Abstract
Background
People living with HIV (PLWH) who have positive tuberculin skin tests (TST) benefit from isoniazid preventive therapy (IPT) whereas those testing TST-negative do not. Revised World Health Organization guidelines explicitly state that assessment of TST is not a requirement for initiation of IPT. However, it is not known what proportions of patients will benefit from IPT if implemented without targeting according to TST status. We therefore determined the proportions of PLWH who test TST-positive.
Methodology/Principal Findings
We systematically reviewed the literature published between January 1990 and February 2012 to determine the proportions of patients without active tuberculosis attending HIV care services in low and middle-income countries who tested TST-positive (≥5 mm induration). Proportions were also determined for different CD4 count strata. Data from 19 studies with 9,478 PLWH from sub-Saharan Africa, Asia and Central and South America were summarized. The vast majority were not receiving antiretroviral therapy (ART). A sub-analysis was conducted of 5 studies (5,567 subjects) from high TB prevalence countries of PLWH with negative TB screens attending HIV care and treatment settings for whom CD4 stratified data were available. The median proportion of PLWH testing TST-positive overall was 22.8% (range, 19.5–32.6%). The median (range) proportions with CD4 cell counts of <200, 200–499 or ≥500 cells/µL who tested positive were 12.4% (8.2–15.3%), 28.4% (20.1–36.9%) and 37.4% (31.3–56.3%), respectively. Heterogeneity in the data precluded calculation of pooled summary estimates.
Conclusions/Significance
In most settings, if IPT is administered to PLWH pre-ART without assessment of TST status, only a minority of those treated are likely to benefit, especially among those with the lowest CD4 cell counts. This may be inefficient use of resources and cost-effectiveness analyses should take this into account. Local knowledge of TST response rates may help inform policies. New simple means of identifying those who will benefit from IPT are needed to permit appropriate targeting of this intervention.
Introduction
HIV-associated tuberculosis (TB) remains a substantial challenge to global health [1], accounting for an estimated 12% (1.1 million) of the overall TB caseload and one quarter (0.35 million) of HIV/AIDS deaths worldwide [2]. Four main programme interventions are recommended to prevent HIV-associated TB [3]. These include scale-up of antiretroviral therapy (ART) [4] used in combination with intensified case finding, isoniazid preventive therapy [IPT] and infection control, the latter interventions being referred to by the acronym the ‘three I’s strategy’ [5]. However, a large majority of cases are in sub-Saharan Africa where the capacity to deliver the needed control interventions is limited [6]. For many years, implementation of IPT in particular has been very poor [7].
A meta-analysis of placebo-controlled trials reported that IPT in people living with HIV (PLWH) conferred an overall risk reduction of 33% (95%CI, 13–49%) [8]. A beneficial effect was only observed among those who tested tuberculin skin test (TST) positive in whom there was a substantially greater risk reduction of 64% (95%CI, 39–78%). In contrast, no benefit was observed among PLWH (n = 2,490) in these trials with negative or anergic TST reactions [8]. Based on this evidence, WHO guidelines between 1998 and 2010 emphasized the main use of IPT as being for prophylaxis of those who are TST-positive [9].
With low uptake of IPT and recognition that the process of TST assessment was a major programmatic stumbling block, the 2011 revision of the WHO IPT guidelines made a strong explicit recommendation that “TST is not a requirement for initiating IPT in people living with HIV” although it “can be used where feasible” [10]. Eliminating the need for assessment of TST status has simplified implementation substantially. However, this means that the intervention is no longer targeted at those who will derive benefit and a small proportion of PLWH who are unlikely to derive benefit might be harmed. The cost-effectiveness of IPT with or without use of TST assessment in a clinical population is likely to be strongly associated with the prevalence of positive TST tests. We therefore conducted this systematic review.
The proportion of PLWH testing TST-positive is likely to be associated with the prevalence of TB in the local community, the prevailing force of infection, social mixing patterns and the clinical population sampled. Prevalence also increases with age [11] but may be attenuated in those with suppression of cell-mediated immune responses. Progressive CD4 cell count loss that characterizes HIV progression is strongly associated with increasing proportions of anergic TST responses [12]. We therefore conducted this systematic literature review and meta-analysis to determine the proportion of PLWH in HIV care and treatment settings who test TST positive and how this proportion varies across a range of CD4 cell count strata.
