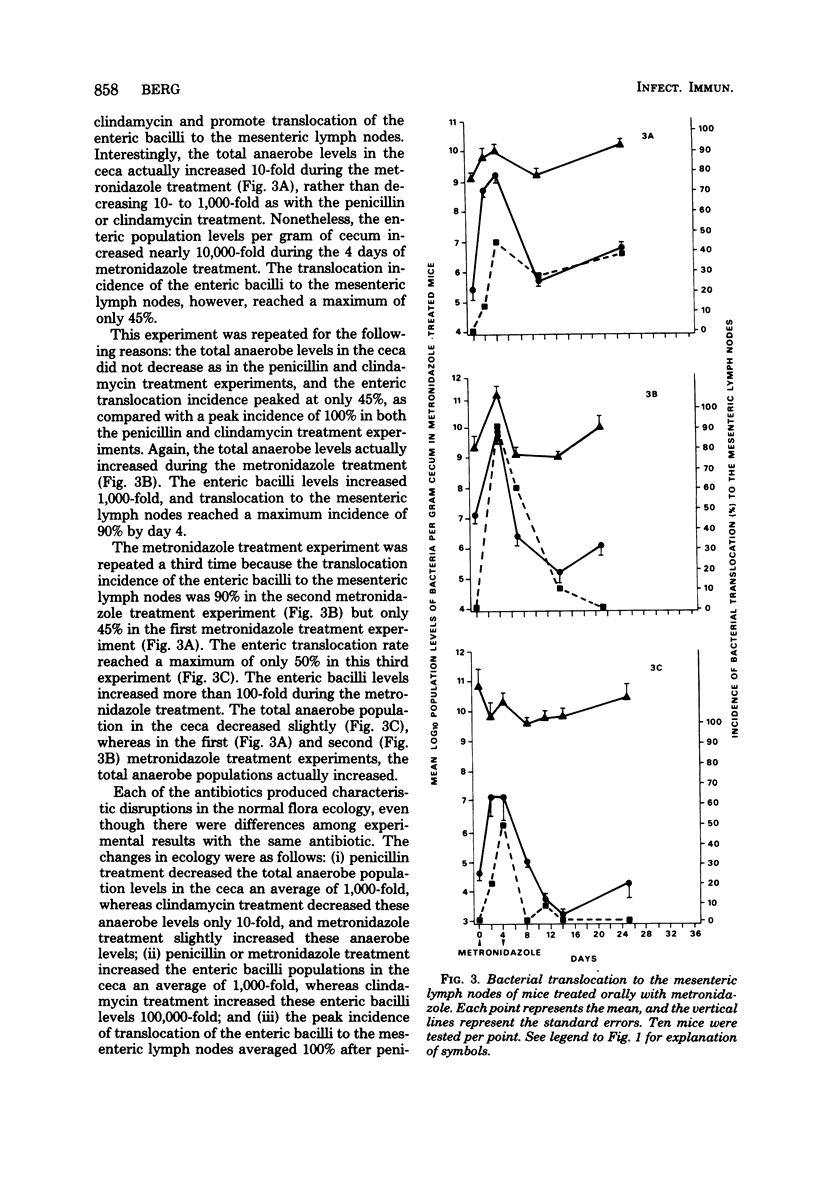
Abstract
Specific pathogen-free mice were treated orally with antibiotics to determine whether the resulting disruption of the normal flora ecology would allow certain gram-negative enteric bacteria to overpopulate the ceca, thereby promoting the translocation of these bacteria from the gastrointestinal tract. The mice were treated orally with penicillin, clindamycin, or metronidazole for 4 days, and then the antibiotic was discontinued. The mice were tested at various intervals for viable enteric bacilli translocating from the gastrointestinal tract to the mesenteric lymph nodes. Penicillin treatment decreased the total anaerobe population levels in the ceca an average of 1,000-fold, whereas clindamycin treatment decreased these anaerobe levels only 10-fold, and metronidazole treatment slightly increased the anaerobe levels. Penicillin or metronidazole treatment slightly increased the anaerobe levels. Penicillin or metronidazole treatment increased the enteric bacilli populations in the ceca an average of 1,000-fold, whereas clindamycin treatment increased the enteric bacilli populations 100,000-fold. The peak incidence of translocation of the enteric bacilli to the mesenteric lymph nodes averaged 100% after penicillin treatment, 97% after clindamycin treatment, and 62% after metronidazole treatment. Thus, oral treatment of mice with penicillin, clindamycin, or metronidazole for only 4 days disrupts the normal flora ecology, allowing an overgrowth in the ceca of the gram-negative enteric bacilli and promoting their translocation to the mesenteric lymph nodes.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aranki A., Freter R. Use of anaerobic glove boxes for the cultivation of strictly anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1329–1334. doi: 10.1093/ajcn/25.12.1329. [DOI] [PubMed] [Google Scholar]
- Baines E. J. Metronidazole: its past, present and future. J Antimicrob Chemother. 1978 Sep;4 (Suppl 100):97–111. doi: 10.1093/jac/4.suppl_c.97. [DOI] [PubMed] [Google Scholar]
- Berg R. D. Antagonism among the normal anaerobic bacteria of the mouse gastrointestinal tract determined by immunofluorescence. Appl Environ Microbiol. 1978 Jun;35(6):1066–1073. doi: 10.1128/aem.35.6.1066-1073.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D., Garlington A. W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979 Feb;23(2):403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D. Inhibition of Escherichia coli translocation from the gastrointestinal tract by normal cecal flora in gnotobiotic or antibiotic-decontaminated mice. Infect Immun. 1980 Sep;29(3):1073–1081. doi: 10.1128/iai.29.3.1073-1081.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. D. Mechanisms confining indigenous bacteria to the gastrointestinal tract. Am J Clin Nutr. 1980 Nov;33(11 Suppl):2472–2484. doi: 10.1093/ajcn/33.11.2472. [DOI] [PubMed] [Google Scholar]
