Abstract
A natural male sterile mutant of Salvia miltiorrhiza (Labiatae, Sh-B) was found during field survey in 2002. Our objective was to analyze its genetic mechanism for producing F1 hybrid seeds and to develop a molecular marker linked to male sterile gene for selection of a hybrid parent line. The segregation ratios of male sterile plants to fertile plants in the progenies of both testcross and backcross were 1:1 in continuous experiments conducted in 2006–2009. The male sterile Sh-B was heterozygous (Msms). The male sterile plants could capture most pollen (2 granule/cm2·24 h) with row ratio (female : male 2 : 1) within 45-cm distance and harvest the largest amount of 6495 g hybrid seeds per hectare. We also developed DNA markers linked to the male sterile gene in a segregating population using bulked segregant analysis (BSA) and amplified fragment length polymorphism (AFLP) techniques. The segregating population was subjected to BSA-AFLP with 128 primer combinations. One out of fourteen AFLP markers (E11/M4208) was identified as tightly linked to the dominant male sterile gene with a recombination frequency of 6.85% and at a distance of 6.89 cM. This marker could be converted to PCR-based assay for large-scale selection of fertile plants in MAS (marker-assisted selection) at the seedling stage. Blastn analysis indicated that the male sterile gene sequence showed higher identity with nucleotides in Arabidopsis chromosome 1–5, and was more likely to encode S-adenosylmethionine-dependent methyltransferase, in which DNA methylation regulated the development of plant gametogenesis.
Introduction
In the past several decades, tremendous economic benefits has been obtained worldwide by using male sterility as a genetic tool to control pollination and to produce hybrid F1 seeds in many crops [1]. However, hybrid medicinal plant cultivars have few reports and not been produced extensively in large-scale production of medicinal materials. To date, only three medicinal plant species with male sterility have been reported, Salvia miltiorrhiza Bunge [2], [3], Platycodon grandiforus (Jacq.) A. DC. [4], [5], [6], and Lycium chinense Miller [7].
The roots and rhizomes of S. miltiorrhiza have been used as traditional Chinese herb drug (known as Danshen) for removing blood stasis, alleviating pain, promoting the circulation of blood, promoting menstruation, tranquilizing the brain, and treating cardiovascular and cerebrovascular diseases [8], [9]. Due to increasing demand in recent years, its cultivation area is rapidly expanding and out-of-order introduction among regions is generally occurring, which makes seeds contamination and degradation more severely and thus resulting in inconstant quality of medicinal materials. Moreover, there is no hybrid cultivar till now for S. miltiorrhiza, which also is disadvantageous to quality control of medicinal materials even for those individuals growing in the same field.
Since a natural male sterile mutant of S. miltiorrhiza (Sh-B) was firstly found during our field survey in 2002, research has been conducted to determine its pollen development [2] and biological characteristics [3]. Amplified fragment length polymorphism (AFLP) technique, one of the most efficient molecular marker systems for screening genes of interest [10], [11], [12], is employed in this study to screen the markers that might link with the dominant male sterile gene.
Therefore, the present investigation is undertaken to determine: 1) the segregation in progenies of both backcrosses and testcross for producing F1 hybrid seeds; 2) screening AFLP markers that associate with male sterility gene in S. miltiorrhiza.
Materials and Methods
Plant Material
The male sterility of S. miltiorrhiza (Sh-B) was derived from natural mutants found in field survey in 2002 [3]. The segregating population (273 individuals) was constructed by consecutively backcrossing between Sh-B and fertile plants at a ratio of 1∶1 (fertile : sterile). An F2 population obtained from testcross between sterile and inbred fertile plants was used to practical test of the markers linked to the male sterile gene. Both male sterile and fertile plants were grown on the experimental farm of Northwest A & F University.
