Abstract
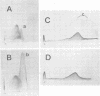
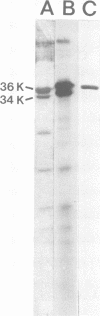
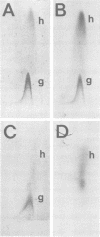
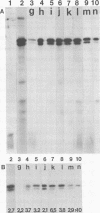
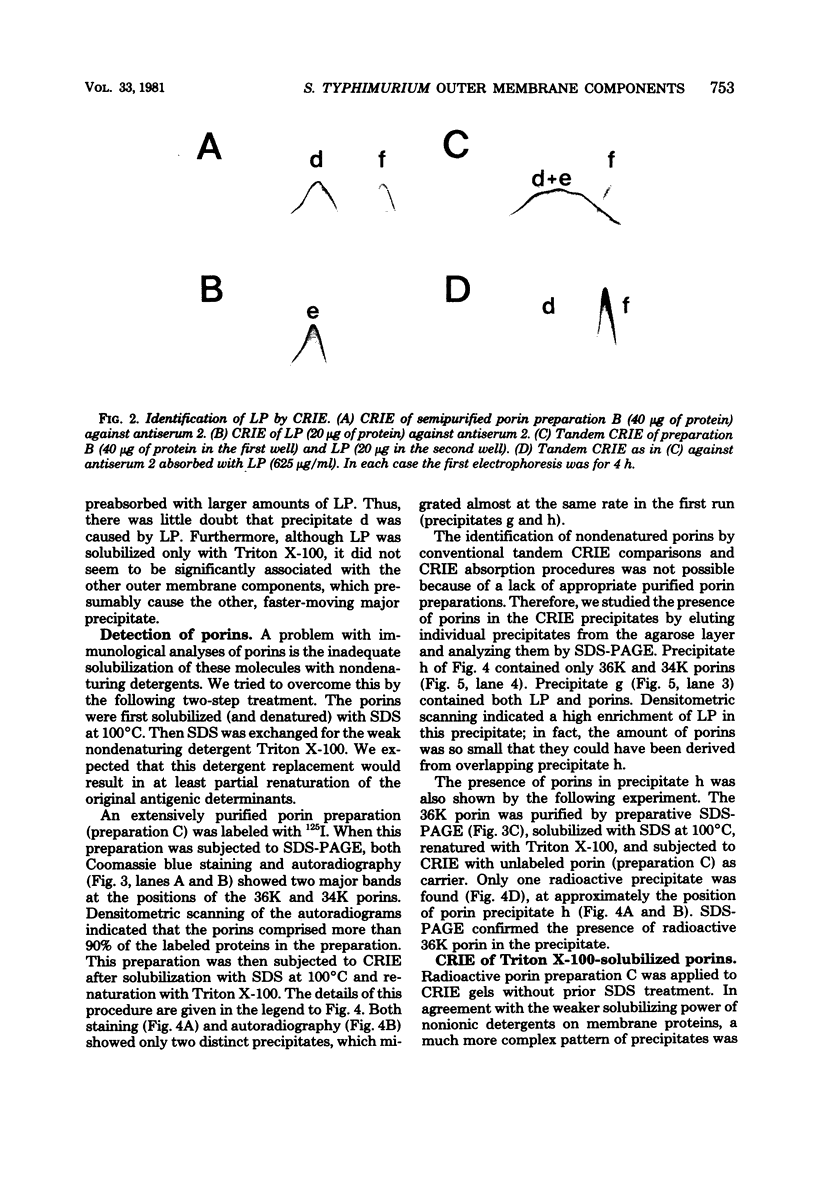
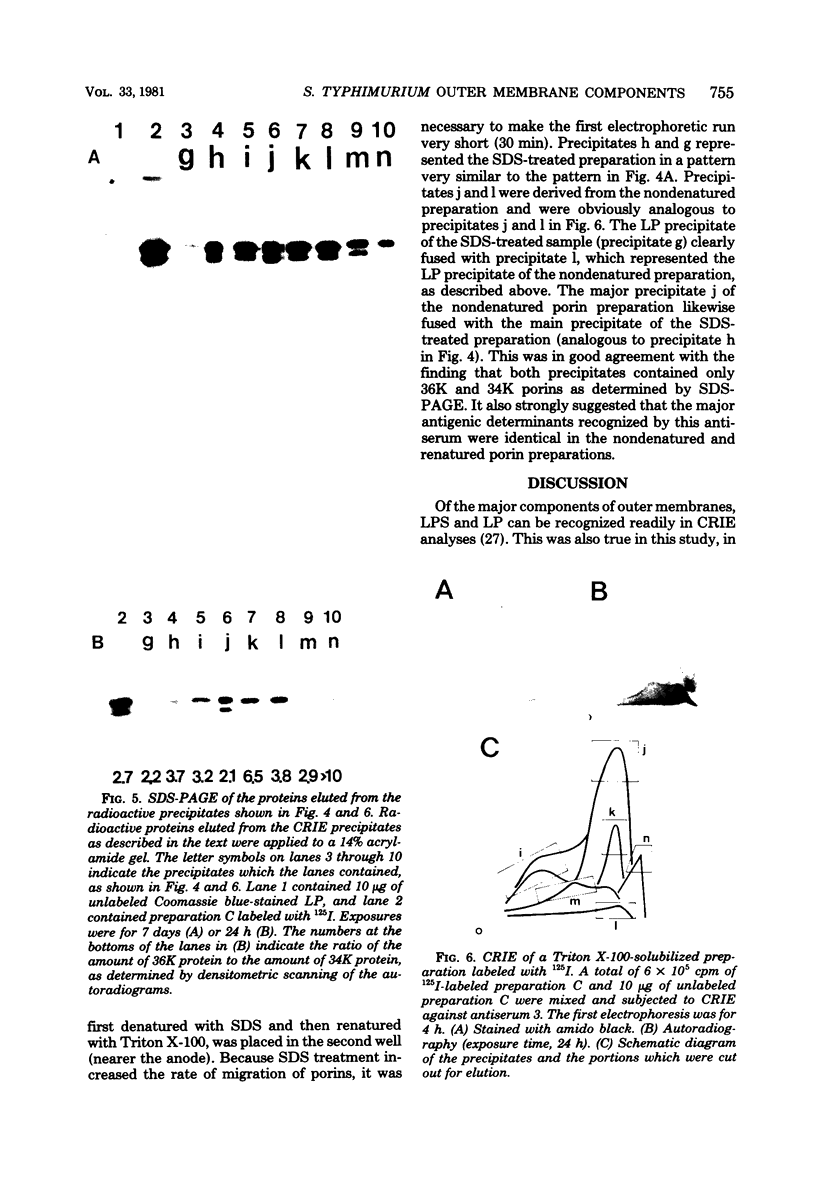
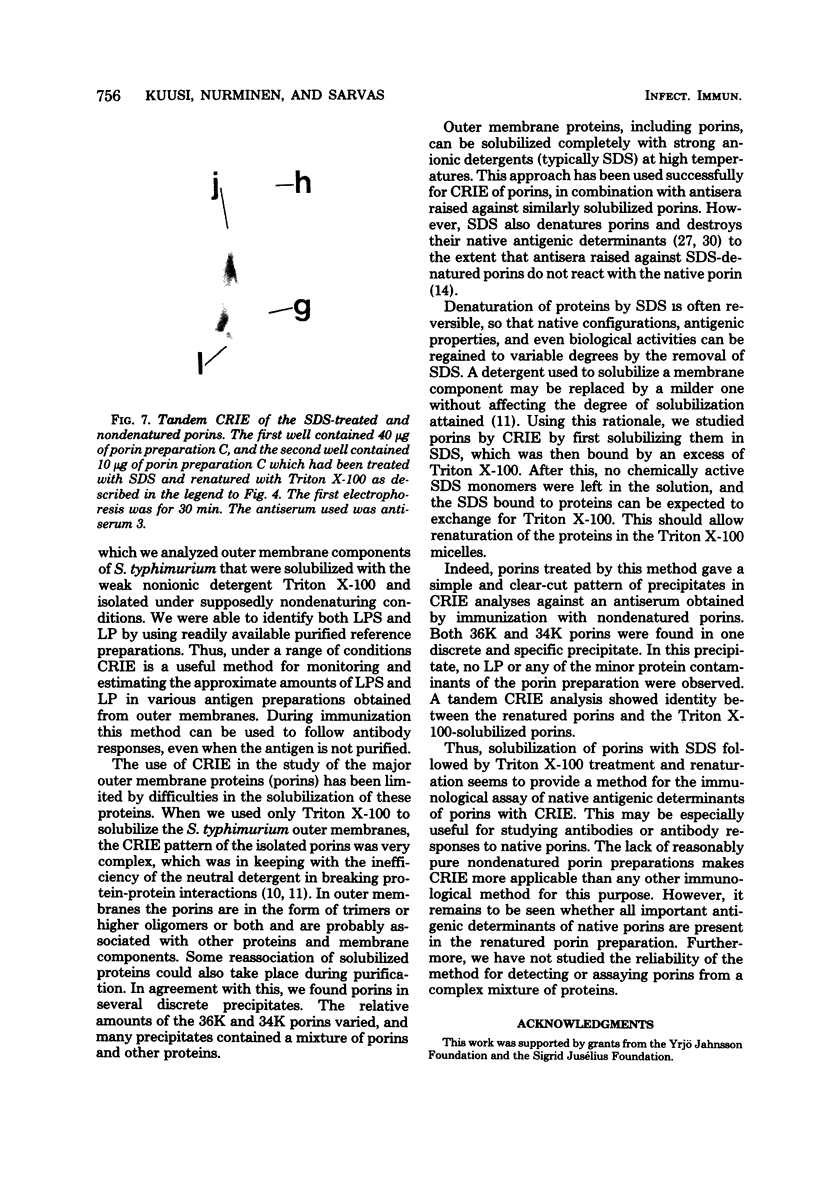
We used crossed immunoelectrophoresis to study detergent-solubilized components of the outer membrane of Salmonella typhimurium under nondenaturing conditions. The antisera used were raised against nondenatured outer membrane preparations. Lipopolysaccharide and lipoprotein were identified easily as discrete precipitates when they were solubilized with Triton X-100. However, solubilization of the porins with Triton X-100 resulted in a complex precipitate pattern, indicating incomplete dissociation of protein-protein interactions. A clear-cut pattern was obtained when the porins were first solubilized and denatured with hot sodium dodecyl sulfate, followed by removal of the sodium dodecyl sulfate and renaturation in the presence of Triton X-100. Our findings suggested that crossed immunoelectrophoresis can be used to study the antigenicity of nondenatured porins and the antibody responses to them.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjerrum O. J., Bog-Hansen T. C. The immunochemical approach to the characterization of membrane proteins. Human erythrocyte membrane proteins analysed as a model system. Biochim Biophys Acta. 1976 Nov 11;455(1):66–89. doi: 10.1016/0005-2736(76)90154-1. [DOI] [PubMed] [Google Scholar]
