Abstract
Nitrate reductase (NR) activity increased up to 14-fold in response to treatment of Arabidopsis thaliana seedlings with the cytokinin benzyladenine. NR induction was observed in seedlings germinated directly on cytokinin-containing medium, seedlings transferred to cytokinin medium, and seedlings grown in soil in which cytokinin was applied directly to the leaves. About the same level of induction was seen in both wild-type and Nia2-deletion mutants, indicating that increased NR activity is related to the expression of the minor NR gene, Nia1. The steady-state Nia1 mRNA level was increased severalfold in both wild-type and mutant seedlings after benzyladenine treatment. Transcript levels of the Nia2 gene, which is responsible for 90% of the NR activity in developing wild-type seedlings, did not show any changes upon cytokinin treatment. Nuclear run-on assays demonstrated that Nia1 gene transcription increased dramatically after cytokinin treatment.
NR (EC 1.6.6.1) is the first enzyme of the nitrate assimilation pathway in higher plants. It reduces the major plant N source, NO3−, into NO2−, which then is further reduced to NH3 by NO2− reductase. This vital, energy-consuming process is tightly regulated and responsive to various factors, including NO3−, NH3, light, diurnal rhythms, plastid factors, photosynthesis status, and phytohormones (for reviews, see Redinbaugh and Campbell, 1991; Pelsy and Caboche, 1992; Crawford, 1995).
The major phytohormone that affects NR levels is cytokinin. Cytokinin induction of NR activity was first described in Agrostemma githago embryos (Borriss, 1967; Kende et al., 1971). Since then, similar effects have been observed in many other species (for review, see Gaudinova, 1990). Cytokinin stimulated NR activity in haploid Nicotiana plumbaginifolia (tobacco) cultures and was used to increase the efficiency of ClO3− selection for NR-deficient mutants (Márton et al., 1982a). Among the different hormones tested, only cytokinins stimulated NR activity in etiolated corn leaves (Rao et al., 1984). Banowetz (1992) found that application of BA to shoots enhanced the NO3− induction of NR in etiolated wheat seedlings in a concentration-dependent manner, and root-applied BA enhanced NO3− induction in both etiolated and light-grown seedlings.
Early results showed that cytokinin induction of NR activity involves de novo synthesis of NR (Rao et al., 1984). In split-root cultures of barley (Hordeum vulgare L.), external application of various cytokinins resulted in up to a 25% increase in NR mRNA in roots and up to a 100% increase in shoots of barley (Samuelson et al., 1995). In nuclear run-on assays, the addition of BA partially reversed the ABA suppression of NR gene transcription in barley (Lu et al., 1992). Protein-synthesis inhibitors depressed BA enhancement of NR activity but did not inhibit BA-enhanced NR transcription. However, posttranscriptional and translational regulation of NR activity may also occur. Using a tobacco cell-suspension culture, Suty et al. (1993) found that cytokinin exerted a specific effect on NR mRNA accumulation through modulation of polyadenylation.
Arabidopsis thaliana has two NR genes, Nia1 and Nia2 (Cheng et al., 1986, 1988; Wilkinson and Crawford, 1993). Nia2 is responsible for 90% of the total NR activity in seedlings, whereas Nia1 accounts for the remaining 10% (Wilkinson and Crawford, 1991). However, in the Nia2-deletion mutant G5, in which the entire Nia2 gene is missing, Nia1 activity alone permits normal growth on NO3− (Wilkinson and Crawford, 1993). The tissue-specific expression of the two Arabidopsis NR genes and their regulation in response to NO3−, light, and circadian rhythm were investigated by Cheng et al. (1991). The basal levels of expression of the two genes in the absence of NO3− are quite different. In addition, these genes exhibit differences in the kinetics of their induction by light. In subsequent work with reporter gene fusions (Chen et al., 1992; Lin and Cheng, 1997), Suc was found to induce the transcription of the Nia1 gene, and the NO3−-responsive regions in both NR gene promoters were identified.