Genetic Analysis and Field Production of F1 Hybrid Seeds
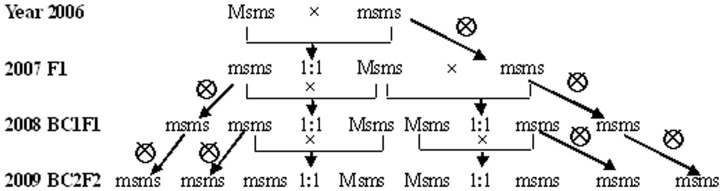
Male sterile line Sh-B was crossed with three fertile lines (A-20, A-249, A-207) to determine the fertility of F1s and to observe F2 segregation for male sterile and fertile plants (Fig. 1, Table 1). The sterile F1 seeds (half of total) were backcrossed to self-pollination progenies of fertile lines to determine the BC1F1 segregation ratios of male-sterile and fertile plants. The BC1F1s were assessed by both testcross and backcross and BC2F2 segregation ratios of male-sterile and fertile plants were determined only in progenies of BC1F1 families that segregated for male sterile plants.
Figure 1. Genetic pattern of male sterility of Salvia miltiorrhiza deduced by segregation result in Table 1 through backcross and testcross.
Table 1. Segregation of male fertile and sterile plants of Salvia miltiorrhiza (Sh-B) in backcross and testcross populations tested over three years.
| Cross* | Year | No. of plants observed | Segregation ratio | Probability | |
| Fertile plants | Sterile plants | ||||
| (Sh-B×A-20) F1 | 2007 | 155 | 148 | 1:1 | 0.17 |
| (Sh-B×A-249)F1 | 2007 | 174 | 169 | 1:1 | 0.22 |
| (Sh-B×A-107)F1 | 2007 | 207 | 193 | 1:1 | 0.13 |
| (Sh-B×A-20) BC1F1 | 2008 | 206 | 195 | 1:1 | 0.21 |
| (Sh-B×A-249)BC1F1 | 2008 | 193 | 189 | 1:1 | 0.29 |
| (Sh-B×A-107)BC1F1 | 2008 | 205 | 198 | 1:1 | 0.18 |
| (Sh-B×A-20) BC2F2 | 2009 | 206 | 200 | 1:1 | 0.15 |
| (Sh-B×A-249)BC2F2 | 2009 | 194 | 185 | 1:1 | 0.22 |
| (Sh-B×A-107)BC2F2 | 2009 | 195 | 193 | 1:1 | 0.62 |
| Sh-B×A-20Sh-BA | 2009 | 215 | 211 | 1:1 | 0.22 |
| Sh-B×A-107Sh-BA | 2009 | 209 | 202 | 1:1 | 0.18 |
| Sh-B×A-249Sh-BA | 2009 | 207 | 199 | 1:1 | 0.15 |
A-20
|
2008 | 120 | 0 | ||
A-249
|
2008 | 140 | 0 | ||
A-107
|
2008 | 150 | 0 | ||
A-20Sh-BA
|
2008 | 180 | 0 | ||
A-107Sh-BA
|
2008 | 170 | 0 | ||
A-249Sh-BA
|
2008 | 190 | 0 | ||
A-20, A-249 and A-107 were sterile and inbred lines of Salvia miltiorrhiza with different biological characteristics; BC1F1 represented F1 progeny with one backcross; BC2F2 represented F2 progeny with twice backcross;  represented self pollination; A-20Sh-BA represented half of fertile plants obtained from sterile seeds after hybridization between A-20 × Sh-B; A-20Sh-BA was self pollination of A-20Sh-BA progeny.
represented self pollination; A-20Sh-BA represented half of fertile plants obtained from sterile seeds after hybridization between A-20 × Sh-B; A-20Sh-BA was self pollination of A-20Sh-BA progeny.
Given that half of F1 plants were fertile, identified till to flowering and they were removed from the field before harvest. To obtain more seedlings with uniform male sterility used to produce F1 hybrid seeds, the asexual reproduction method by using root cuttings as ‘seeds’ for sterile plants was chosen due to its higher propagation index (general 4–5 root cuttings/one root) than that of stem base cuttings (general 2–3 cuttings/one base).
