Abstract
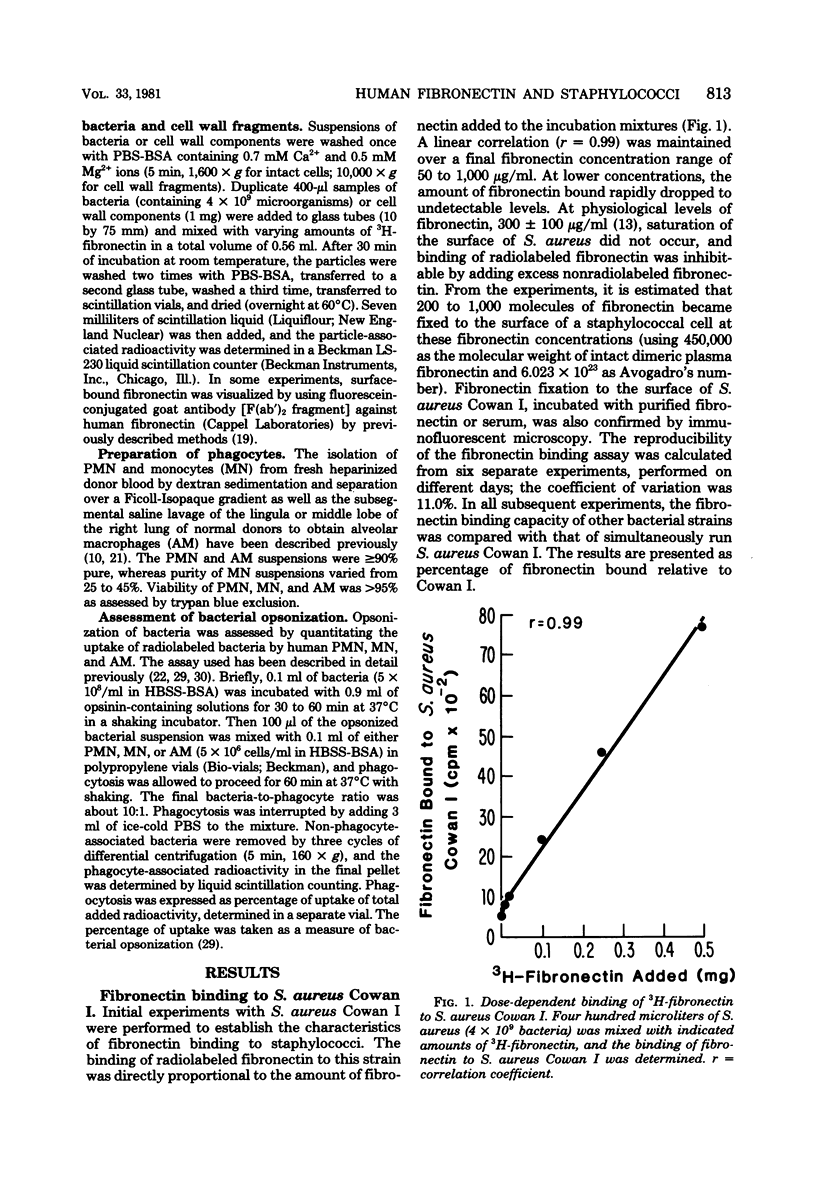
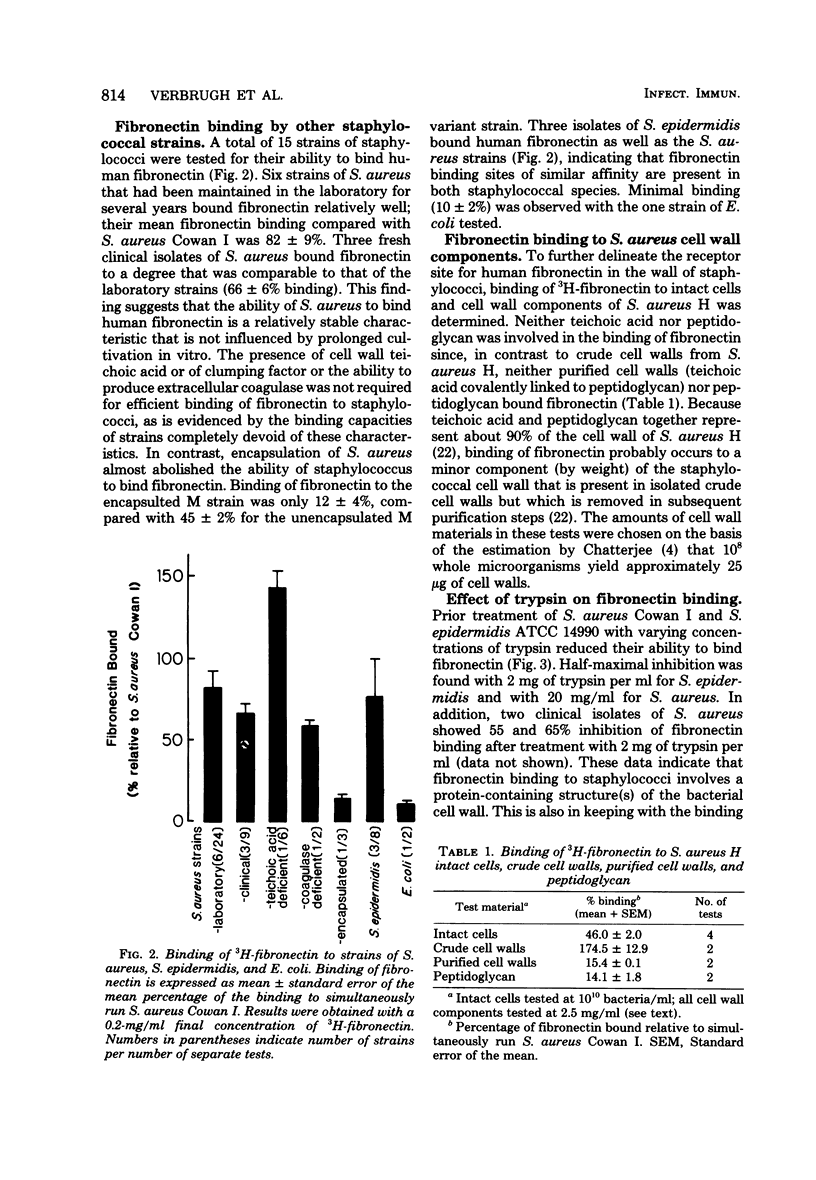
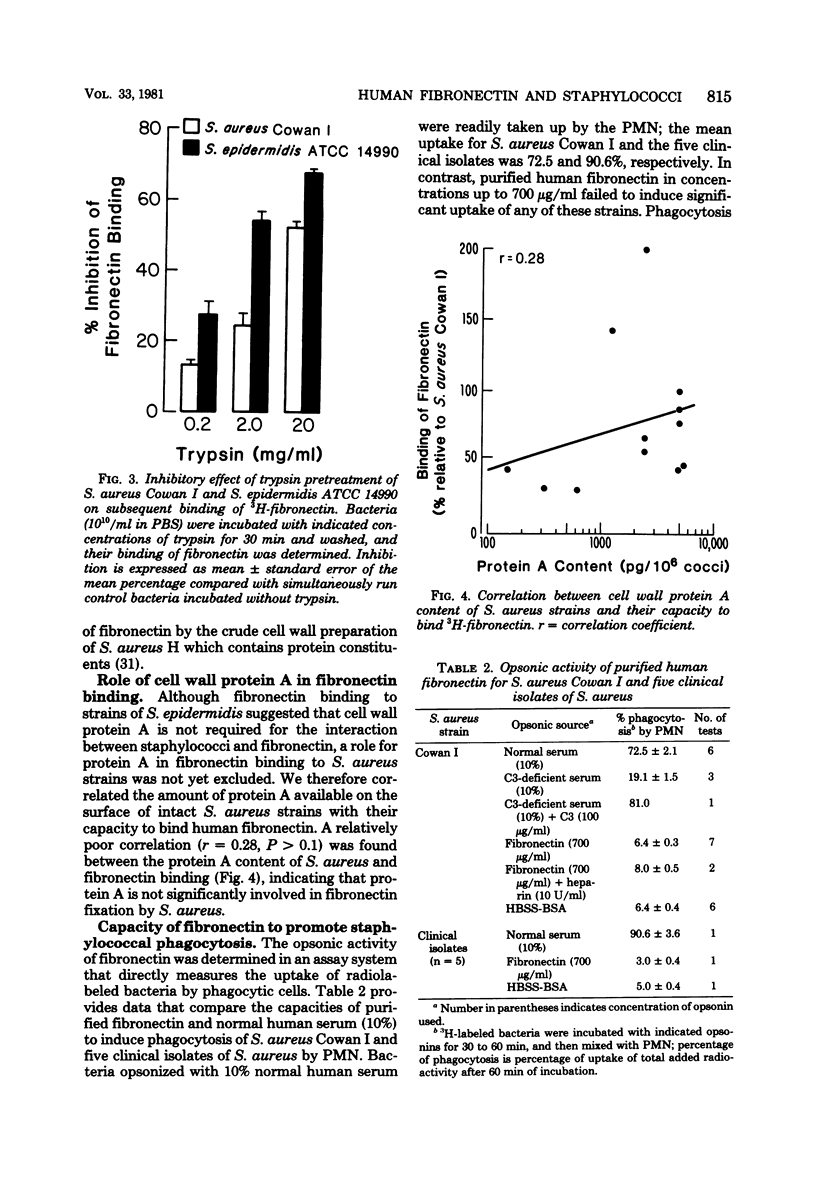
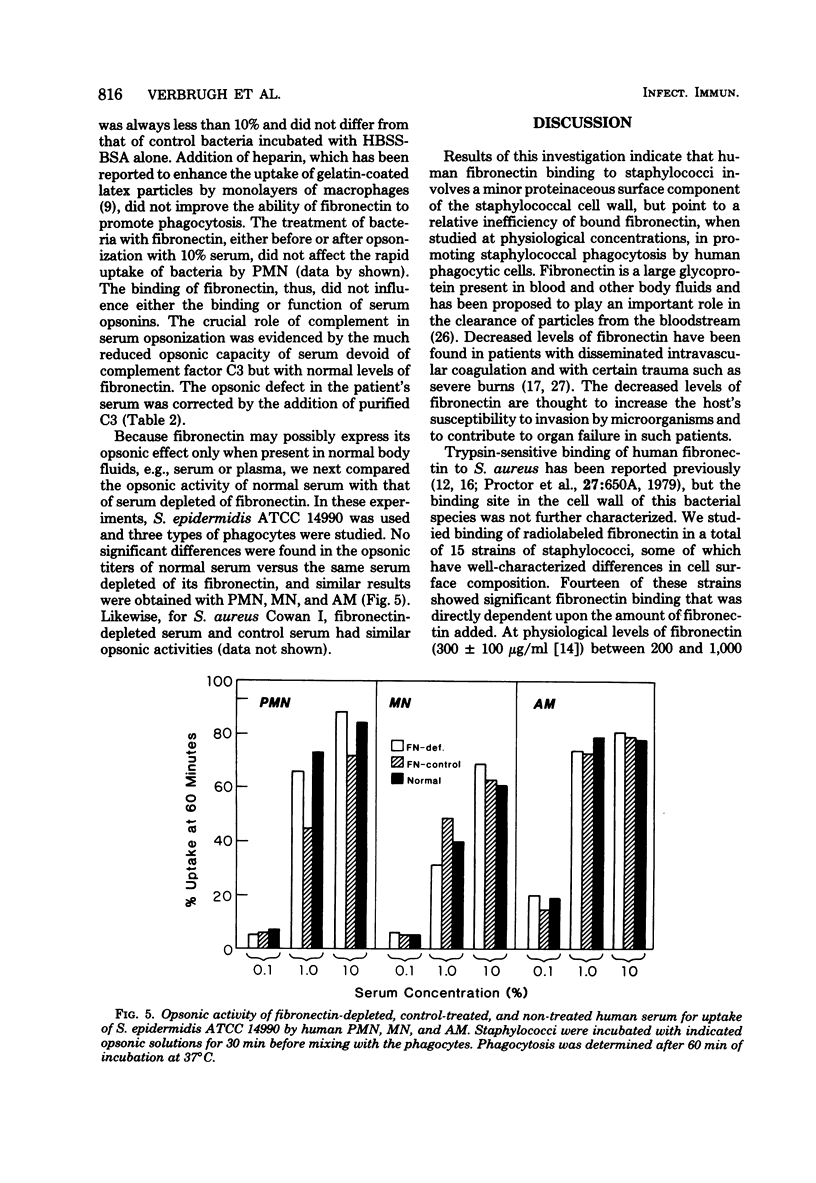
The interaction between human fibronectin and 17 strains of staphylococci was studied in an attempt to elucidate the staphylococcal cell wall component(s) involved in fibronectin binding and to determine the influence of fibronectin upon phagocytosis by three types of phagocytic cells. Purified, radiolabeled fibronectin bound to a similar degree to six laboratory strains and three fresh clinical isolates of Staphylococcus aureus; similar binding of fibronectin was found with S. aureus strains deficient in cell wall teichoic acid or clumping factor and coagulase, as well as with three strains of S. epidermidis. There was minimal binding of fibronectin to encapsulated S. aureus and to Escherichia coli. Fibronectin bound to intact cells and to a crude cell wall preparation of S. aureus H, but not to purified cell walls or peptidoglycan. Trypsinization of staphylococci prevented subsequent fibronectin binding, but binding did not correlate well with the protein A content in S. aureus cell walls. At physiological concentrations, fibronectin binding to staphylococci did not promote phagocytosis of bacteria by human polymorphonuclear leukocytes, monocytes, or alveolar macrophages. Also, depletion of fibronectin from normal human serum did not result in a measurable loss of opsonic activity for staphylococci. It is concluded that fibronectin binding to staphylococci involves a surface protein shared among strains of S. aureus and S. epidermidis, and that in comparison to C3b and IgG, fibronectin plays a relatively minor role as an opsonin for staphylococci.