Abstract
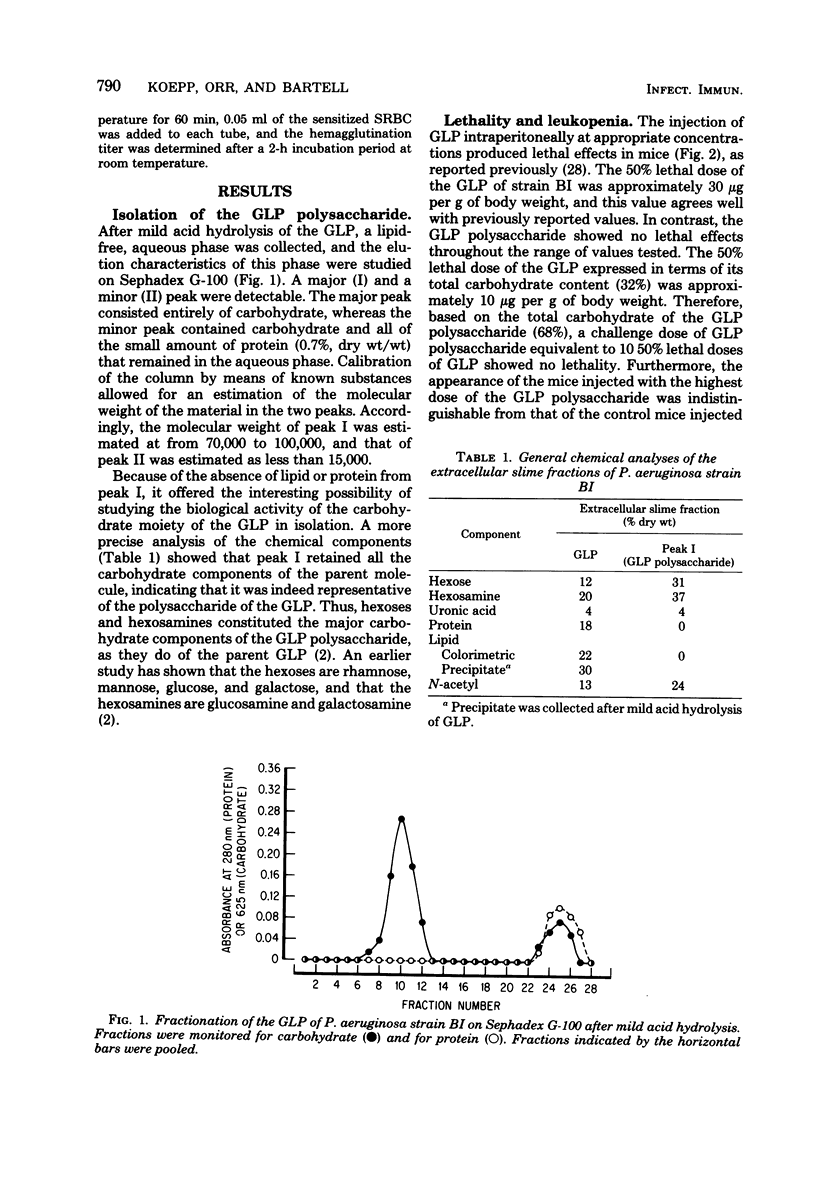
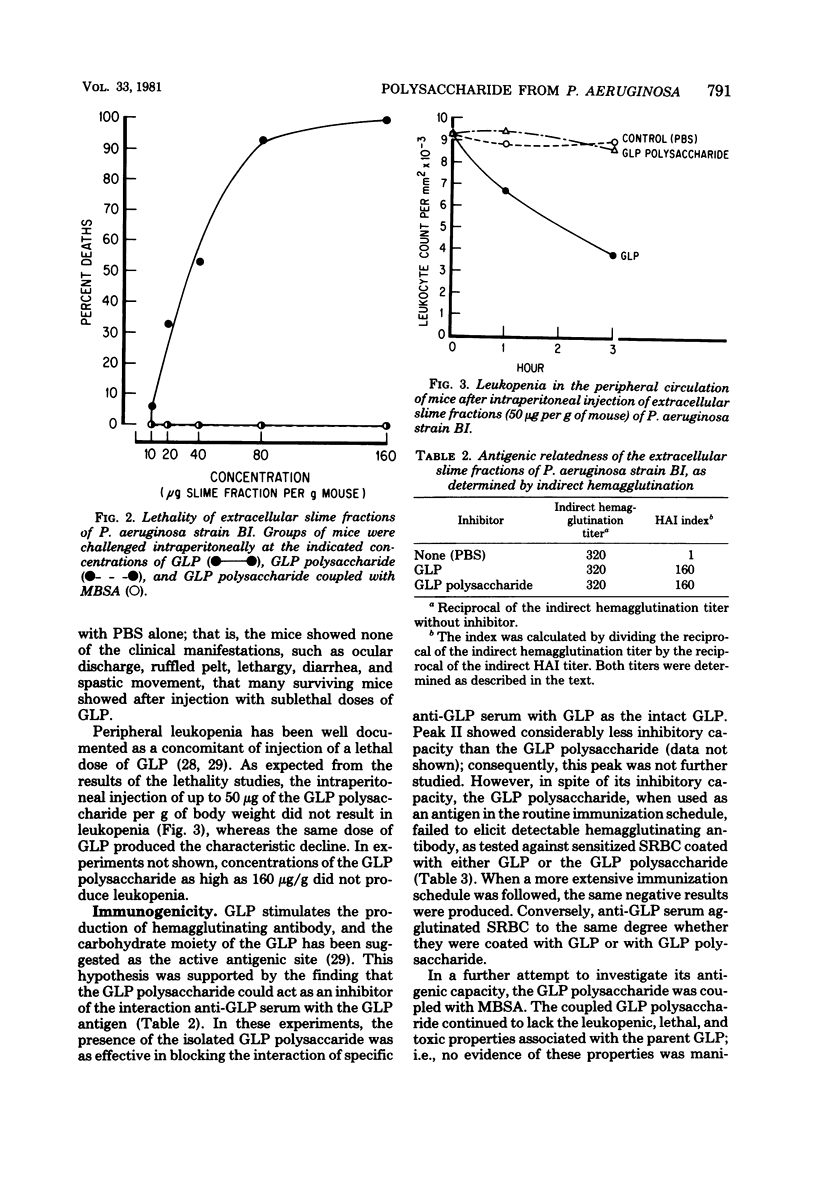
The polysaccharide moiety was isolated by mild acid hydrolysis from the slime glycolipoprotein of Pseudomonas aeruginosa strain BI. After gel filtration, the polysaccharide obtained from the Carbohydrate peak fractions was found to be lipid- and protein-free. Analyses indicated that the polysaccharide contained the carbohydrate components of the parent glycolipoprotein. Molecular size of the polysaccharide was estimated by gel filtration as 70,000 to 100,000. The polysaccharide showed no indications of toxicity in mice at doses far in excess of the lethal dose for the parent glycolipoprotein, nor did the mice develop the leukopenia that characteristically follows intraperitoneal injection of glycolipoprotein. The polysaccharide acted as an inhibitor of indirect hemagglutination of glycolipoprotein-coated erythrocytes in the presence of anti-glycolipoprotein serum; however, it was not antigenic itself in rabbits. Coupled with methylated bovine serum albumin, the polysaccharide continued to lack the leukopenic and toxic properties of the parent glycolipoprotein, but the coupled polysaccharide was capable of stimulating indirect hemagglutinating antibody against both the polysaccharide and the glycolipoprotein coating erythrocytes. Moreover, the antibody to the coupled polysaccharide protected mice against challenge with lethal doses of viable P. aeruginosa with the same effectiveness as anti-glycolipoprotein serum.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E., Lam G. K. Polysaccharide depolymerase associated with bacteriophage infection. J Bacteriol. 1966 Jul;92(1):56–62. doi: 10.1128/jb.92.1.56-62.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxerbaum B., Kagumba A., Matthews L. W. Selective inhibition of phagocytic activity of rabbit alveolar macrophages by cystic fibrosis serum. Am Rev Respir Dis. 1973 Oct;108(4):777–783. doi: 10.1164/arrd.1973.108.4.777. [DOI] [PubMed] [Google Scholar]
- Castillo F. J., Bartell P. F. Studies on the bacteriophage 2 receptors of Pseudomonas aeruginosa. J Virol. 1974 Oct;14(4):904–909. doi: 10.1128/jvi.14.4.904-909.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coligan J. E., Fraser B. A., Kindt T. J. Immunochemistry of streptococcal group C polysaccharide and the nature of its crossreaction with the Forssman glycolipid. Prog Clin Biol Res. 1978;23:601–612. [PubMed] [Google Scholar]