To obtain the largest yield of F1 hybrid seeds from male sterile plants, four row ratios (female: male = 2∶1, 2∶2, 3∶2, 4∶4) were investigated. Each row ratio field experiment was conducted in an isolated region to avoid pollen contamination each other. Pollen flow was also observed at full-flowering stage to determine its spread distance (30, 45, 60 and 75 cm were estimated), corresponding with different row ratios. Pollen was captured by sticky microscope slides (length 76.2, width 25.4 and thickness 1.1 mm) coating with vaseline, which were placing on tripods with the same height as flower from last afternoon 6:00 PM to the next afternoon 6:00 PM. The number of pollen grains was then calculated under microscope within the range of all slide surface. In addition, both seed setting rate (percentage of seeding flowers/all flowers) and seed index (the number of seeds/all flowers), as well as the yield of F1 hybrid seeds were all calculated.
DNA Extraction and AFLP Analysis
Fresh young leaves of S. miltiorrhiza from each plant were collected and DNA was extracted by using an improved cetyltrimethylammonium bromide (CTAB) method [13]. To screen AFLP markers linking to male sterile gene, equivalent amounts of DNA from eight randomly selected sterile individuals were pooled to construct sterile bulk and another eight fertile individuals for fertile bulk. The AFLP procedure followed was the one described by Vos et al. [14].
AFLP Fragment Cloning and Sequencing
Bands containing targeted AFLP fragments were exercised from polyacrylamide gel and then were placed in a 0.5 ml eppendorf tube. The gel slices were crushed with a pipette tip and boiled at 100°C for 15 minutes after adding 50 µl of double-distilled water. The tubes were centrifuged at a velocity of 10,000 rotation per minute for 5 minutes. 5 µl of the supernatant was used as template solution for selective amplification with the same primer combination. The re-amplified PCR product was analyzed with a 1.2% agarose gel. A UNIQ-10 EZ Spin Column DNA Gel Extraction Kit (Sangon, Shanghai) and a pGEM-T Easy Vector (Tiangen, Beijing) were used to purify and clone the polymorphism fragments. Then white colonies growing on Amp+/X-gal/IPTG LB solid media plates were selected and cultured overnight in LB liquid media. Colonies containing target fragments were sequenced by Sangon Biotech (Shanghai) Co. Ltd.
The homology of the sequenced AFLP markers linked to male sterile gene was determined using BLASTn by comparison with database at NCBI (http://www.ncbi.nlm.nih.gov/BLAST).
Linkage Analysis
The polymorphic AFLP markers were identified in the complete F1 mapping population only those exhibiting reproducible polymorphisms between fertile DNA bulks and sterile DNA bulks. The fertility and molecular marker data were combined for linkage analysis using the software package MAPMAKER/EXP 3.0 [15], [16]. The recombinant frequencies between male sterile gene and AFLP markers were calculated through two-point tests and linkage map was constructed by three-point or multiple-point tests with a minimum LOD threshold of 3.0. The recombination values were converted into centiMorgans (cM) by using Kosambi mapping function [17].
Results and Discussion
Segregation Analysis
The segregation of male sterility of both testcross and backcross progenies were all showed a 1∶1 ratio suggesting a dominant control (Ms) (Fig. 1, Table 1). The male sterile Sh-B was heterozygous (Msms). Given that all male sterile plants (Msms) were stable sterile, it was impossible to perform self-pollination and to obtain homozygous male sterile plants (MsMs).
F1 Hybrid Seeds Production
The male sterile line (as female) and a fertile inbred line (as male) were planted together with four row ratios (2∶1, 2∶2, 3∶2, 4∶4) in each isolation area for production of F1 hybrid seeds (Table 2). The male sterile plants could capture most pollen flow (2 pollen granule/cm2·24 h) with row ratio (female:male 2∶1) within 45 cm in one day and harvest 6495 g seeds per hectare. Seeds collected from male sterile line formed the F1 hybrid, but half of them were fertile.
