Abstract
Here we report the result of a genetic screen for mutants resistant to the microtubule poison methyl benzimidazol-2-yl carbamate (MBC) that were also temperature sensitive for growth. In total the isolated mutants were distributed in ten complementation groups. Cloning experiments revealed that most of the mutants were in essential genes encoding various 26S proteasome subunits. We found that the proteasome mutants are multi-drug resistant due to stabilization of the stress-activated transcription factor Pap1. We show that the ubiquitylation and ultimately the degradation of Pap1 depend on the Rhp6/Ubc2 E2 ubiquitin conjugating enzyme and the Ubr1 E3 ubiquitin-protein ligase. Accordingly, mutants lacking Rhp6 or Ubr1 display drug-resistant phenotypes.
Introduction
Intracellular protein degradation is a regulated process that maintains cellular homeostasis [1]. However, selective destruction of regulatory proteins also provides an important control mechanism for quickly and irreversibly eliminating signalling proteins such as transcription factors [1]. Intracellular protein degradation is therefore relevant for most cellular and physiological functions including apoptosis, cell cycle progression, differentiation and DNA repair [1], and also partakes in cellular stress responses [2].
In eukaryotic cells the major degradation pathway for intracellular proteins is via the ubiquitin-proteasome system (UPS) [3] [1] [4]. This system relies on a cascade of three enzymes termed E1, E2 and E3 that conjugate the small protein ubiquitin to specific target proteins [3] [5]. Subsequently, the proteins, which have been marked with ubiquitin, are targeted to the 26S proteasome, a large proteolytic particle found in the nucleus and cytosol of all eukaryotic cells [4]. At the 26S proteasome the ubiquitin chains are released while the substrate is degraded.
The 26S proteasome is composed of two subcomplexes, the proteolytically active 20S core particle and 19S regulatory complexes that bind to one or both ends of the 20S particle [4]. Structurally the 20S core is built from 28 subunits, arranged as four stacked heptameric rings, forming a cylindrical structure [6]. The two outer rings each contain seven different α subunits (α1- α7) and the two inner rings each contain seven different β subunits (β1– β7), forming an overall α1–7β1–7β1–7α1–7 structure [6]. Some of the β subunits are threonine-type proteases that expose their active sites towards a central chamber inside the 20S cylinder [6].
The 19S regulatory complex is an asymmetric particle composed of about 19 different subunits distributed between two subcomplexes called the base and the lid [4]. Some of these subunits are responsible for binding ubiquitylated substrates, while others are involved in recycling ubiquitin, by cleaving the ubiquitin moieties from the substrate during degradation. The 19S particle also contains six different ATPase subunits that function in unfolding and translocation of the protein substrates into the 20S cylinder [7]–[8].
In the fission yeast Schizosaccharomyces pombe a number of mutants have been isolated by their ability to be resistant to the mitotic poison methyl benzimidazol-2-yl carbamate (MBC) and also be temperature sensitive for growth, and were named mts for “MBC resistant and temperature sensitive”. Most of the mts mutants identified by this screen were found to be in different subunits of the 26S proteasome [9]–[12]. Although the 26S proteasome, through degradation of various substrates, is involved in multiple cellular pathways, the reason for the enrichment of 26S proteasome mutants in the screen has remained elusive.
The S. pombe homolog of the human AP-1 transcription factor, Pap1, is one of the major stress activated transcription factors in fission yeast [13]. Overexpression of Pap1 results in resistance to a number of different drugs such as staurosporine [14] and brefeldin A [15]. Conversely, mutants lacking Pap1 are hypersensitive to drugs such as caffeine [16].
Here, we characterize six novel mts mutants. Five of these mutants are in subunits of the 26S proteasome, while one is in the nuclear export receptor, Crm1. We show that the proteasome mutants are multi-drug resistant. This phenotype depends on the Pap1 transcription factor that is degraded by the ubiquitin pathway, but stabilized in the proteasome mutants. Finally, we also show that the Rhp6/Ubc2, E2 ubiquitin conjugating enzyme and the Ubr1 E3 ubiquitin-protein ligase are responsible for ubiquitylation of Pap1, and targeting Pap1 for degradation by the 26S proteasome.
Materials and Methods
S. pombe Strains, Techniques and Reagents
The S. pombe strains used in this study (Table 1) are derivatives of the wild type heterothallic strains 972h− and 975h+. Standard genetic methods and media were used and S. pombe transformations were performed using the lithium acetate procedure [17]. The PCR mutagenesis was performed according to a previously published procedure [18]. Methyl benzimidazol-2-yl carbamate (MBC) was purchased from Sigma.
Table 1. Fission yeast strains used in this study.
| Strain | Genotype | Reference |
| wild type | leu1-32 ura4-D18 h- | Laboratory stock |
| pap1Δ | pap1::ura4 leu1-32 ura4-D18 h- | [14] |
| cdc25.M35 | cdc25.M35 leu1-32 h- | [44] |
| mts1-1 | mts1-1 leu1-32 ura4-D18 h- | This study |
| mts2-1 | mts2-1 leu1-32 ura4-D18 h- | [9] |
| mts3-1 | mts3-1 leu1-32 ura4-D18 h- | [10] |
| mts4-1 | mts4-1 leu1-32 ura4-D18 h- | [11] |
| mts5-1 | mts5-1 (pad1-1) leu1-32 ura4-D18 h- | [12] |
| mts6-1 | mts6-1 leu1-32 ura4-D18 h- | This study |
| mts7-1 | mts7-1 leu1-32 ura4-D18 h- | This study |
| mts8-1 | mts8-1 leu1-32 ura4-D18 h- | This study |
| mts9-1 | mts9-1 leu1-32 ura4-D18 h- | This study |
| mts10-1 | mts10-1(crm1) leu1-32 ura4-D18 h- | This study |
| ubc1Δ | ubc1::ura4 leu1-32 ura4-D18 h- | [45] |
| rhp6Δ | rhp6(ubc2)::ura4 leu1-32 ura4-D18 h- | [45] |
| ubc3Δ | ubc3::ura4 leu1-32 ura4-D18 h- | [45] |
| ubc4-1 | ubcP1 leu1-32 ura4-D18 h- | [46] |
| ubc6Δ | ubc6::ura4 leu1-32 ura4-D18 h- | [45] |
| ubc8Δ | ubc8::ura4 leu1-32 ura4-D18 h- | This study |
| ubc11-1 | ubcP4 leu1-32 ura4-D18 h- | [46] |
| ubc13Δ | ubc13::ura4 leu1-32 ura4-D18 h- | [47] |
| ubc14Δ | ubc14::ura4 leu1-32 ura4-D18 h- | This study |
| ubc15Δ | ubc15::ura4 leu1-32 ura4-D18 h- | [48] |
| ubc16Δ | ubc16::HYG leu1-32 ura4-D18 h- | This study |
| rhp18Δ | rhp18::ura4 leu1-32 ura4-D18 h- | [45] |
| ubr1Δ | ubr1::ura4 leu1-32 ura4-D18 h- | [45] |
| ubr11Δ | ubr11::ura4 leu1-32 ura4-D18 h- | [45] |
| cdc8-27 | cdc8-27 leu1-32, h- | [49] |
| cdc13-117 | cdc13-117 leu1-32 | [50] |
| sep1Δ | sep1::G418 ura4-D18 leu1-32 h- | [51] |
| cdt2Δ | cdt2::G418 ura4-D18 leu1-32 h- | [52] |
| txl1Δ | txl1::NAT leu1-32 ura4-D18 h- | [53] |
Antibodies
The antibody to tubulin was the TAT1 monoclonal (Sigma). The antibody to actin was from GE Healthcare. The antibody to GFP was purchased from Roche. The antibodies to Obr1 (p25) and Pap1 have been described previously [19]. The antibody to Mts4 has been described previously [11]. The antibody to the 20S alpha subunits was the monoclonal MCP231 from Enzo Life Sciences. Secondary antibodies were from Dako. All antibodies were used in 1∶1000 dilutions.