- Dimitracopoulos G., Bartell P. F. Slime glycolipoproteins and the pathogenicity of various strains of Pseudomonas aeruginosa in experimental infection. Infect Immun. 1980 Nov;30(2):402–408. doi: 10.1128/iai.30.2.402-408.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitracopoulos G., Sensakovic J. W., Bartell P. F. Slime of Pseudomonas aeruginosa: in vivo production. Infect Immun. 1974 Jul;10(1):152–156. doi: 10.1128/iai.10.1.152-156.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORKNER C. E., Jr, FREI E., 3rd, EDGCOMB J. H., UTZ J. P. Pseudomonas septicemia; observations on twenty-three cases. Am J Med. 1958 Dec;25(6):877–889. doi: 10.1016/0002-9343(58)90060-3. [DOI] [PubMed] [Google Scholar]
- Flick M. R., Cluff L. E. Pseudomonas bacteremia. Review of 108 cases. Am J Med. 1976 Apr;60(4):501–508. doi: 10.1016/0002-9343(76)90716-6. [DOI] [PubMed] [Google Scholar]
- Holby N., Olling S. Pseudomonas aeruginosa infection in cystic fibrosis. Bactericidal effect of serum from normal individuals and patients with cystic fibrosis on P. aeruginosa strains from patients with cystic fibrosis or other diseases. Acta Pathol Microbiol Scand C. 1977 Apr;85(2):107–114. [PubMed] [Google Scholar]
- ITAYA K., UI M. COLORIMETRIC DETERMINATION OF FREE FATTY ACIDS IN BIOLOGICAL FLUIDS. J Lipid Res. 1965 Jan;6:16–20. [PubMed] [Google Scholar]
- Kozel T. R., Cazin J., Jr Induction of humoral antibody response by soluble polysaccharide of Cryptococcus neoformans. Mycopathol Mycol Appl. 1974 Oct 15;54(1):21–30. doi: 10.1007/BF02055969. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lynn M., Sensakovic J. W., Bartell P. F. In vivo distribution of Pseudomonas aeruginosa slime glycolipoprotein: association with leukocytes. Infect Immun. 1977 Jan;15(1):109–114. doi: 10.1128/iai.15.1.109-114.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. T. Studies in immuno-chemistry: The preparation and properties of a specific polysaccharide from B. dysenteriae (Shiga). Biochem J. 1936 May;30(5):909–925. doi: 10.1042/bj0300909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly R. J., Anderson P., Ingram D. L., Peter G., Smith D. H. Circulating polyribophosphate in Hemophilus influenzae, type b meningitis. Correlation with clinical course and antibody response. J Clin Invest. 