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Amrani D., Mosesson M. W., Bianco C. Receptors for cold-insoluble globulin (plasma fibronectin) on human monocytes. J Exp Med. 1981 Jan 1;153(1):42–60. doi: 10.1084/jem.153.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenstock F. A., Saba T. M., Weber P., Laffin R. Biochemical and immunological characterization of human opsonic alpha2SB glycoprotein: its identity with cold-insoluble globulin. J Biol Chem. 1978 Jun 25;253(12):4287–4291. [PubMed] [Google Scholar]
- Chatterjee A. N. Use of bacteriophage-resistant mutants to study the nature of the bacteriophage receptor site of Staphylococcus aureus. J Bacteriol. 1969 May;98(2):519–527. doi: 10.1128/jb.98.2.519-527.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furcht L. T., Mosher D. F., Wendelschafer-Crabb G. Immunocytochemical localization of fibronectin (LETS proteins) on the surface of L6 myoblasts: light and electron microscopic studies. Cell. 1978 Feb;13(2):263–271. doi: 10.1016/0092-8674(78)90195-2. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Weissmann G. Nonphagocytic stimulation of human polymorphonuclear leukocytes: role of the plasma membrane. Semin Hematol. 1979 Jul;16(3):175–187. [PubMed] [Google Scholar]
- Grebner J. V., Mills E. L., Gray G. H., Quie P. G. Comparison of phagocytic and chemiluminescence response of human polymorphonuclear neutrophils. J Lab Clin Med. 1977 Jan;89(1):153–159. [PubMed] [Google Scholar]