Recently, we observed unexpectedly high ClO3− sensitivity/NR activity in cultures initiated from Nia2-deficient Arabidopsis seedlings grown on high-concentration cytokinin medium. Increased in vivo NR activity was also detected when cytokinin was applied directly onto the leaves of soil-grown seedlings. As shown here, the induction of NR activity could only be explained by a 10-fold elevation in Nia1 gene expression. An increased contribution of Nia1 to the total NR activity was also observed in wild-type plants grown on high-cytokinin medium. These results indicated that the two NR genes are differentially regulated by cytokinin. The increased NR activity was accompanied by a simultaneous and specific increase in Nia1 transcript level under standard culture conditions (light, Suc, and NO3−). Nuclear run-on assays demonstrated that this Nia1 induction correlated with an increase in Nia1 transcription.
MATERIALS AND METHODS
Plant Material and Growth Conditions
G5, a γ-irradiation-induced Nia2-deletion mutant of Arabidopsis thaliana (L.) Heynh ecotype Columbia, and G′4–3, a Nia1/Nia2 double mutant derived from G5, were kindly provided by Dr. N.M. Crawford (Wilkinson and Crawford, 1991, 1993). Arabidopsis seed-germination and tissue-culture conditions were described previously (Czakó et al., 1993). Plants were cultured on basal Murashige-Skoog medium containing Murashige-Skoog salts, 3% Suc, 0.7% agar, 100 mg L−1 myo-inositol, and 10 mg L−1 thiamine HCl (Márton and Browse, 1991).
NR Activity Assay
In vivo NR activity was assayed by the protocol of Márton et al. (1982b). NR activity was measured in a spectrophotometric assay by determining the amount of NO2− released from the tissue. Seedlings were weighed and transferred into 1 mL of reaction buffer (40 mm KNO3, 0.08 m Na2HPO4, 0.02 m NaH2PO4, and 4% [v/v] n-propanol, pH 7.5) and incubated in the dark for 2 h. The reaction was stopped by the addition of 200 μL of 1% sulfanilamide (dissolved in 3 n HCl) and 200 μL of 0.05% N-(1-napthyl)ethylenediamine hydrochloride. The concentration of NO2− was determined by the A540 of the solution. If the absorbance was higher than 0.5, the reaction solution was diluted 10-fold with reaction buffer and sulfanilamide.
The in vitro NR activity assay was adapted from the protocol of Samuelson and Larsson (1993). Arabidopsis seedlings (2 g fresh weight) were homogenized in 3 mL of extraction buffer (250 mm Tris, pH 8.0, 1 mm EDTA, 1 μm Na2MoO4, 5 μm FAD, 3 mm DTT, 1% [w/v] BSA, 12 mm β-mercaptoethanol, and 250 μm PMSF). The homogenate was centrifuged at 10,000g for 10 min. One-hundred-fifty microliters of the supernatant was added to 850 μL of reaction buffer (40 mm NaNO3, 0.08 m Na2HPO4, 0.02 m NaH2PO4, pH 7.5, and 0.2 mm NADH) and incubated at room temperature for 2 h. Sulfanilamide and N-(1-napthyl)ethylenediamine hydrochloride were added, and NO2− concentration was measured as an in vivo assay.
Gel-Blot Hybridization
RNA isolation was performed as described previously (Czakó et al., 1995). Northern hybridizations were performed according to standard protocols (Sambrook et al., 1989). A 3.1-kb XhoI fragment from plasmid pAt60 was used as the Nia2-specific probe (Wilkinson and Crawford, 1991). A 1.8-kb EcoRI-HindIII fragment from pALCO74 was used as the Nia1-specific probe (Wilkinson and Crawford, 1993). An 801-bp EcoRI fragment from pCG22 was the cyclophilin probe, which was used as an internal control (Lippuner et al., 1994). A 1-kb EcoRI/HindIII fragment of a cDNA clone pCDMC13 was used as the AtDMC1 probe (Klimyuk and Jones, 1997). The probes were labeled using the Megaprime DNA-labeling kit from Amersham. Zeta-probe GT-blotting membranes were obtained from Bio-Rad. PhosphorImager (Storm 860) and image-analyzing software (Image Quant) were obtained from Molecular Dynamics (Sunnyvale, CA).
