI108635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A., Leider J. E., Silverstein S. C. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med. 1975 Nov 1;142(5):1263–1282. doi: 10.1084/jem.142.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Griffin J. A., Silverstein S. C. Studies on the mechanism of phagocytosis. II. The interaction of macrophages with anti-immunoglobulin IgG-coated bone marrow-derived lymphocytes. J Exp Med. 1976 Sep 1;144(3):788–809. doi: 10.1084/jem.144.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest. 1980 Jan;65(1):82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI I. W., MUDD S. THE HEAT-LABILE SERUM FACTOR ASSOCIATED WITH INTRACELLULAR KILLING OF STAPHYLOCOCCUS AUREUS. J Immunol. 1965 Jun;94:852–857. [PubMed] [Google Scholar]
- Lawrence D. A., Weigle W. O., Spiegelberg H. L. Immunoglobulins cytophilic for human lymphocytes, monocytes, and neutrophils. J Clin Invest. 1975 Feb;55(2):368–376. doi: 10.1172/JCI107940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijh P. C., van Zwet T. L., van Furth R. Effect of extracellular serum in the stimulation of intracellular killing of streptococci by human monocytes. Infect Immun. 1980 Nov;30(2):421–426. doi: 10.1128/iai.30.2.421-426.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., Dubbeldeman-Rempt I., van Furth R. Kinetics of intracellular killing of Staphylococcus aureus and Escherichia coli by human granulocytes. Eur J Immunol. 1980 Oct;10(10):750–757. doi: 10.1002/eji.1830101005. [DOI] [PubMed] [Google Scholar]
- Leijh P. C., van den Barselaar M. T., van Zwet T. L., Daha M. R., van Furth R. Requirement of extracellular complement and immunoglobulin for intracellular killing of micro-organisms by human monocytes. J Clin Invest. 1979 Apr;63(4):772–784. doi: 10.1172/JCI109362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leist-Welsh P., Bjornson A. B. Requirements of immunoglobulin and the classical and alternative complement pathways for phagocytosis and intracellular killing of multiple strains of Gram-negative aerobic bacilli. Infect Immun. 1979 Oct;26(1):99–109. doi: 10.1128/iai.26.1.99-109.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner R. P., Jelinek J. Receptors for human gamma G globulin on human neutrophils. J Clin Invest. 1970 Dec;49(12):2165–2171. doi: 10.1172/JCI106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottini G. D., Cian F., Tedesco F., de Nicola G., Patriarca P. Effect of antibodies and complement on the interaction between Escherichia coli 0111:B4 and polymorphonuclear leukocytes. Infection. 1979;7(4):160–165. doi: 10.1007/BF01640933. [DOI] [PubMed] [Google Scholar]
- Scribner D. J., Fahrney D. Neutrophil receptors for IgG and complement: their roles in the attachment and ingestion phases of phagocytosis. J Immunol. 1976 Apr;116(4):892–897. [PubMed] [Google Scholar]
- Solberg C. O., Christie K. E., Larsen B., Tonder O. Influence of antibodies and thermolabile serum factors on the bactericidal activity of human neutrophil granulocytes. Acta Pathol Microbiol Scand C. 1976 Apr;84(2):112–118. doi: 10.1111/j.1699-0463.1976.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Solberg C. O., Hellum K. B. Influence of serum on the bactericidal activity of neutrophil granulocytes. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Oct;81(5):621–626. doi: 10.1111/j.1699-0463.1973.tb02252.x. [DOI] [PubMed] [Google Scholar]
- Solberg C. O. Influence of therapeutic concentrations of phenylbutazone on granulocyte function. Acta Pathol Microbiol Scand B. 1975 Apr;83(2):100–102. doi: 10.1111/j.1699-0463.1975.tb00077.x. [DOI] [PubMed] [Google Scholar]
- Strauss R. R., Paul B. B., Sbarra A. J. Effect of phenylbutazone on phagocytosis and intracellular killing by guinea pig polymorphonuclear leukocytes. J Bacteriol. 1968 Dec;96(6):1982–1990. doi: 10.1128/jb.96.6.1982-1990.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulkens P., Schneider Y. J., Trouet A. The fate of the plasma membrane during endocytosis. Biochem Soc Trans. 1977;5(6):1809–1815. doi: 10.1042/bst0051809. [DOI] [PubMed] [Google Scholar]
- Van Der Meer J. W., Leijh P. C., Van Den Barselaar, Van Furth R. Functions of phagocytic cells in chronic mucocutaneous candidiasis. Br Med J. 1978 Jan 21;1(6106):147–148. doi: 10.1136/bmj.1.6106.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura M., Valdimarsson H. Participation of C3 in intracellular killing of Candida albicans. Scand J Immunol. 1977;6(6-7):591–594. doi: 10.1111/j.1365-3083.1977.tb02137.x. [DOI] [PubMed] [Google Scholar]
- van Rood J. J., van Leeuwen A., Ploem J. S. Simultaneous detection of two cell populations by two-colour fluorescence and application to the recognition of B-cell determinants. Nature. 1976 Aug 26;262(5571):795–797. doi: 10.1038/262795a0. [DOI] [PubMed] [Google Scholar]