- Gudewicz P. W., Molnar J., Lai M. Z., Beezhold D. W., Siefring G. E., Jr, Credo R. B., Lorand L. Fibronectin-mediated uptake of gelatin-coated latex particles by peritoneal macrophages. J Cell Biol. 1980 Nov;87(2 Pt 1):427–433. doi: 10.1083/jcb.87.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoidal J. R., White J. G., Repine J. E. Influence of cationic local anesthetics on the metabolism and ultrastructure of human alveolar macrophages. J Lab Clin Med. 1979 May;93(5):857–866. [PubMed] [Google Scholar]
- King B. F., Biel M. L., Wilkinson B. J. Facile penetration of the Staphylococcus aureus capsule by lysostaphin. Infect Immun. 1980 Sep;29(3):892–896. doi: 10.1128/iai.29.3.892-896.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978 Dec 14;276(5689):718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- LI I. W., MUDD S. THE HEAT-LABILE SERUM FACTOR ASSOCIATED WITH INTRACELLULAR KILLING OF STAPHYLOCOCCUS AUREUS. J Immunol. 1965 Jun;94:852–857. [PubMed] [Google Scholar]
- Lanser M. E., Saba T. M., Scovill W. A. Opsonic glycoprotein (plasma fibronectin) levels after burn injury. Relationship to extent of burn and development of sepsis. Ann Surg. 1980 Dec;192(6):776–782. doi: 10.1097/00000658-198012000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosesson M. W., Umfleet R. A. The cold-insoluble globulin of human plasma. I. Purification, primary characterization, and relationship to fibrinogen and other cold-insoluble fraction components. J Biol Chem. 1970 Nov 10;245(21):5728–5736. [PubMed] [Google Scholar]
- Mosher D. F., Proctor R. A. Binding and factor XIIIa-mediated cross-linking of a 27-kilodalton fragment of fibronectin to Staphylococcus aureus. Science. 1980 Aug 22;209(4459):927–929. doi: 10.1126/science.7403857. [DOI] [PubMed] [Google Scholar]