Nuclei Isolation and Run-on Assay
We adapted a nuclei isolation and run-on assay protocol from Brusslan et al. (1993). Seven grams of 12-d-old seedling leaves, harvested on ice, was ground for 6 min using a mortar and pestle in 40 mL of ice-cold modified Honda buffer (0.44 m Suc, 25 mm Tris, pH 8.5, 10 mm MgCl2, 10 mm spermine, 2.5% [w/v] Ficoll 400, 4% dextran 40, 0.5% [v/v] Triton X-100, and 10 mm β-mercaptoethanol), and the homogenate was filtered through four layers of cheesecloth and one layer of Miracloth (Calbiochem). The filtrate was centrifuged at 4°C and 4500 rpm for 7 min, and the pellet was washed once with 5 mL of the same buffer minus spermine and once in a washing buffer containing 50 mm Tris, pH 8.5, 5 mm MgCl2, 10 mm β-mercaptoethanol, and 20% (v/v) glycerol. The pellet was then resuspended in 200 μL of the same washing buffer.
For in vitro transcription, 50 μL of nuclei (5 × 107) was used in a 100-μL reaction with 100 mm (NH4)2SO4, 5 mm MgCl2, 500 μm ATP, GTP, and CTP, 30 μm UTP, 20 μL of 111 TBq/mmol (200 μCi) [α-32P]UTP (ICN), 0.1 mm phosphocreatine, 1 mg of phosphocreatine kinase, and 80 units of RNasin (Promega). The mixture was incubated at 30°C for 30 min. The reaction was stopped by the addition of equal volumes of run-on stop buffer (2% SDS, 10 mm EDTA, 20 mm Tris, pH 7.4, and 200 μg/mL proteinase K), incubated at 42°C for 30 min, and purified by phenol-chloroform; 100 μg of yeast tRNA was added before ethanol precipitation. The RNA pellet was resuspended in 100 μL of H2O and hybridized to DNA blots. The blots were prepared by digestion of 10 μg of plasmid DNAs, separation on 0.9% agarose gels, and transfer to Zeta-probe nylon membranes by capillary transfer.
RESULTS AND DISCUSSION
The Induction of NR Activity by Cytokinin
In previous experiments we found that the NR activity was several times higher in Arabidopsis tissue cultures grown on high-concentration cytokinin-containing (regeneration) medium than in plantlets grown on hormone-free medium (data not shown). To investigate cytokinin induction of NR activity, seedlings of three A. thaliana ecotypes, Columbia, RLD, and Landsberg erecta, were tested for NR activity on Murashige-Skoog basal medium that provided an inductive amount of NO3−. Three hormone combinations were applied: 1 mg L−1 BA, 1 mg L−1 BA plus 0.1 mg L−1 NAA, and 0.1 mg L−1 NAA. The seedlings were subjected to two types of treatments. In treatment 1, seeds were germinated directly on hormone-containing medium. In treatment 2, 5-d-old seedlings germinated on hormone-free medium were transferred onto hormone-containing or hormone-free medium. The in vivo NR activity was measured 5 and 12 d after hormone treatment.
The induction of NR activity correlated with cytokinin treatment in all three wild-type ecotypes (Table I). NR activity in RLD and Landsberg erecta ecotypes was induced 3- to 14-fold on 1 mg L−1 BA-containing medium. This extent of induction was observed after 5 and 12 d in the two hormone treatments (treatments 1 and 2). The induction of NR activity in the Columbia ecotype was less dramatic than the other two ecotypes in this experiment, but all of the BA-treated seedlings showed higher NR activity. Treatment with 1 mg L−1 BA plus 0.1 mg L−1 NAA resulted in approximately the same increase in NR activity as BA treatment alone. There were no reports about auxin effects on NR activity in previous literature. In our experiments we observed a transient and moderate increase in NR activity on 0.1 mg L−1 NAA medium. This increase was seen only in seedlings treated with NAA for 5 d; it completely disappeared after 12 d, and the magnitude of increase was significantly less than that for BA-treated seedlings (an average 2.2-fold increase with NAA versus 5.6-fold with BA, Table I).
Table I.