Plasmids and Purification
The expression constructs used here were wild type cDNA encoding Pap1 and ubiquitin N-terminally tagged with GFP and 6His, respectively, subcloned to the pREP41 S. pombe expression vector. 6His-tagged ubiquitin was purified on Ni2+-NTA agarose beads (Qiagen) under denaturing conditions in 8 M urea as described by the manufacturer.
The S. pombe cDNA library was generously supplied by Prof. Peter A. Fantes (Edinburgh, UK).
Protein Degradation Assays
Protein degradation kinetics were determined by SDS-PAGE and blotting of extracts prepared from cultures treated with cycloheximide (CHX), as described previously [20].
Results
Isolation of the mts Mutants
We carried out a screen to isolate mutants that were resistant to the mitotic poison methyl benzimidazol-2-yl carbamate (MBC) and also temperature sensitive for growth. In total 24 mutants were obtained. Crossing them to each other demonstrated that the mutants lay in 10 complementation groups (mts1-mts10) (Table 2). Genetic analyses showed that in each case the temperature sensitive and drug resistant phenotypes co-segregated demonstrating that the same mutation was responsible for both phenotypes.
Table 2. The mts complementation groups.
| Mtsgroup | No. ofalleles | Encoded protein | Function |
| mts1 | 3 | Rpn9 | 19S lid proteasome subunit, |
| mts2 | 4 | Rpt2 | 19S base proteasome subunit |
| mts3 | 1 | Rpn12 | 19S lid proteasome subunit |
| mts4 | 6 | Rpn1 | 19S base proteasome subunit |
| mts5 | 1 | Rpn11/Pad1 | 19S lid proteasome subunit |
| mts6 | 2 | β2/Pup1 | 20S proteasome subunit |
| mts7 | 1 | α4/Pre6 | 20S proteasome subunit |
| mts8 | 1 | β1/Pre3 | 20S proteasome subunit |
| mts9 | 1 | β7/Pre4 | 20S proteasome subunit |
| mts10 | 4 | Crm1 | Nuclear export receptor |
Cloning of mts1, mts6, mts7, mts8, mts9 and mts10
Previous published work identified mts2, mts3, mts4 and mts5/pad1 mutants to be in different subunits of the 26S proteasome [9]–[12]. All four mutant strains were in different subunits of the 19S regulatory complex. The mts2 + gene encodes the Rpt2 base ATPase subunit, mts3+ the Rpn12 lid subunit, mts4+ the base non-ATPase Rpn1 and mts5+ the lid Rpn11/Pad1 deubiquitylating subunit (Table 2). To identify the genes encoding the mts1, mts6, mts7, mts8, mts9 and mts10 mutants the temperature sensitive phenotype of each mutant strain was rescued by transformation with a fission yeast cDNA library in an S. pombe expression vector. After isolating and sequencing plasmids it was observed that five of the mutant strains (mts1, mts6, mts7, mts8 and mts9) were rescued by cDNAs that encoded different subunits of the 26S proteasome. The mts1+ gene was found to encode the lid Rpn9 subunit. The mts6, mts7, mts8 and mts9 were rescued by cDNA encoding the 20S proteasome core subunits β2, α4, β1 and β7, respectively (Table 2). Finally, the cDNA that complemented the mts10 temperature sensitive phenotype encoded the nuclear export protein Crm1 (Table 2). To demonstrate that the cloned cDNAs encoded the authentic genes and not extragenic suppressors, each of the mts strains were crossed to mutants in closely linked genes: cdt2 for mts1, cdc8 for mts6, cdc13 for mts7, sep1 for mts8, txl1 for mts9 and sep1 for mts10. In each case strong linkage was observed indicating that the temperature sensitive mutations were in the authentic genes (data not shown). In all cases deleting the mts genes resulted in lethality, revealing that the mutants were all conditional mutant alleles of essential genes. As nine out of ten genes encoded subunits of the 26S proteasome (Table 2) this raised the important question of why the screen was so biased for isolation of mutants in different subunits of the 26S proteasome.