- Mosher D. F., Williams E. M. Fibronectin concentration is decreased in plasma of severely ill patients with disseminated intravascular coagulation. J Lab Clin Med. 1978 May;91(5):729–735. [PubMed] [Google Scholar]
- Peterson P. K., Kim Y., Wilkinson B. J., Schmeling D., Michael A. F., Quie P. G. Dichotomy between opsonization and serum complement activation by encapsulated staphylococci. Infect Immun. 1978 Jun;20(3):770–775. doi: 10.1128/iai.20.3.770-775.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Verhoef J., Sabath L. D., Quie P. G. Effect of protein A on staphylococcal opsonization. Infect Immun. 1977 Mar;15(3):760–764. doi: 10.1128/iai.15.3.760-764.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Verhoef J., Schmeling D., Quie P. G. Kinetics of phagocytosis and bacterial killing by human polymorphonuclear leukocytes and monocytes. J Infect Dis. 1977 Oct;136(4):502–509. doi: 10.1093/infdis/136.4.502. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Douglas S. D., Quie P. G., Verhoef J. The key role of peptidoglycan in the opsonization of Staphylococcus aureus. J Clin Invest. 1978 Mar;61(3):597–609. doi: 10.1172/JCI108971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quie P. G., Messner R. P., Williams R. C., Jr Phagocytosis in subacute bacterial endocarditis. Localization of the primary opsonic site to Fc fragment. J Exp Med. 1968 Oct 1;128(4):553–570. doi: 10.1084/jem.128.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E., Hayman E. G. Fibronectin: current concepts of its structure and functions. Coll Relat Res. 1981;1(1):95–128. doi: 10.1016/s0174-173x(80)80011-2. [DOI] [PubMed] [Google Scholar]