- Bjerrum O. J. Immunochemical investigation of membrane proteins. A methodological survey with emphasis placed on immunoprecipitation in gels. Biochim Biophys Acta. 1977 Aug 9;472(2):135–195. doi: 10.1016/0304-4157(77)90016-8. [DOI] [PubMed] [Google Scholar]
- Bjerrum O. J., Lundahl P. Detergent-containing gels for immunological studies of solubilized erythrocyte membrane components. Scand J Immunol Suppl. 1973;1:139–143. doi: 10.1111/j.1365-3083.1973.tb03794.x. [DOI] [PubMed] [Google Scholar]
- Bock E. Non-plasma proteins in cerebrospinal fluid. Scand J Immunol Suppl. 1973;1:119–124. [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Helenius A., Fries E., Garoff H., Simons K. Solubilization of the Semliki Forest virus membrane with sodium deoxycholate. Biochim Biophys Acta. 1976 Jun 17;436(2):319–334. doi: 10.1016/0005-2736(76)90197-8. [DOI] [PubMed] [Google Scholar]
- Helenius A., McCaslin D. R., Fries E., Tanford C. Properties of detergents. Methods Enzymol. 1979;56:734–749. doi: 10.1016/0076-6879(79)56066-2. [DOI] [PubMed] [Google Scholar]