Table 2. Pollen flow and seed set of male sterile plants of Salvia miltiorrhiza when producing hybrid F1 seeds with different row ratios.
| Row ratio female:male | Pollen flow at distance (cm) (gradule/24 h) | Seed setting rate (%) | Seed index | Seed yield per individual (g) | Seed yield per hectare (g) | ||||
| 30 | 45 | 60 | 75 | Average | |||||
| 2:1 | 2 | 2 | 2 | 54.7 | 0.30 | 0.50 | 6495 | ||
| 2:2 | 2 | 2 | 2 | 54.2 | 0.29 | 0.32 | 3120 | ||
| 3:2 | 2 | 2 | 1 | 1.67 | 47.6 | 0.27 | 0.49 | 5730 | |
| 4:4 | 2 | 2 | 1 | 1 | 1.5 | 44.0 | 0.23 | 0.42 | 4095 |
According to the inheritance of male sterility, a two-step method for hybrid seed production has been developed. The first is production of F1 hybrid and the second is asexual reproduction of male sterile plant roots. Given the fact that half of F1 hybrid seeds are fertile that will be identified till to flowering [3]. There are two asexual reproduction methods for male sterile plants except for tissue culture, cutting of stem base connecting to root and root cutting. Cutting of stem base has lower propagation coefficient (2–3) than root cutting (4–5), thus resulting in less application in production. When we obtained sufficient roots cuttings as male sterile “seeds”, F1 hybrid seeds can be produced in large scale.
Marker-assisted selection (MAS) of simple traits and quantitative traits is helpful to improve the efficiency of breeding programs for many crops [18]. To further use this male sterile system for hybrid seed production, it is very important to breed maternal parents. The problem of male sterility of Salvia miltiorrhiza was that the maternal parents are segregated to 50% male-fertile and 50% male-sterile plants in hybrid seeds. Therefore, the fertile should be identified and removed as early as possible before flowering stage. Development of a large-scale selection system combined with stable PCR-based assay will exclude all male-fertile plants at seedling stage before planting in the seed production field. If cheap, fast and reliable PCR-based markers were available for the male sterile gene, it would greatly improve the efficiency of breeding program. Consequently, conversion of a polymorphic AFLP fragment tightly linked to male sterile gene into a gene-specific marker is the next step for marker-assisted selection breeding in Salvia miltiorrhiza.
Identification of AFLP Marker Linked to Male Sterile Gene
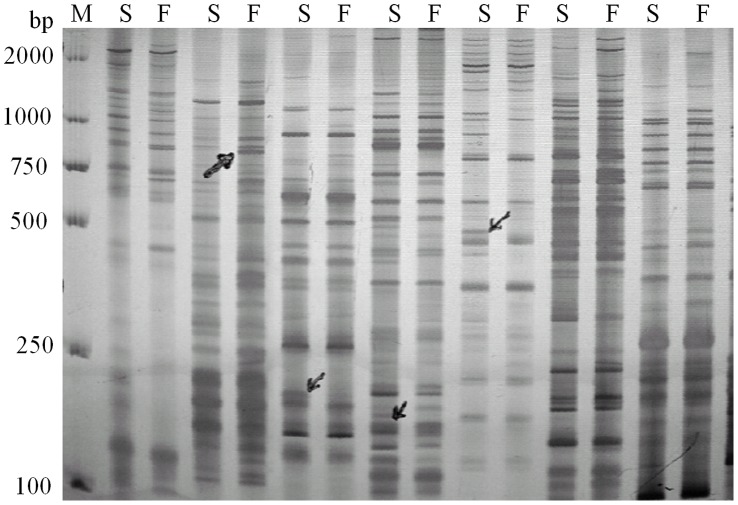
In AFLP analysis, the fertile and sterile DNA bulks were used to identify putative markers linked to male sterile gene. The assays involved two common enzymes (EcoRI and MseI). A total of 128 pairs of primer combinations were used with E+3/M+3. Fourteen primer combinations revealed polymorphism between the fertile and sterile DNA bulks (Fig. 2). Each primer combinations amplified fragments ranging from 25 to 120 with an average of 37.90. These fragments were widely dispersed and ranged in size from 100 to 1000 bp, mainly concentrating on ca. 500 bp.