In vivo NR activity from hormone-treated wild-type seedlings
| Medium | NR Activity
|
|||
|---|---|---|---|---|
| Treatment
1
|
Treatment 2
|
|||
| 5 d | 12 d | 5 d | 12 d | |
| nm NO2− mg−1 seedlings | ||||
| Columbia wild type | ||||
| φa | 610 ± 167 | 1608 ± 242 | 1785 ± 271 | 1025 ± 232 |
| NAAb | 928 ± 290 | 1485 ± 477 | 1950 ± 155 | 813 ± 135 |
| BAc | 1700 ± 387 | 1903 ± 290 | 3304 ± 382 | 2438 ± 341 |
| BA + NAAd | 1904 ± 616 | 2194 ± 660 | 3482 ± 257 | 3150 ± 355 |
| RLD wild type | ||||
| φ | 422 ± 132 | 905 ± 233 | 896 ± 187 | 855 ± 166 |
| NAA | 1182 ± 380 | 1519 ± 240 | 2023 ± 167 | 503 ± 67 |
| BA | 1470 ± 345 | 5501 ± 787 | 4478 ± 800 | 4991 ± 788 |
| BA + NAA | 5993 ± 1426 | 4012 ± 665 | 5799 ± 986 | 3290 ± 896 |
| Landsberg erecta | ||||
| φ | 498 ± 86 | 927 ± 128 | 1102 ± 290 | 523 ± 90 |
| NAA | 1919 ± 321 | 861 ± 120 | 1722 ± 251 | 442 ± 104 |
| BA | 3027 ± 689 | 4054 ± 585 | 8213 ± 1029 | 4168 ± 824 |
| BA + NAA | 5110 ± 819 | 3318 ± 373 | 5566 ± 1336 | 4083 ± 1034 |
The average NR activity of five seedlings from each treatment are expressed as means ± se (n = 5).
φ, Murashige-Skoog basal medium without any phytohormone.
NAA, Murashige-Skoog basal medium plus 0.1 mg L−1 NAA.
BA, Contained 1 mg L−1 BA.
BA + NAA, Contained 1 mg L−1 BA plus 0.1 mg L−1 NAA.
To distinguish the response of each NR gene to cytokinin, mutants carrying a major deletion in the Nia2 gene were subjected to the same hormone treatment as described for the wild type. G5 in the A. thaliana Columbia ecotype (Wilkinson and Crawford, 1991) has a deletion of at least 25 kb at the chl3–5 locus, including the entire Nia2 gene. The other mutant, G′4–3, is a double mutant derived from G5 (Wilkinson and Crawford, 1993). In addition to the deletion at the chl3–5 locus, G′4–3 also carries a point mutation in the coding region of Nia1, rendering its NR activity 10% that of the G5 mutant. This residual NR activity (about 1% of the wild type) is sufficient to allow some growth in soil. On NH4+/NO3−-based synthetic medium in sterile cultures, the G5 and G′4–3 mutants showed about 44 and 22% of the wild-type NR activity, respectively (Tables I and II), indicating that the contribution of Nia1 gene to NR activity is much higher than that reported for soil-grown seedlings (Wilkinson and Crawford, 1993).
Table II.
NR activity from hormone-treated mutant seedlings
| Mediuma | NR Activity
|
|||
|---|---|---|---|---|
| Treatment 1
|
Treatment 2
|
|||
| 5 d | 12 d | 5 d | 12 d | |
| nm NO2− mg−1 seedlings | ||||
| G5 mutant | ||||
| φ | 270 ± 38 | 589 ± 214 | 380 ± 54 | 366 ± 62 |
| NAA | 274 ± 87 | 669 ± 193 | 530 ± 50 | 377 ± 55 |
| BA | 1179 ± 301 | 1526 ± 360 | 1629 ± 242 | 1652 ± 244 |
| BA + NAA | 1137 ± 296 | 1682 ± 105 | 1790 ± 440 | 2091 ± 433 |
| G′ 4–3 mutant | ||||
| φ | 136 ± 49 | 395 ± 72 | 293 ± 59 | 273 ± 26 |
| NAA | 145 ± 30 | 506 ± 78 | 781 ± 115 | 531 ± 31 |
| BA | 994 ± 277 | 2518 ± 499 | 2067 ± 112 | 1768 ± 135 |
| BA + NAA | 855 ± 182 | 1626 ± 295 | 1735 ± 191 | 1925 ± 301 |
The average NR activity of five seedlings from each treatment are expressed as means ± se (n = 5).