- Hertz J. B., Hølby N., Andersen V., Baekgaard P. Crossed immunoelectrophoretic analysis of Bordetella pertussis antigens and of corresponding antibodies in human sera. Acta Pathol Microbiol Scand B. 1976 Dec;84B(6):386–394. doi: 10.1111/j.1699-0463.1976.tb01957.x. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Suzuki H., Nishimura Y., Yasuda S. On the process of cellular division in Escherichia coli: a mutant of E. coli lacking a murein-lipoprotein. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1417–1420. doi: 10.1073/pnas.74.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstra H., Dankert J. Preparation and quantitative determination of antibodies against major outer mambranes proteins of Escherichia coli O26 K60. J Gen Microbiol. 1980 Apr;117(2):437–447. doi: 10.1099/00221287-117-2-437. [DOI] [PubMed] [Google Scholar]
- Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium. Identification of proteins exposed on cell surface. Biochim Biophys Acta. 1977 Feb 4;464(3):589–601. doi: 10.1016/0005-2736(77)90033-5. [DOI] [PubMed] [Google Scholar]
- Kroll J. Tandem-crossed immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:57–59. [PubMed] [Google Scholar]
- Kuusi N. A technical improvement for crossed immunoelectrophoresis. J Immunol Methods. 1979;31(3-4):361–364. doi: 10.1016/0022-1759(79)90149-2. [DOI] [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxen H., Valtonen M., Mäkelä P. H. Immunization with major outer membrane proteins in experimental salmonellosis of mice. Infect Immun. 