The mts Mutants are Multi-drug Resistant
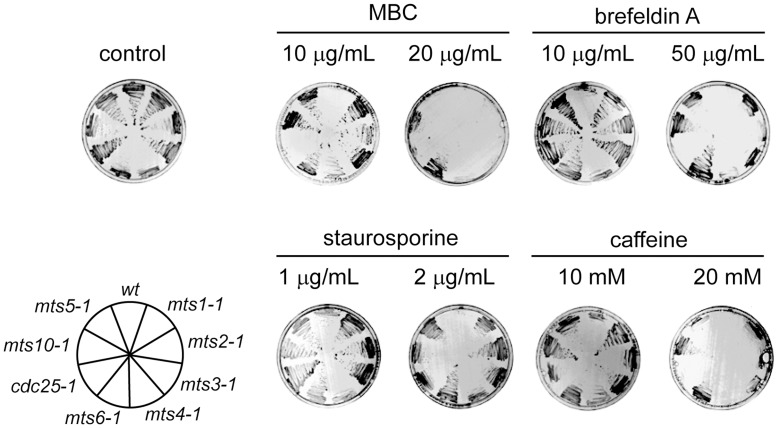
The mts mutants were isolated on account of their resistance to the mitotic poison MBC. We were interested to ask if the mutants were resistant to other drugs. When the mts strains were streaked on complete media containing the drugs brefeldin A, staurosporine or caffeine at the permissive temperature of 25°C, the mts mutants were found to be more resistant than wild type cells (Fig. 1). To show that this was not an effect of the temperature sensitivity of the mutants, a temperature sensitive mutant in cdc25, a gene encoding a cell cycle regulated phosphatase, was included as a control. The mts mutants all showed the same spectrum of resistance although the level of resistance varied with the different mutants (Fig. 1). The cdc25 mutant was moderately resistant to staurosporine, but did not display the multi-drug resistant phenotype of the mts mutants (Fig. 1). These data confirm that the mts mutants are multi-drug resistant.
Figure 1. The mts mutants are multi-drug resistant.
The indicated yeast strains (lower left panel) were streaked onto solid medium containing MBC, brefeldin A, staurosporine or caffeine at the shown concentrations and incubated for 48 hours at room temperature. On the control medium lacking drugs (upper left panel) all the strains grew. When the indicated drugs were added to the media the growth of wild type cells was compromised, while the mts mutants displayed resistance.
The Pap1 Protein is Stabilized in the mts Mutants at the Permissive Temperature
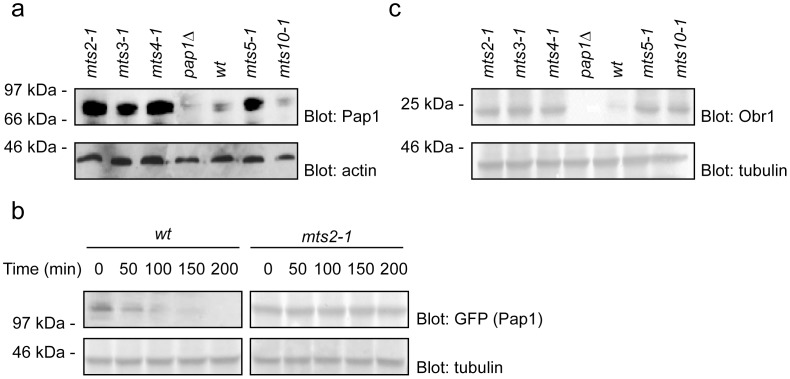
The multi-drug resistant phenotype has been observed before for cells in which the Pap1 transcription factor is overexpressed [14]–[15]. Indeed temperature and cold sensitive alleles of the crm1 (mts10) gene display multi-drug resistance that depends on the presence of a wild type pap1+ gene [19]. This raised the possibility that the proteasome mutants were multi-drug resistant due to the Pap1 protein being a substrate of the proteasome. Hence, Pap1 could be stabilized in the proteasome mutants because proteolysis is defective in these strains. To test this hypothesis the steady state levels of the Pap1 protein were determined in the mts2-1, mts3-1, mts4-1, mts5-1 and wild type cells by Western blot analysis using an antibody to the Pap1 protein. The Pap1 protein was significantly more abundant in extracts prepared from the mts mutants compared to those prepared from wild type cells (Fig. 2a). In contrast, the level of Pap1 protein in the crm1/mts10 mutant strain was unchanged compared to wild type (Fig. 2a). This is consistent with a different mechanism for multi-drug resistance in the crm1-1 (mts10-1) strain compared to the proteasome mutants. Thus, in the crm1-1 strain nuclear export of the Pap1 protein is impaired leading to drug resistance, as previously described [13].
Figure 2. Stabilization of Pap1 in the mts mutants leads increased obr1+ expression.
(a) To compare the steady state levels of Pap1 cell extracts of the indicated strains were prepared and analyzed by SDS-PAGE and Western blotting using antibodies to Pap1. Actin served as a loading control. Compared to wild type cells, the Pap1 levels were increased in the proteasome mutants, but not in the mts10-1 (crm1) mutant. A pap1Δ mutant was included as a control. (b) The degradation kinetics of GFP-tagged Pap1 was followed by blotting of wild type (wt) and mts2-1 cultures treated with cycloheximide (CHX). α-tubulin served as a loading control. In wild type cells Pap1 was rapidly degraded with a half-life of about 50 minutes. In the mts2-1 background Pap1 was stabilized (c) To compare the steady state levels of the Pap1 target Obr1 cell extracts of the indicated strains were prepared and analyzed by SDS-PAGE and Western blotting using antibodies to Obr1. Tubulin served as a loading control. Compared to wild type cells, the Obr1 levels were increased in the proteasome mutants and, as expected, in the mts10-1 (crm1) mutant. No Obr1 was detected in the pap1Δ mutant.
To further demonstrate that the Pap1 protein was stabilized in the proteasome mts mutants, the degradation of Pap1 was followed in cultures treated with the translation inhibitor cycloheximide. The Pap1 protein was fused to GFP and expressed in wild type cells and the mts2-1 proteasome mutant strain at the restrictive temperature of 36°C in the presence of cycloheximide. Extracts were prepared at intervals and analyzed by SDS-PAGE and blotting using an antibody to GFP. As expected the Pap1-GFP fusion protein was stabilized in the proteasome mutant compared to wild type cells (Fig. 2b).
The mts Mutants Contain Elevated Levels of the Pap1 Target Obr1
Although the Pap1 protein is stabilized in the mts mutants, it is important to demonstrate that this increased amount of Pap1 protein augments the gene expression of Pap1 target genes. The obr1+ gene contains a consensus Pap1 DNA binding motif in its promoter and has been shown to be transcribed specifically by Pap1 [19]. To show that the increased amount of Pap1 protein resulted in an increase in obr1 + expression, extracts were prepared from the mts strains and analysed by Western blotting with antibodies to Obr1. Indeed, the level of the Obr1 was significantly upregulated in the mts mutants as compared to wild type cells (Fig. 2c). This demonstrates that the increased amount of Pap1 protein observed in the mts mutants results in an increase in Pap1-mediated gene expression.