See Table I for explanation of media.
NR activity in both mutants was induced more than 2.6-fold by BA and BA plus NAA treatment in seeds germinated directly on hormone-containing medium or in seedlings transferred to hormone medium after germination (Table II). In G5, all of the seedlings transferred to BA medium and the 5-d-old seedlings germinated directly on BA medium had a more than 4-fold increase in NR activity. The G′4–3 double mutant showed even higher induction by cytokinin, with seven of eight different treatments (Table II, 5 and 12 d, treatments 1 and 2, BA and BA plus NAA) and exhibited a more than 6-fold increase in NR activity. Again, this induction was not observed on medium containing NAA only. Thus, the effect of cytokinin on induction of Nia1 activity is clear.
Additional experiments demonstrated that in vitro NR activity was also increased similarly by cytokinin treatment to the above in vivo NR activity. Twelve-day-old Columbia wild-type seedlings showed a 2.3-fold increase in in vitro NR activity when treated with 1 mg L−1 BA (460.5 ± 26.5 versus 1066.5 ± 49.5 nm NO2− h−1 mg−1 tissue in BA-treated seedlings; 16.4 μg of proteins were extracted out of 1 mg of tissue). The G5 mutant showed a 4.5-fold increase in in vitro NR activity following the same treatment (117.1 ± 4.5 versus 523.2 ± 76.2 nm NO2− h−1 mg−1 tissue in BA-treated seedlings). The in vitro results suggest that any indirect effects on NR activity, such as cofactor availability or changes in overall posttranscriptional regulation, do not account significantly for the increased NR activities.
We also examined the NR activity of soil-grown seedlings with and without cytokinin treatment. About 100 Arabidopsis seedlings grown in a 4-inch (diameter) pot for 10 d were sprayed with 2 mL of a 1 mg L−1 BA solution. Both the Columbia wild type and the G5 mutant demonstrated higher NR activity when assayed after 48 h. The in vivo NR activity was 1.7-fold higher in BA-treated Columbia seedlings (98.1 ± 14.5 versus 166.6 ± 61.0 nm NO2− mg−1) and 4.5-fold higher in BA-treated G5 seedlings (20.2 ± 17.2 versus 91.2 ± 37.6 nm NO2− mg−1). This result shows that cytokinin induction occurs not only under sterile culture conditions but also in soil-grown seedlings given an external application of cytokinin.
When the seedlings were germinated on different concentrations of BA medium, we observed that the minimum concentration of hormone needed to induce a significant increase of in vivo NR activity was 0.05 mg L−1 (1.3 × 10−7 m, P < 0.05). This result is comparable to previous reports using other plant species (Kende et al., 1971; Banowetz, 1992).
To estimate the contribution of the major NR gene Nia2 to the increased NR activity in cytokinin-treated wild-type plants, the NR activity of the Nia2-deletion mutant G5, which expresses only the Nia1 gene (Columbia background), was subtracted from the NR activity of the Columbia wild type of the same treatment. For example, in the 5-d-old seedlings germinated directly on 1 mg L−1 BA medium (Tables I and II, treatment 1), the total NR activity (Columbia) changed from 610 to 1700 nm NO2− mg−1 seedlings (up by 1090 nm NO2− mg−1 seedlings); the Nia1 activity (G5) changed from 270 to 1179 nm NO2− mg−1 seedlings (up by 909 nm NO2− mg−1 seedlings); therefore, the derived “Nia2 activity” changed from 340 to 519 nm NO2− mg−1 seedlings (up by 179 nm NO2− mg−1 seedlings). The above calculation suggested that the increased NR activity derives predominantly from the Nia1 gene and the contribution of the Nia1 gene to the total NR activity is much higher after cytokinin induction. However, this calculation may not accurately reflect the Nia2 activity in Columbia wild type because the G5 mutant may have different Nia1 expression, but these data prompted us to study Nia1 and Nia2 expression at the RNA level.