1979 Sep;25(3):857–862. doi: 10.1128/iai.25.3.857-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Nurminen M., Lounatmaa K., Sarvas M., Mäkelä P. H., Nakae T. Bacteriophage-resistant mutants of Salmonella typhimurium deficient in two major outer membrane proteins. J Bacteriol. 1976 Aug;127(2):941–955. doi: 10.1128/jb.127.2.941-955.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Friedman-Kien A. E., Salton M. R. Antigenic analysis of Neisseria gonorrhoeae by crossed immunoelectrophoresis. Infect Immun. 1976 Apr;13(4):1273–1288. doi: 10.1128/iai.13.4.1273-1288.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Siegel J., Salton M. R., Owen P. Immunochemical analysis of inner and outer membranes of Escherichia coli by crossed immunoelectrophoresis. J Bacteriol. 1978 Jan;133(1):306–319. doi: 10.1128/jb.133.1.306-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker B. A., Nurminen M., Mäkelä P. H. Mutants defective in the 33K outer membrane protein of Salmonella typhimurium. J Bacteriol. 1979 Aug;139(2):376–383. doi: 10.1128/jb.139.2.376-383.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S. B., Nurminen M., Lindberg A. A. Artificial Salmonella vaccines: O-antigenic oligosaccharide-protein conjugates induce protection against infection with Salmonella typhimurium. Infect Immun. 1979 Sep;25(3):863–872. doi: 10.1128/iai.25.3.863-872.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]