- Saba T. M., Jaffe E. Plasma fibronectin (opsonic glycoprotein): its synthesis by vascular endothelial cells and role in cardiopulmonary integrity after trauma as related to reticuloendothelial function. Am J Med. 1980 Apr;68(4):577–594. doi: 10.1016/0002-9343(80)90310-1. [DOI] [PubMed] [Google Scholar]
- Scovill W. A., Annest S. J., Saba T. M., Blumenstock F. A., Newell J. C., Stratton H. H., Powers S. R. Cardiovascular hemodynamics after opsonic alpha-2-surface binding glycoprotein therapy in injured patients. Surgery. 1979 Aug;86(2):284–293. [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis: recognition and ingestion. Semin Hematol. 1975 Jan;12(1):83–116. [PubMed] [Google Scholar]
- Verbrugh H. A., van Dijk W. C., Peters R., van Erne M. E., Daha M. R., Peterson P. K., Verhoef J. Opsonic recognition of staphylococci mediated by cell wall peptidoglycan: antibody-independent activation of human complement and opsonic activity of peptidoglycan antibodies. J Immunol. 1980 Mar;124(3):1167–1173. [PubMed] [Google Scholar]
- Verhoef J., Peterson P., Kim Y., Sabath L. D., Quie P. G. Opsonic requirements for staphylococcal phagocytosis. Heterogeneity among strains. Immunology. 1977 Aug;33(2):191–197. [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Kim Y., Peterson P. K., Quie P. G., Michael A. F. Activation of complement by cell surface components of Staphylococcus aureus. Infect Immun. 1978 May;20(2):388–392. doi: 10.1128/iai.20.2.388-392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Sisson S. P., Kim Y., Peterson P. K. Localization of the third component of complement on the cell wall of encapsulated Staphylococcus aureus M: implications for the mechanism of resistance to phagocytosis. Infect Immun. 1979 Dec;26(3):1159–1163. doi: 10.1128/iai.26.3.1159-1163.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