Increased Nia1 Steady-State RNA Level in Cytokinin-Treated Plants
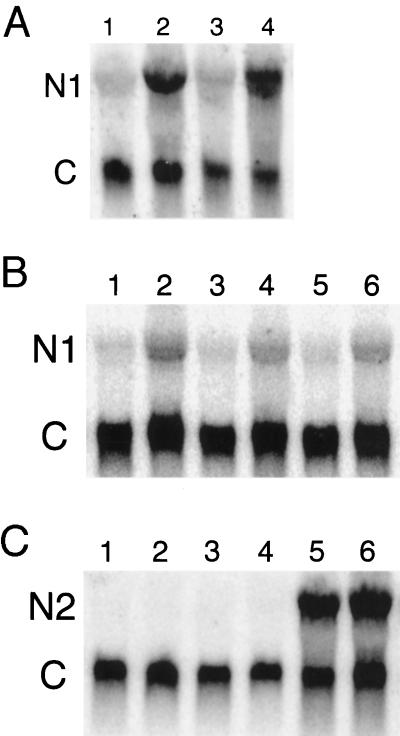
Total RNA was extracted from 12-d-old seedlings germinated directly on hormone-containing medium. Nia1 transcript levels were as much as 10-fold higher in both G5 and G′4–3 mutant seedlings treated with BA than in nontreated ones (Fig. 1A). Nia1 transcript levels also increased significantly in all three wild types (Fig. 1B) grown on BA medium. However, Nia2 RNA did not show any noticeable increase (Fig. 1C). Therefore, the increased NR activity in these plants may be due to an increased mRNA level of the Nia1 gene, not the Nia2 gene. However, the possible contribution of increased Nia1 and Nia2 mRNA stability to the high NR activity and high mRNA levels was not investigated in these experiments.
Figure 1.
Autoradiograms of an RNA gel blot from wild-type and mutant plants hybridized with Nia1- and Nia2-coding region-specific probes separately. N1, Nia1 transcript; N2, Nia2 transcript; and C, constitutive cyclophilin control. In A, lane 1 is the total RNA extracted from G′4–3 mutant seedlings without BA treatment; lane 2 is the RNA of G′4–3 seedlings treated with BA; lane 3 is the G5 mutant without BA treatment; and lane 4 is the G5 mutant with BA. In B, lanes 1, 3, and 5 are Columbia, RLD, and Landsberg erecta wild type, respectively, without BA treatment. Lanes 2, 4, and 6 were treated with BA. In C, lanes 1, 3, and 5 are G′4–3, G5, and Columbia wild-type seedlings without BA and lanes 2, 4, and 6 are seedlings with BA.
Increased Nia1 Transcription in Cytokinin-Treated Plants
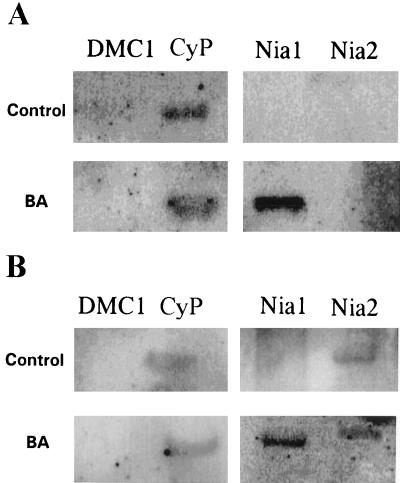
To examine the transcriptional regulation of Nia1 in cytokinin-induced plants, nuclei were isolated from seedlings germinated directly on medium with or without hormone, and nuclear run-on assays were performed. Nia1 transcripts were not detectable in mutant G5 seedlings without cytokinin treatment but were present at high levels in G5 seedlings treated with cytokinin (Fig. 2A). The positive internal control was cyclophilin, the expression of which is constant under various growing conditions and developmental stages (Lippuner et al., 1994). The meiosis-specific AtDMC1 gene is expressed only during gametogenesis in floral development and was used as a negative internal control (Klimyuk and Jones, 1997). Similarly, Nia1 transcription is also induced by cytokinin in Columbia wild type (Fig. 2B), whereas Nia2 transcription did not show any significant difference in the treated versus untreated seedlings (Fig. 2B). Previous work by others with corn and barley seedlings had suggested that the induction of NR activity by cytokinin was regulated at the level of transcription (Rao et al., 1984; Lu et al., 1992). Our results here show that this is true in Arabidopsis as well. However, this transcriptional regulation is due to a specific increase only in Nia1 transcription.