1975 Oct;56(4):1012–1022. doi: 10.1172/JCI108148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Sadoff J. C. Protective immunity induced in mice by immunization with high-molecular-weight polysaccharide from Pseudomonas aeruginosa. Infect Immun. 1978 Dec;22(3):919–925. doi: 10.1128/iai.22.3.919-925.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Zolyomi S., Sadoff J. C. Isolation and characterization of a high-molecular-weight polysaccharide from the slime of Pseudomonas aeruginosa. Infect Immun. 1978 Dec;22(3):908–918. doi: 10.1128/iai.22.3.908-918.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pseudomonas aeruginosa infections: persisting problems and current research to find new therapies. Ann Intern Med. 1975 Jun;82(6):819–831. doi: 10.7326/0003-4819-82-6-819. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., Lee C. J., Rastogi S. C., Schiffman G., Henrichsen J. Comparative immunogenicity of group 6 pneumococcal type 6A(6) and type 6B(26) capsular polysaccharides. Infect Immun. 1979 Dec;26(3):1116–1122. doi: 10.1128/iai.26.3.1116-1122.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUEOKA N., CHENG T. Y. Fractionation of nucleic acids with the methylated albumin column. J Mol Biol. 1962 Mar;4:161–172. doi: 10.1016/s0022-2836(62)80048-5. [DOI] [PubMed] [Google Scholar]
- Sensakovic J. W., Bartell P. F. Biological activity of fragments derived from the extracellular slime glycolipoprotein of Pseudomonas aeruginosa. Infect Immun. 1975 Oct;12(4):808–812. doi: 10.1128/iai.12.4.808-812.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensakovic J. W., Bartell P. F. The slime of Pseudomonas aeruginosa: biological characterization and possible role in experimental infection. J Infect Dis. 1974 Feb;129(2):101–109. doi: 10.1093/infdis/129.2.101. [DOI] [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Meyer R. D., Armstrong D. Pseudomonas aeruginosa vaccine in cancer patients. Ann Intern Med. 1973 Oct;79(4):518–527. doi: 10.7326/0003-4819-79-4-518. [DOI] [PubMed] [Google Scholar]
- Young L. S. Role of antibody in infections due to Pseudomonas aeruginosa. J Infect Dis. 1974 Nov;130 (Suppl)(0):S111–S118. doi: 10.1093/infdis/130.supplement.s111. [DOI] [PubMed] [Google Scholar]