The Pap1 Protein is the Cause of the Multi-drug Resistant Phenotype of the mts Mutants
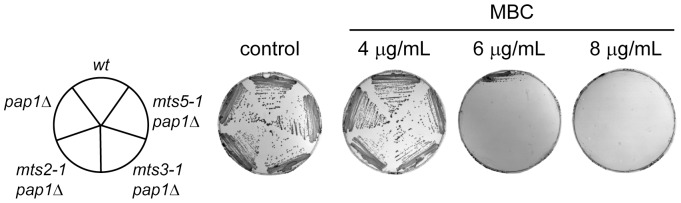
If the observed stabilization of the Pap1 protein is the true cause of the multi-drug resistance observed in the mts mutants then this phenotype should be lost in strains which have been deleted for the pap1 + gene. Cells lacking Pap1 are viable although they are stress sensitive [21]. Therefore the pap1 null strain was crossed to each of the mts2-1, mts3-1 and mts5-1 mutants to construct pap1Δmts double mutants. The MBC drug resistance phenotype was then investigated for each of the double mutant strains. The pap1Δmts double mutants were all as sensitive to MBC as the pap1Δ strain, and were now more sensitive to MBC than wild type cells (Fig. 3). Similar results were obtained with brefeldin A, staurosporine and caffeine (data not shown). This strongly suggests that the observed multi-drug resistance phenotype observed in the mts mutants is the result of the stabilization of Pap1, and that the Pap1 protein is the primary target whose misregulation results in a multi-drug resistant phenotype in the proteasome mutants.
Figure 3. The multi-drug resistance of the mts mutants depends on Pap1.
The indicated yeast strains (left panel) were streaked onto solid medium containing MBC at the shown concentrations and incubated for 48 hours at room temperature. On the control medium lacking drugs all the strains grew. In the presence of 6 µg/mL MBC some growth of the wild type was still apparent, while no growth of the other strains was observed.
The Pap1 Protein is Polyubiquitylated
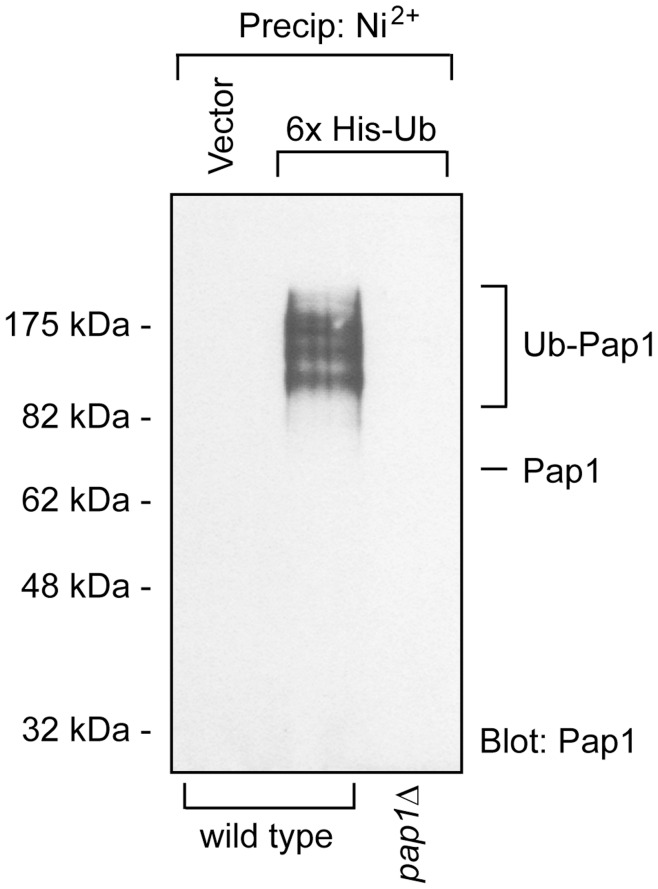
If the Pap1 transcription factor is a target of the 26S proteasome one would expect that it should be polyubiquitylated. To test this, 6His-tagged ubiquitin was expressed in S. pombe cells and purified on a nickel resin. The tagged ubiquitin conjugates were resolved by SDS-PAGE and analysed by Western blotting using antibodies to Pap1. Indeed we found that Pap1 was heavily ubiquitylated (Fig. 4).
Figure 4. Pap1 is ubiquitylated.
To determine the ubiquitylation status of Pap1, wild type cells and as a control a pap1Δ strain were transformed to express 6His-tagged ubiquitin. The 6His-tagged ubiquitin was then precipitated using Ni2+ agarose under denaturing conditions in 8 M urea. The precipitates were then washed three times in a denaturing buffer and analyzed by Western blotting using antibodies to Pap1. Prior to precipitation, the protein concentrations were determined and normalized. In total 5 mg cell protein was used for each precipitation. Ubiquitylated species of Pap1 were detected in wild type cells expressing the 6His-tagged ubiquitin, but not in the pap1Δ strain or in the vector control.
Rhp6/Ubc2 Functions as the E2 Ubiquitin-conjugating Enzyme for Pap1
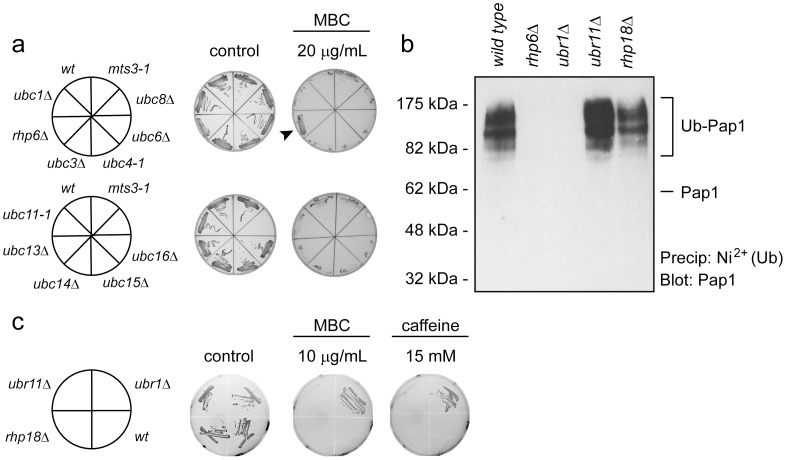
Ubiquitin is added to substrate proteins by the action of an E1, E2 and E3 enzyme cascade. We were interested in investigating which E2s and E3s were involved in the addition of the ubiquitin chain to Pap1. We postulated that the relevant E2 involved in targeting Pap1 protein for ubiquitylation and degradation should show resistance to MBC in an analogous manner to the mts proteasome mutants. The fission yeast genome encodes several different ubiquitin-specific E2 proteins. We obtained 8 null or conditional mutants that were generated previously (ubc1, ubc2, ubc3, ubc4, ubc6, ubc11, ubc13 and ubc15), and constructed three additional null mutants (ubc8, ubc14 and ubc16) for this study. The ORFs of ubc8+ and ubc14+ were replaced with the ura4+ gene, while the ubc16+ ORF was replaced with the hygromycin resistance gene. When these strains were streaked on plates containing MBC, only the mutant in ubc2, also known as rhp6, displayed MBC resistance (Fig. 5a), suggesting that Rhp6 is involved in Pap1 ubiquitylation.