Figure 2.
Nuclear run-on assay of the G5 mutant (A) and Columbia wild type (B). DMC1, Meiosis-specific gene used as a negative control; and CyP, cyclophilin constitutive control.
In conclusion, total NR activity dramatically increased in Arabidopsis cultures containing cytokinin or a high-cytokinin/low-auxin hormone combination. The relative contribution of the Nia1 gene to the total NR activity increased significantly, indicating a strong cytokinin response of Nia1 but not Nia2. At the same time, the steady-state levels of the Nia1 mRNA increased significantly and specifically. Specific induction of NR activity by cytokinin is accompanied by increased transcription of the Nia1 gene in both mutant and wild-type plants, as judged from nuclear run-on assays. The level of Nia2 transcripts did not change as a result of cytokinin treatment. Since increased NR activity was observed during more than 2 weeks in culture, the role of cell division cannot be excluded. Epigenetic changes and/or higher Nia1 expression preferentially in the dividing adventitious meristem cell population could also explain the observed dramatic changes in total NR activity and in transcription of the Nia1 gene. Further investigations using a series of truncated promoter-reporter gene fusions and tissue printing to investigate the tissue-specific expression of Nia1, as well as epigenetic studies to search for the mechanism of the long-term effect of cytokinin on Nia1 expression, are under way.
ACKNOWLEDGMENTS
We thank Dr. J.Q. Wilkinson for plasmid pAt60, Dr. N.M. Crawford for pALCO74, Dr. C. Gasser for pCG22, and Dr. V. Klimyuk for pCDMC13. We also thank Drs. M. Czako and B. Krizek for critical reading of this manuscript.
Abbreviation:
- NR
nitrate reductase
Footnotes
This work was funded in part by the U.S. Department of Energy (grant no. DE-FG02-92ER20073) and the U.S. Department of Agriculture (grant no. 93-3705-9442).
LITERATURE CITED
- Banowetz GM. The effect of endogenous cytokinin content on benzyladenine enhanced nitrate reductase induction. Physiol Plant. 1992;86:341–348. [Google Scholar]
- Borriss H. Untersuchungen uber die Steuerung der Enzymaktivitat in pflanzlichen Embryonen durch Cytokinine. Wiss Z Univ Rostock Math-Naturwiss Reihe. 1967;16:629–639. [Google Scholar]
- Brusslan JA, Karlin-Neumann GA, Huang L, Tobin EM. An Arabidopsis mutant with a reduced level of cab140 RNA is a result of cosuppression. Plant Cell. 1993;5:667–677. doi: 10.1105/tpc.5.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CL, Acedo GN, Dewdney J, Goodman HM, Conlking MA. Differential expression of the two Arabidopsis nitrate reductase genes. Plant Physiol. 1991;96:275–279. doi: 10.1104/pp.96.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CL, Dewdney J, Kleinhofs A, Goodman HM. Cloning and nitrate induction of nitrate reductase. Proc Natl Acad Sci USA. 1986;83:6825–6828. doi: 10.1073/pnas.83.18.6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CL, Dewdney J, Nam HG, den-Boer BGW, Goodman HM. A new locus (NIA1) in Arabidopsis thaliana encoding nitrate reductase. EMBO J. 1988;7:3309–3314. doi: 10.1002/j.1460-2075.1988.tb03201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford NM. Nitrate: nutrient and signal for plant growth. Plant Cell. 1995;7:859–868. doi: 10.1105/tpc.7.7.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czakó M, Marathe RP, Xiang C, Guerra DJ, Bishop GJ, Jones JDG, Marton L. Variable expression of the herpes simplex virus thymidine kinase gene in Nicotiana tabacum affects negative selection. Theor Appl Genet. 1995;91:1242–1247. doi: 10.1007/BF00220935. [DOI] [PubMed] [Google Scholar]
- Czakó M, Wilson J, Yu X, Marton L. Sustained root culture for generation and vegetative propagation of transgenic Arabidopsis thaliana. Plant Cell Rep. 1993;12:603–609. doi: 10.1007/BF00232807. [DOI] [PubMed] [Google Scholar]