Figure 2. Selective amplification in male sterile (S) and fertile (F) populations of Salvia miltiorrhiza by AFLP.
Arrows represented differently expressed bands between them.
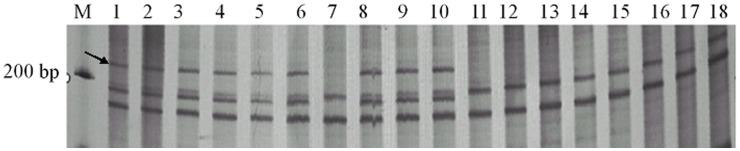
Examination of eight fertile and eight sterile individuals in the bulks indicated that one of fourteen AFLP markers, E11/M4208 (E11: 5′-GACTGCGTACCAATTCACC-3′, M4: 5′-GATGAGTCCTGAGTAACAG-3′), was associated with male sterile gene (Fig. 3). This AFLP marker was confirmed in the 136 fertile and 137 sterile plants from F1 mapping population (χ 2-test 3.84, Probability 0.137) and then was cloned and sequenced. Electrophoresis results indicated that this marker segregated in sterile and fertile plants corresponding with the expected Mendelian ratio of 1∶1. Linkage analysis confirmed that this AFLP marker was tightly linked to male sterile gene with a recombination frequency of 6.85% and at a distance of 6.89 cM.
Figure 3. AFLP amplification profiles in male sterile and fertile plant populations generated by primer combination E11/M4208.
Lanes: 1–9 sterile individuals; 10–18 fertile individuals; The arrow represented the band that tightly linked with dominant male sterile gene Of Salvia miltiorrhiza.
As mentioned above, half of fertile plants containing in F1 hybrid populations need to be removed from the field as early as possible, however, they were identified as far as flowering stage in nowadays. The identified E11/M4208 marker can easily be converted into sequence characterized amplified region (SCAR) marker, which is a fast, cheap and reliable PCR-based assay used to identify large amount of individuals in a target population. It will greatly facilitate the identification of fertile plants as early in seedling stage by using this SCAR marker and make the large-scale production of male sterile seedlings through seeds possible because those fertile ones can be identified and then removed before transplanted in field. Under this circumstance, large scale F1 hybrid seed production using sexual propagation was realized and thus reduced the great deal of labor cost in cutting root.
Sequence Features of Male Sterile Gene Amplified by E11/M4 Primer Combination
The fragment (208 bp) amplified by E11/M4 primer combination was submitted to the NCBI website (http://www.ncbi.nlm.nih.gov/) for nucleotide-nucleotide BLAST (Blastn) analysis. The sequence was identical to nucleotides in Arabidopsis genome ranging from 84 to 100% and in Oryza sativa japonica genome with the percentage of 82–100% (Table 3).
Table 3. Blast hits of AFLP fragment E11/M4208 that tightly linked with male sterile gene in Salvia miltiorrhiza.