Figure 5. Pap1 is ubiquitylated by Rhp6/Ubc2 and Ubr1.
(a) To identify which E2 ubiquitin conjugating enzyme is responsible for ubiquitylating Pap1, the indicated E2 null mutants were streaked onto solid medium containing MBC and grown for 48 hours at room temperature. Since the rhp6Δ cells grow in the presence of MBC (arrowhead) it was a candidate E2 in Pap1 ubiquitylation. (b) To identify the E2 and E3 enzymes responsible for ubiquitylating Pap1, the indicated strains were transformed to express 6His-tagged ubiquitin. The 6His-tagged ubiquitin was then precipitated using Ni2+ agarose under denaturing conditions and analyzed by Western blotting using antibodies to Pap1. Ubiquitylated species of Pap1 were detected in wild type, ubr11Δ and rhp18Δ cells, but not in cells lacking the E2 ubiquitin conjugating enzyme Rhp6 and in cells lacking the E3 ubiquitin-protein ligase Ubr1. (c) The indicated yeast strains (left panel) were streaked onto solid medium containing MBC or caffeine at the shown concentrations and incubated for 48 hours at room temperature. On the control medium lacking drugs all the strains grew. In the presence of drugs the growth of wild type cells was compromised, while the ubr1Δ mutant displayed resistance.
To verify that Rhp6 is involved in Pap1 ubiquitylation, the expression plasmid for 6His-tagged ubiquitin was introduced in the rhp6 null strain. Indeed Pap1 was no longer ubiquitylated in the rhp6Δ mutant (Fig. 5b). These data are consistent with the observation that the rhp6Δ mutant is the only E2 mutant of those tested to show MBC resistance and leads to the conclusion that Rhp6 functions as the major, or perhaps sole, E2 conjugating enzyme for ubiquitylation of Pap1.
The Ubr1 E3 Ubiquitin-protein Ligase Targets Pap1 for Degradation
Since the budding yeast Rhp6 ortholog, Rad6, has previously been shown to interact with the E3 ubiquitin ligases called Ubr1 [22], Ubr2 [23] and Rad18 [24], we reasoned that the fission yeast orthologs Ubr1, Ubr11 and Rhp18, respectively, are likely candidates as E3s for ubiquitylation of Pap1. We therefore transformed ubr1Δ, ubr11Δ and rhp18Δ mutants with the expression plasmid for 6His-tagged ubiquitin and purified ubiquitin-conjugates as above. The results showed that Pap1 remained ubiquitylated in the rhp18 and ubr11 null strains, whereas no ubiquitylated Pap1 was detected in the ubr1Δ strain (Fig. 5b). This suggests that Ubr1, but not Ubr11 and Rhp18, functions as E3 ubiquitin-protein ligase in targeting Pap1 for degradation. Accordingly, we found that ubr1Δ cells are resistant to MBC and caffeine, whereas the rhp18 and ubr11 null strains are not (Fig. 5c). Collectively, this leads to the conclusion that Pap1 ubiquitylation and subsequent degradation is catalyzed by the Rhp6 E2 ubiquitin conjugating enzyme and the Ubr1 E3 ubiquitin-protein ligase. Moreover, the complete lack of ubiquitylated Pap1 in the ubr1Δ strain implies that the Ubr1 protein plays a major role in targeting the Pap1 protein for degradation.
Discussion
Here we have described a genetic screen for mutants in fission yeast that showed resistance to the mitotic poison MBC that were also temperature sensitive for growth. Mutants in ten different essential genes were isolated. Nine of the genes encode different subunits of the 26S proteasome, while one encoded the nuclear export factor Crm1. The proteasome mutants were obtained in all the different subcomplexes which make up the 26S proteasome. Four mutants, mts6, mts7, mts8 and mts9 were in subunits of the 20S catalytic complex. Two mutants, mts2 and mts4, were in subunits of the 19S regulatory base subcomplex. The mts2 strain had mutations in one of the ATPase subunits (Rpt2) while mts4 had mutations in the Rpn1 nonATPase subunit. The remaining three mts genes mts1, mts3 and mts5 encoded different subunits of the 19S regulatory lid sub-complex. All the mts mutants were conditional alleles of essential genes. Curiously, budding yeast null mutants in RPN9 are viable [25]. We found that in S. pombe the RPN9 ortholog mts1 + is an essential gene. The isolation of mutants in all the different sub-complexes of the 26S proteasome would seem to indicate that a general defect in 26S proteasome function was responsible for the MBC drug resistance seen at the permissive temperature. Previous studies in budding yeast have also noted that mutants in proteasome assembly factors are resistant to alkylating agents [26], presumably this occurs via a similar mechanism as the one described here. In this paper we describe the mechanism which is responsible for the observed drug resistance. The Pap1 stress-activated transcription factor appears, by genetic and biochemical criteria, to be the sole, or at least primary, target for the different mts proteasome mutants that results in the drug resistant phenotype. In the mts proteasome mutants Pap1 protein is stabilized, resulting in increased Pap1-dependent activity and the observed multi-drug resistant phenotype. Up regulation of the Pap1 transcription factor has been implicated in the multi-drug resistant phenotype in a number of different screens, probably due to the upregulation of ABC transporters such as Bfr1 [27] [15] [28] that mediate drug efflux [13] [28].
The Pap1 protein is known to be tightly regulated at many different levels. Thus, Pap1 is activated by oxidation, but is also regulated on the level of its subcellular localization. In addition, the Pap1 protein is rapidly turned over by the UPS [29]–[31]. Hence, under non stressed conditions Pap1 is and located in the cytosol, while stress conditions trigger its nuclear translocation. However, as we show here, this regulation requires that the Pap1 levels are kept balanced by the UPS.