- Berg R. D., Owens W. E. Inhibition of translocation of viable Escherichia coli from the gastrointestinal tract of mice by bacterial antagonism. Infect Immun. 1979 Sep;25(3):820–827. doi: 10.1128/iai.25.3.820-827.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden R. N., Heel R. C., Speight T. M., Avery G. S. Metronidazole in anaerobic infections: a review of its activity, pharmacokinetics and therapeutic use. Drugs. 1978 Nov;16(5):387–417. doi: 10.2165/00003495-197816050-00002. [DOI] [PubMed] [Google Scholar]
- Freter R., Abrams G. D. Function of various intestinal bacteria in converting germfree mice to the normal state. Infect Immun. 1972 Aug;6(2):119–126. doi: 10.1128/iai.6.2.119-126.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERSH E. M., BODEY G. P., NIES B. A., FREIREICH E. J. CAUSES OF DEATH IN ACUTE LEUKEMIA: A TEN-YEAR STUDY OF 414 PATIENTS FROM 1954-1963. JAMA. 1965 Jul 12;193:105–109. doi: 10.1001/jama.1965.03090020019005. [DOI] [PubMed] [Google Scholar]
- Hartley C. L., Clements H. M., Linton K. B. Effects of cephalexin, erythromycin and clindamycin on the aerobic Gram-negative faecal flora in man. J Med Microbiol. 1978 May;11(2):125–135. doi: 10.1099/00222615-11-2-125. [DOI] [PubMed] [Google Scholar]
- Lee A., Gemmell E. Changes in the mouse intestinal microflora during weaning: role of volatile fatty acids. Infect Immun. 1972 Jan;5(1):1–7. doi: 10.1128/iai.5.1.1-7.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh D. A., Simmons K. Effect of clindamycin and lincomycin therapy on faecal flora. J Clin Pathol. 1978 May;31(5):439–443. doi: 10.1136/jcp.31.5.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee R. F., Jr, Smith C. B., Wilcox C., Finland M. Comparative studies of antibacterial activity in vitro and absorption and excretion of lincomycin and clinimycin. Am J Med Sci. 1968 Nov;256(5):279–292. doi: 10.1097/00000441-196811000-00002. [DOI] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R. J. The fecal flora of various strains of mice. Its bearing on their susceptibility to endotoxin. J Exp Med. 1962 Jun 1;115:1149–1160. doi: 10.1084/jem.115.6.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., Dubos R. Alterations in the mouse cecum and its flora produced by antibacterial drugs. J Exp Med. 1968 Jul 1;128(1):97–110. doi: 10.1084/jem.128.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Waaij D., Berghuis-de Vries J. M., Lekkerkerk-van der Wees Colonization resistance of the digestive tract and the spread of bacteria to the lymphatic organs in mice. J Hyg (Lond) 1972 Jun;70(2):335–342. doi: 10.1017/s0022172400022385. [DOI] [PMC free article] [PubMed] [Google Scholar]