| GenBank Acc. No. | Blast hits | Organism | E - value | Identities (%) |
| NC003071.7 | Hypothetical protein, 2640 bp at 5′ side: ethanolaminephosphotransferase; 314 bp at 3′ side: MADS-box protein | Arabidopsis thaliana, chromosome 2 | 0.12 | 93 |
| NC003076.8 | zeaxanthin epoxidase (ZEP) (ABA1) | Arabidopsis thaliana, chromosome 5 | 5.2 | 95 |
| NC003075.7 | dicarboxylate carrier 2 | Arabidopsis thaliana, chromosome 4 | 5.2 | 91 |
| NC003074.8 | hypothetical protein | Arabidopsis thaliana, chromosome 3 | 5.2 | 84 |
| NC003070.9 | S-adenosylmethionine-dependent methyltransferase domain-containing protein | Arabidopsis thaliana, chromosome 1 | 5.2 | 100 |
| NC008401.2 | 5016 bp at 5′ side: Hypothetical protein; 256 bp at 3′ side: Hypothetical protein | Oryza sativa Japonica Group DNA, chromosome 8 | 1.4 | 82 |
| NC008398.2 | 38938 bp at 5′ side: Hypothetical protein; 11051 bp at 3′ side: Hypothetical protein | Oryza sativa Japonica Group DNA, chromosome 5 | 1.4 | 92 |
| NC008400.2 | Hypothetical protein | Oryza sativa Japonica Group DNA, chromosome 7 | 4.8 | 100 |
Both phosphoethanolamine N-methyltransferase (PEAMT) and choline/ethanolamine phosphotransferase play key roles in phosphatidylcholine (Ptd-Cho) and glycinebetaine (GlyBet)biosynthesis [19]. The PEAMT gene was found highly expressing in Salicornia europaea pollen, root, petiole and leaf, which activity was induced by low temperature, abscisic acid (ABA) and NaCl [20]. MADS-box protein was also found highly expressing in flower bud of cytoplasmic male sterile soybean (Glycine max L. Merr.) plants [21].
Zeaxanthin epoxidase (ZEP) plays an important role in ABA biosynthesis and participates xanthophyll cycle in higher plants, which can catalyze zeaxanthin into anthraxanthin and violaxanthin by using excessive light energy and confirm photosynthesis can be smoothly proceeded under lower light [22]. The anthraxanthin is the expansion section of stamen that producing pollens, which function would affect the activity of pollens.
S-adenosylmethionine (AdoMet) is a common co-substrate involved in methyl group transfers. Transmethylation, transsulfuration, and aminopropylation are the metabolic pathways that use AdoMet. On the other hand, DNA methylation involves the development of plant gametogenesis [23]. Depletion of A. thaliana MET1 results in immense epigenetic diversification of gametes and this diversity seems to be a consequence of passive postmeiotic demethylation, leading to gametes with fully demethylated and hemidemethylated DNA [24].
Funding Statement
Funding was provided by the Education Ministry National Higher Education Fund for Doctoral Program (20100204120027), the National Higher Education Fund for Basic Research (QN2009065) (www.nwsuaf.edu.cn) and the Department of Science and Technology of Shaanxi Government (2011K16-02-06)(http://www.sninfo.gov.cn:8083/initSnFirstPageList.do?method=initSnFirstPageList). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Zhou K, Wang S, Feng Y, Ji W, Wang G (2008) A new male sterile mutant LZ in wheat (Triticum aestivum L.). Euphytica 159: 403–410. [Google Scholar]
- 2. Shu Z, Liang Z, Sun Q, Zhang X, Fu L (2006) Identification and pollen development anatomy of the male sterility line Sh-B in Salvia miltiorrhiza. . Acta Bot Boreal-Occident Sin 26: 2202–2207. [Google Scholar]
- 3. Shu Z, Liang Z, Sun Q, Fu L, Zhang X, et al. (2007) Biological characteristics of Salvia miltiorrhiza male sterile line Sh-B. J Northwest A & F Univer (Nat Sci Ed) 35: 175–179. [Google Scholar]
- 4. Wang ZF, Su XH, Shan CG, Yan SL (2007) Discovery and identification for male-sterile Chinese bellflower. Res Prac Chin Med 21: 8–10. [Google Scholar]
- 5. Wu JR, Yan YZ, Quan XL, Pu J, Wu SQ (2008) Discovery and identification of male sterility Platycodon grandiflorum . Bull Bot Res 28: 716–719. [Google Scholar]
- 6. Liu ZG, Zhang Y, Yang YL (2009) Discovery and identification of male sterile Platycodon grandiflorum . Northern Horticul 1: 40–43. [Google Scholar]
- 7. Qin K, Tian Y, Li YX, Hong FY, Zhong SY, et al. (2006) Discovery and identification of male sterility Lycium barbarum germplasm YX-1. Acta Bot Boreal-Occident Sin 26: 1838–1841. [Google Scholar]
- 8. Dong J, Wan G, Liang Z (2010) Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J Biotechn 148: 99–104. [DOI] [PubMed] [Google Scholar]
- 9.National Committee of China Pharmacopeia (2010) China Pharmacopoeia. Beijing: Chemical Industry Press. 52 p.