We propose that the fission yeast Rhp6/Ubc2 functions as the major E2 ubiquitin conjugation enzyme for Pap1 degradation, while Ubr1, but not the related Ubr11 and Rhp18, functions as E3 ubiquitin-protein ligase in targeting Pap1 for degradation. These data are in perfect agreement with recent results showing that Ubr1 regulates the fission yeast oxidative stress response by targeting Pap1 for degradation [31]. Intriguingly, the budding yeast orthologues of Rhp6 and Ubr1, called Rad6 and Ubr2, respectively, were found to regulate degradation of Rpn4, a transcription factor driving expression of most proteasome components [32]. Since Pap1 and Rpn4 are not related, this is most likely coincidental. Accordingly, in Saccharomyces cerevisiae degradation of the Pap1 orthologue, Yap1, was recently shown to depend on another ligase called Not4 [33].
Since budding yeast Yap1 transactivates expression of Rpn4 [34], it is possible that in the fission yeast mts mutants the impaired degradation of Pap1 leads to an increase in proteasome expression. If this is indeed the case, such a mechanism would only blunt the response we observe here. Moreover the existence of such a regulatory mechanism is not obvious, since the S. pombe genome does not encode any obvious orthologue of budding yeast Rpn4, the closest relative being Rsv2 that induces stress-related genes during spore formation [35]. Therefore it is currently unclear how proteasome gene expression is regulated in S. pombe. However, previous studies have shown that proteasome expression does not depend on Pap1 [36]. Accordingly, we did not observe any changes in proteasome levels in a pap1Δ strain (Fig. S1).
Originally Ubr1 was shown to be required for degradation of proteins carrying destabilizing residues in their N-terminus via the so-called N-end rule [37]–[38]. However, Ubr1 also recognizes misfolded proteins [39]–[40] and substrates carrying internal degradation signals [41]. More recently, Ubr1 was also linked to the Johanson-Blizzard syndrome [42], an autosomal recessive disorder that involves pancreatic dysfunction and mental retardation, that has been suggested to be connected with impaired transcription factor degradation [41].
Since all the components identified here (Pap1, Rhp6, Ubr1 and the 26S proteasome) are known to have orthologs in mammalian cells, it is likely that multi-drug resistance could occur by a similar mechanism in mammalian cells. Interestingly, it has been reported that the mammalian Pap1 ortholog, the c-Fos transcription factor, is targeted for degradation by the Ubr1 ortholog in human cells [41]. Thus, interference with this pathway, either genetically or by inhibitors, like bortezomib (Velcade), used in cancer therapy [43], may increase drug and/or stress tolerance also in mammalian cells.
Supporting Information
Proteasome levels are unchanged in a pap1Δ mutant. Whole cell extracts from wild type (wt) and pap1Δ strains were analyzed by SDS-PAGE and Western blotting using antibodies to the 26S proteasome subunit Rpn1/Mts4 and 20S α subunits. Antibodies to tubulin were used to ensure an even loading. No significant differences between the strains in proteasome levels were observed.
(TIF)
Acknowledgments
We thank Dr Peter A. Fantes, Dr Olaf Nielsen, Dr David Beach, Dr Masayuki Yamamoto, Dr Genevieve Thon, Dr Wei Xiao and Dr Fumiaki Yamao for sharing strains and reagents.
Funding Statement
This work has been supported by grants to RHP and CG from the Medical Research Council, the Lundbeck Foundation and the Danish Natural Science Research Council. TT has been supported by Cancer Research UK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Glickman MH, Ciechanover A (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82: 373–428. 10.1152/physrev.00027.2001 [doi]. [DOI] [PubMed]
- 2.Kriegenburg F, Poulsen EG, Koch A, Kruger E, Hartmann-Petersen R (2011) Redox control of the ubiquitin-proteasome system: from molecular mechanisms to functional significance. Antioxid Redox Signal 15: 2265–2299. 10.1089/ars.2010.3590 [doi]. [DOI] [PubMed]
- 3.Pickart CM (2001) Mechanisms underlying ubiquitination. Annu Rev Biochem 70: 503–533. 70/1/503 [pii];10.1146/annurev.biochem.70.1.503 [doi]. [DOI] [PubMed]
- 4.Finley D (2009) Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem 78: 477–513. 10.1146/annurev.biochem.78.081507.101607 [doi]. [DOI] [PMC free article] [PubMed]
- 5.Komander D (2009) The emerging complexity of protein ubiquitination. Biochem Soc Trans 37: 937–953. BST0370937 [pii];10.1042/BST0370937 [doi]. [DOI] [PubMed]
- 6.Groll M, Ditzel L, Lowe J, Stock D, Bochtler M, et al.. (1997) Structure of 20S proteasome from yeast at 2.4 A resolution. Nature 386: 463–471. 10.1038/386463a0 [doi]. [DOI] [PubMed]
- 7.Braun BC, Glickman M, Kraft R, Dahlmann B, Kloetzel PM, et al.. (1999) The base of the proteasome regulatory particle exhibits chaperone-like activity. Nat Cell Biol 1: 221–226. 10.1038/12043 [doi]. [DOI] [PubMed]
- 8. Strickland E, Hakala K, Thomas PJ, DeMartino GN (2000) Recognition of misfolding proteins by PA700, the regulatory subcomplex of the 26 S proteasome. J Biol Chem 275: 5565–5572. [DOI] [PubMed] [Google Scholar]
- 9.Gordon C, McGurk G, Dillon P, Rosen C, Hastie ND (1993) Defective mitosis due to a mutation in the gene for a fission yeast 26S protease subunit. Nature 366: 355–357. 10.1038/366355a0 [doi]. [DOI] [PubMed]
- 10. Gordon C, McGurk G, Wallace M, Hastie ND (1996) A conditional lethal mutant in the fission yeast 26 S protease subunit mts3+ is defective in metaphase to anaphase transition. J Biol Chem 271: 5704–5711. [DOI] [PubMed] [Google Scholar]
- 11. Wilkinson CR, Wallace M, Seeger M, Dubiel W, Gordon C (1997) Mts4, a non-ATPase subunit of the 26 S protease in fission yeast is essential for mitosis and interacts directly with the ATPase subunit Mts2. J Biol Chem 272: 25768–25777. [DOI] [PubMed] [Google Scholar]
- 12. Penney M, Wilkinson C, Wallace M, Javerzat JP, Ferrell K, et al. (1998) The Pad1+ gene encodes a subunit of the 26 S proteasome in fission yeast. J Biol Chem 273: 23938–23945. [DOI] [PubMed] [Google Scholar]
- 13. Toone WM, Kuge S, Samuels M, Morgan BA, Toda T, et al. (1998) Regulation of the fission yeast transcription factor Pap1 by oxidative stress: requirement for the nuclear export factor Crm1 (Exportin) and the stress-activated MAP kinase Sty1/Spc1. Genes Dev 12: 1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toda T, Shimanuki M, Yanagida M (1991) Fission yeast genes that confer resistance to staurosporine encode an AP-1-like transcription factor and a protein kinase related to the mammalian ERK1/MAP2 and budding yeast FUS3 and KSS1 kinases. Genes Dev 5: 60–73. [DOI] [PubMed] [Google Scholar]
- 15. Turi TG, Webster P, Rose JK (1994) Brefeldin A sensitivity and resistance in Schizosaccharomyces pombe. Isolation of multiple genes conferring resistance. J Biol Chem 269: 24229–24236. [PubMed] [Google Scholar]
- 16.Calvo IA, Gabrielli N, Iglesias-Baena I, Garcia-Santamarina S, Hoe KL, et al.. (2009) Genome-wide screen of genes required for caffeine tolerance in fission yeast. PLoS One 4: e6619. 10.1371/journal.pone.0006619 [doi]. [DOI] [PMC free article] [PubMed]
- 17. Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194: 795–823. [DOI] [PubMed] [Google Scholar]
- 18.Bahler J, Wu JQ, Longtine MS, Shah NG, McKenzie A, III, et al.. (1998) AID-YEA292>3.0.CO;2-Y [pii];10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y [doi].