- Gaudinova A. Effect of cytokinin on nitrate reductase activity. In: Kutacek M, Elliot MC, Machackova M, editors. Molecular Aspects of Hormonal Regulation of Plant Development. The Hague, The Netherlands: SPB Academic Publishing; 1990. pp. 225–231. [Google Scholar]
- Kende H, Hahn H, Kays SE. Plant Physiol. 1971;48:702–706. doi: 10.1104/pp.48.6.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimyuk VI, Jones JDG. AtDMC1, the Arabidopsis homologue of the yeast DMC1 gene: characterization, transposon-induced allelic variation and meiosis-associated expression. Plant J. 1997;11:1–14. doi: 10.1046/j.1365-313x.1997.11010001.x. [DOI] [PubMed] [Google Scholar]
- Lin Y, Cheng CL. A chlorate-resistant mutant defective in the regulation of nitrate reductase gene expression in Arabidopsis defines a new HY locus. Plant Cell. 1997;9:21–35. doi: 10.1105/tpc.9.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippuner V, Chou IT, Varian-Scott S, Ettinger WF, Theg SM, Gasser CS. Cloning and characterization of chloroplast and cytosolic forms of cyclophilin from Arabidopsis thaliana. J Biol Chem. 1994;269:7863–7868. [PubMed] [Google Scholar]
- Lu J, Ertl JR, Chen C. Transcriptional regulation of nitrate reductase mRNA levels by cytokinin-abscisic acid interactions in etiolated barley leaves. Plant Physiol. 1992;98:1255–1260. doi: 10.1104/pp.98.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márton L, Browse J. Facile transformation of Arabidopsis. Plant Cell Rep. 1991;10:235–239. doi: 10.1007/BF00232565. [DOI] [PubMed] [Google Scholar]
- Márton L, Dung TM, Mendel RR, Maliga P. Nitrate reductase-deficient cell lines from haploid protoplast cultures of Nicotiana plumbaginifolia. Mol Gen Genet. 1982a;186:301–304. [Google Scholar]
- Márton L, Sidorov V, Biasini G, Maliga P. Complementation in somatic hybrids indicates four types of nitrate-reductase deficient lines in Nicotiana plumbaginifolia. Mol Gen Genet. 1982b;187:1–3. [Google Scholar]
- Pelsy F, Caboche M. Molecular genetics of nitrate reductase in higher plants. Adv Genet. 1992;30:1–40. [Google Scholar]
- Rao LVM, Datta N, Hahadevan M, Guha-Mukherjee S, Sopory SK. Influence of cytokinin and phytochrome on nitrate reductase activity in etiolated leaves of maize. Photochemistry. 1984;23:1875–1879. [Google Scholar]
- Redinbaugh MG, Campbell WH. Higher plant responses to environmental nitrate. Physiol Plant. 1991;82:640–650. [Google Scholar]
- Sambrook J, Frisch EF, Maniatis T. Molecular Cloning, a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Samuelson ME, Campbell WH, Larsson CM. The influence of cytokinins in nitrate regulation of nitrate reductase activity and expression in barley. Physiol Plant. 1995;93:533–539. [Google Scholar]
- Samuelson ME, Larsson CM. Nitrate regulation of zeatin riboside in barley roots: effects of inhibitors of N assimilation, and comparison with ammonium. Plant Sci. 1993;93:77–84. [Google Scholar]
- Suty L, Moureaux T, Leydecker MT, Teyssentier de la Serve B. Cytokinin affects nitrate reductase expression through the modulation of polyadenylation of the nitrate reductase mRNA transcript. Plant Sci. 1993;90:11–19. [Google Scholar]
- Wilkinson JQ, Crawford NM. Identification of the Arabidopsis CHL3 gene as the nitrate reductase structural gene Nia2. Plant Cell. 1991;3:461–471. doi: 10.1105/tpc.3.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson JQ, Crawford NM. Identification and characterization of a chlorate resistant mutant of Arabidopsis with mutations in both Nia1 and Nia2 nitrate reductase structural genes. Mol Gen Genet. 1993;239:289–297. doi: 10.1007/BF00281630. [DOI] [PubMed] [Google Scholar]