- 10. Liu ZW, Fu TD, Tu JX, Chen BY (2005) Inheritance of seed colour and identification and AFLP markers linked to the seed in rapeseed (Brassica napus L.). Theor Appl Genet 110: 303–310. [DOI] [PubMed] [Google Scholar]
- 11. Jiang T, Zhou B, Gao F, Guo B (2011) Genetic linkage maps of white birches (Betula platyphylla Suk. and B. pendula Roth) based on RAPD and AFLP markers. Mol Breeding 27: 347–356. [Google Scholar]
- 12. McNeil MD, Hermann S, Jackson PA, Aitken KS (2011) Conversion of AFLP markers to high-throughput markers in a complex polyploid, sugarcane. Mol Breeding 27: 395–407. [Google Scholar]
- 13. Wang C, Liang Z, Li D, Wang H, Shu Z (2007) Optimization of DNA extraction method from Salvia miltiorrhiza Bge. Pharmaceu Biotechn 14: 29–33. [Google Scholar]
- 14. Vos P, Hogers R, Bleeker M, Reijans M, Lee Tv d, et al. (1995) AFLP: A new technique for DNA fingerprinting. Nucl Acids Res 23: 4407–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, et al. (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1: 174–181. [DOI] [PubMed] [Google Scholar]
- 16.Lincoln S, Daly M, Lander E (1992) Constructing genetic maps with MAPMAKER/EXP 3.0. Whitehead Technical Institute, Cambridge, MA.
- 17. Kosambi DD (1944) The estimation of map distance from recombination values. Ann Eugen 12: 172–175. [Google Scholar]
- 18. Francia E, Tacconi G, Crosatti C, Barabaschi D, Bulgarelli D, et al. (2005) Marker assisted selection in crop plants. Plant Cell Tissue Organ Cult 82: 317–342. [Google Scholar]
- 19. McNeil SD, Nuccio ML, Ziemak MJ, Hanson AD (2001) Enhanced synthesis of choline and glycine betaine in transgenic tobacco plants that overexpress phosphoethanolamine Nmethyltransferase. Proc Natl Acad Sci USA 98: 10001–10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma SJ, Su Q, Wu S, An LJ (2008) Isolation and transient expression assay of PEAMT gene promoter from Salicornia europaea . China Biotechn 28: 69–73. [Google Scholar]
- 21. Han LT, Jiang W, Yang SP, Yu DY, Gai JY (2010) Isolation and analysis of MADS-box gene from soybean (Glycine max L. Merr.) cytoplasmic male sterile line. Acta Agron Sin 36: 905–910. [Google Scholar]
- 22. Havaux M, Dall'Osto L, Cuine S, Giuliano G, Bassi R (2004) The effect of zeaxanthin as the only xanthophyll on the structure and function of the photosynthetic apparatus in Arabidopsis thaliana . J Biol Chem 279: 13878–13888. [DOI] [PubMed] [Google Scholar]
- 23.Baroux C, Spillane C, Grossniklaus U (2002) Genomic imprinting during seed development. Adv Genet Academic Press San Diego USA 165–214. [DOI] [PubMed]
- 24. Saze H, Scheid OM, Paszkowski J (2003) Maintenance of CpG me-thylation is essential for epigenetic inheritance during plant gametogenesis. Nature Genet 34: 65–69. [DOI] [PubMed] [Google Scholar]