- 19. Toda T, Shimanuki M, Saka Y, Yamano H, Adachi Y, et al. (1992) Fission yeast pap1-dependent transcription is negatively regulated by an essential nuclear protein, crm1. Mol Cell Biol 12: 5474–5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartmann-Petersen R, Wallace M, Hofmann K, Koch G, Johnsen AH, et al.. (2004) The Ubx2 and Ubx3 cofactors direct Cdc48 activity to proteolytic and nonproteolytic ubiquitin-dependent processes. Curr Biol 14: 824–828. 10.1016/j.cub.2004.04.029 [doi];S0960982204002994 [pii]. [DOI] [PubMed]
- 21.Han TX, Xu XY, Zhang MJ, Peng X, Du LL (2010) Global fitness profiling of fission yeast deletion strains by barcode sequencing. Genome Biol 11: R60. gb-2010-11-6-r60 [pii];10.1186/gb-2010-11-6-r60 [doi]. [DOI] [PMC free article] [PubMed]
- 22. Dohmen RJ, Madura K, Bartel B, Varshavsky A (1991) The N-end rule is mediated by the UBC2(RAD6) ubiquitin-conjugating enzyme. Proc Natl Acad Sci U S A 88: 7351–7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ju D, Wang X, Xu H, Xie Y (2008) Genome-wide analysis identifies MYND-domain protein Mub1 as an essential factor for Rpn4 ubiquitylation. Mol Cell Biol 28: 1404–1412. MCB.01787-07 [pii];10.1128/MCB.01787-07 [doi]. [DOI] [PMC free article] [PubMed]
- 24. Bailly V, Lauder S, Prakash S, Prakash L (1997) Yeast DNA repair proteins Rad6 and Rad18 form a heterodimer that has ubiquitin conjugating, DNA binding, and ATP hydrolytic activities. J Biol Chem 272: 23360–23365. [DOI] [PubMed] [Google Scholar]
- 25. Glickman MH, Rubin DM, Fried VA, Finley D (1998) The regulatory particle of the Saccharomyces cerevisiae proteasome. Mol Cell Biol 18: 3149–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Tallec B, Barrault MB, Guerois R, Carre T, Peyroche A (2009) Hsm3/S5b participates in the assembly pathway of the 19S regulatory particle of the proteasome. Mol Cell 33: 389–399. S1097-2765(09)00037-9 [pii];10.1016/j.molcel.2009.01.010 [doi]. [DOI] [PubMed]
- 27. Arioka M, Kouhashi M, Yoda K, Takatsuki A, Yamasaki M, et al. (1998) Multidrug resistance phenotype conferred by overexpressing bfr2+/pad1+/sks1+ or pap1+ genes and mediated by bfr1+ gene product, a structural and functional homologue of P-glycoprotein in Schizosaccharomyces pombe. Biosci Biotechnol Biochem 62: 390–392. [DOI] [PubMed] [Google Scholar]
- 28. Nagao K, Taguchi Y, Arioka M, Kadokura H, Takatsuki A, et al. (1995) bfr1+, a novel gene of Schizosaccharomyces pombe which confers brefeldin A resistance, is structurally related to the ATP-binding cassette superfamily. J Bacteriol 177: 1536–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shimanuki M, Saka Y, Yanagida M, Toda T (1995) A novel essential fission yeast gene pad1+ positively regulates pap1(+)-dependent transcription and is implicated in the maintenance of chromosome structure. J Cell Sci 108 (Pt 2): 569–579. [DOI] [PubMed] [Google Scholar]
- 30.Herrero E, Ros J, Belli G, Cabiscol E (2008) Redox control and oxidative stress in yeast cells. Biochim Biophys Acta 1780: 1217–1235. S0304-4165(07)00294-2 [pii];10.1016/j.bbagen.2007.12.004 [doi]. [DOI] [PubMed]
- 31.Kitamura K, Taki M, Tanaka N, Yamashita I (2011) Fission yeast Ubr1 ubiquitin ligase influences the oxidative stress response via degradation of active Pap1 bZIP transcription factor in the nucleus. Mol Microbiol 80: 739–755. 10.1111/j.1365-2958.2011.07605.x [doi]. [DOI] [PubMed]
- 32.Ju D, Wang L, Mao X, Xie Y (2004) Homeostatic regulation of the proteasome via an Rpn4-dependent feedback circuit. Biochem Biophys Res Commun 321: 51–57. 10.1016/j.bbrc.2004.06.105 [doi];S0006-291X(04)01385-3 [pii]. [DOI] [PubMed]
- 33.Gulshan K, Thommandru B, Moye-Rowley WS (2012) Proteolytic degradation of the Yap1 transcription factor is regulated by subcellular localization and the E3 ubiquitin ligase Not4. J Biol Chem 287: 26796–26805. M112.384719 [pii];10.1074/jbc.M112.384719 [doi]. [DOI] [PMC free article] [PubMed]
- 34.Owsianik G, Balzi lL, Ghislain M (2002) Control of 26S proteasome expression by transcription factors regulating multidrug resistance in Saccharomyces cerevisiae. Mol Microbiol 43: 1295–1308. 2823 [pii]. [DOI] [PubMed]
- 35.Mata J, Wilbrey A, Bahler J (2007) Transcriptional regulatory network for sexual differentiation in fission yeast. Genome Biol 8: R217. gb-2007-8-10-r217 [pii];10.1186/gb-2007-8-10-r217 [doi]. [DOI] [PMC free article] [PubMed]
- 36.Chen D, Wilkinson CR, Watt S, Penkett CJ, Toone WM, et al.. (2008) Multiple pathways differentially regulate global oxidative stress responses in fission yeast. Mol Biol Cell 19: 308–317. E07-08-0735 [pii];10.1091/mbc.E07-08-0735 [doi]. [DOI] [PMC free article] [PubMed]
- 37. Varshavsky A (1997) The N-end rule pathway of protein degradation. Genes Cells 2: 13–28. [DOI] [PubMed] [Google Scholar]
- 38.Tasaki T, Sriram SM, Park KS, Kwon YT (2012) The N-end rule pathway. Annu Rev Biochem 81: 261–289. 10.1146/annurev-biochem-051710-093308 [doi]. [DOI] [PMC free article] [PubMed]
- 39.Theodoraki MA, Nillegoda NB, Saini J, Caplan AJ (2012) A network of ubiquitin ligases is important for the dynamics of misfolded protein aggregates in yeast. J Biol Chem. M112.341164 [pii];10.1074/jbc.M112.341164 [doi]. [DOI] [PMC free article] [PubMed]
- 40.Khosrow-Khavar F, Fang NN, Ng AH, Winget JM, Comyn SA, et al.. (2012) The yeast ubr1 ubiquitin ligase participates in a prominent pathway that targets cytosolic thermosensitive mutants for degradation. G3 (Bethesda ) 2: 619–628. 10.1534/g3.111.001933 [doi];GGG_001933 [pii]. [DOI] [PMC free article] [PubMed]
- 41.Sasaki T, Kojima H, Kishimoto R, Ikeda A, Kunimoto H, et al.. (2006) Spatiotemporal regulation of c-Fos by ERK5 and the E3 ubiquitin ligase UBR1, and its biological role. Mol Cell 24: 63–75. S1097-2765(06)00564-8 [pii];10.1016/j.molcel.2006.08.005 [doi]. [DOI] [PubMed]
- 42.Zenker M, Mayerle J, Lerch MM, Tagariello A, Zerres K, et al.. (2005) Deficiency of UBR1, a ubiquitin ligase of the N-end rule pathway, causes pancreatic dysfunction, malformations and mental retardation (Johanson-Blizzard syndrome). Nat Genet 37: 1345–1350. ng1681 [pii];10.1038/ng1681 [doi]. [DOI] [PubMed]
- 43.Kisselev AF, van der Linden WA, Overkleeft HS (2012) Proteasome inhibitors: an expanding army attacking a unique target. Chem Biol 19: 99–115. S1074-5521(12)00012-9 [pii];10.1016/j.chembiol.2012.01.003 [doi]. [DOI] [PMC free article] [PubMed]
- 44.Fantes P (1979) Epistatic gene interactions in the control of division in fission yeast. Nature 279: 428–430. 279428a0 [pii];10.1038/279428a0 [doi]. [DOI] [PubMed]
- 45.Kitamura K, Katayama S, Dhut S, Sato M, Watanabe Y, et al.. (2001) Phosphorylation of Mei2 and Ste11 by Pat1 kinase inhibits sexual differentiation via ubiquitin proteolysis and 14-3-3 protein in fission yeast. Dev Cell 1: 389–399. S1534-5807(01)00037-5 [pii]. [DOI] [PubMed]
- 46. Seino H, Kishi T, Nishitani H, Yamao F (2003) Two ubiquitin-conjugating enzymes, UbcP1/Ubc4 and UbcP4/Ubc11, have distinct functions for ubiquitination of mitotic cyclin. Mol Cell Biol 23: 3497–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown M, Zhu Y, Hemmingsen SM, Xiao W (2002) Structural and functional conservation of error-free DNA postreplication repair in Schizosaccharomyces pombe. DNA Repair (Amst) 1: 869–880. S1568786402001118 [pii]. [DOI] [PubMed]
- 48. Nielsen IS, Nielsen O, Murray JM, Thon G (2002) The fission yeast ubiquitin-conjugating enzymes UbcP3, Ubc15, and Rhp6 affect transcriptional silencing of the mating-type region. Eukaryot Cell 1: 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurahashi H, Imai Y, Yamamoto M (2002) Tropomyosin is required for the cell fusion process during conjugation in fission yeast. Genes Cells 7: 375–384. 526 [pii]. [DOI] [PubMed]
- 50. Booher R, Beach D (1988) Involvement of cdc13+ in mitotic control in Schizosaccharomyces pombe: possible interaction of the gene product with microtubules. EMBO J 7: 2321–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buck V, Ng SS, Ruiz-Garcia AB, Papadopoulou K, Bhatti S, et al.. (2004) Fkh2p and Sep1p regulate mitotic gene transcription in fission yeast. J Cell Sci 117: 5623–5632. 117/23/5623 [pii];10.1242/jcs.01473 [doi]. [DOI] [PubMed]
- 52.Liu C, Poitelea M, Watson A, Yoshida SH, Shimoda C, et al.. (2005) Transactivation of Schizosaccharomyces pombe cdt2+ stimulates a Pcu4-Ddb1-CSN ubiquitin ligase. EMBO J 24: 3940–3951. 7600854 [pii];10.1038/sj.emboj.7600854 [doi]. [DOI] [PMC free article] [PubMed]
- 53.Andersen KM, Jensen C, Kriegenburg F, Lauridsen AM, Gordon C, et al.. (2011) Txl1 and Txc1 are co-factors of the 26S proteasome in fission yeast. Antioxid Redox Signal 14: 1601–1608. 10.1089/ars.2010.3329 [doi]. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Proteasome levels are unchanged in a pap1Δ mutant. Whole cell extracts from wild type (wt) and pap1Δ strains were analyzed by SDS-PAGE and Western blotting using antibodies to the 26S proteasome subunit Rpn1/Mts4 and 20S α subunits. Antibodies to tubulin were used to ensure an even loading. No significant differences between the strains in proteasome levels were observed.
(TIF)