Methods
Systematic Search Strategy
We conducted the search and review process according to a predefined protocol and the study conformed to the PRISMA statement checklist [13]. Embase, Global Health, Medline and Web of Science were searched for potentially relevant citations using a defined search strategy (Table S1). The search included articles published in English between January 1st 1990 and February 5th 2012, covering the majority of the epidemic [14]. Initial searches found no relevant data published before this period. The bibliographies of review articles on interferon-γ-release assays were specifically searched for additional articles. Abstracts from the World Conference on Lung Health of the International Union Against Tuberculosis and Lung Disease (IUATLD) published between 2004 and 2011 were searched by hand for additional relevant citations.
Citations identified through the search process were compiled into Endnote X4 and duplicates removed. Titles and abstracts were screened for eligibility according to pre-defined inclusion and exclusion criteria and full-texts were reviewed for eligibility by ADK and SDL. The authors of eligible studies were contacted as required to clarify or modify the stratification of their data by CD4 cell count.
A study was eligible for inclusion if it was conducted in a low-income or middle-income country (as defined by the World Bank list of economies current on 1st July 2011) [15]; the study subjects were PLWH aged 15 years or older; the study reported TST results (positive if ≥5 mm induration) for individuals stratified into at least two CD4 cell count strata (CD4 cell counts <200 and ≥200 cells/µL); data were reported on PLWH without evidence of current active TB disease, and if the study contained 50 or more subjects meeting the above criteria. If two eligible studies presented overlapping samples, only the study with a larger sample size was included. Studies using a two-step TST-testing were excluded as this methodology is not used in public health programmes in resource-limited settings.
Data from eligible studies were abstracted directly into a structured Microsoft Excel spreadsheet. Study characteristics recorded included title, authors, year of publication, study period, study location, mean or median patient age, bacille Calmette et Guerin (BCG) scar status, clinical setting, ART status, TST methodology, inclusion of special populations or sub-groups and the total number of PLWH with TST-positive and TST-negative results stratified by CD4 counts (either using <200 and ≥200 cells/µL or using <200, 200–499 and ≥500 cells/µL).
Sub-analysis of Studies
For inclusion in a sub-analysis and potential meta-analysis, we selected those studies in high TB prevalence settings which were of sufficient size, were conducted among PLWH accessing HIV care and treatment services and in whom active TB had been excluded. This selection reflects the PLWH who are specified in WHO guidelines as being the key targets for IPT [10]. High TB prevalence was defined as an average ≥100 cases per 100,000 population using WHO estimates for the five-year period spanning the study mid-point [7]. A minimum of 40 PLWH were required in each CD4 cell count stratum to limit imprecision around estimates (anticipated range, 10%–50% testing TST-positive). Studies evaluating TB diagnostic assays or conducted in TB clinics were excluded due to the likelihood for selection bias during enrolment.
Two reviewers (ADK and SDL) assessed the quality of studies using a graded checklist (Table S2). A study was scored “good quality” if it received 70% or more of available points, “medium quality” if it received between 50–69% and “lower quality” if less than 50%. The primary outcome of interest for our study was the proportion of PLWH who tested TST-positive among those in each of the following CD4 cell count strata: <200, 200–499 and ≥500 cells/µL. Risk of bias in individual studies and across studies was also assessed.
Data-analysis
All statistical analyses were conducted using STATA 10. The proportions of PLWH testing TST-positive were calculated with corresponding 95% confidence intervals (95% CI). These data were stratified by geographic region, country TB prevalence (<100, 100–249 or ≥250 cases per 100,000 population) and CD4 cell count (<200 and ≥200 cells/µL or <200, 200–499 and ≥500 cells/µL). Findings were presented using forest plots. The median and corresponding range of proportions of patients testing TST-positive were calculated. I-squared statistics [16] were calculated to assess the heterogeneity of the data stratified by CD4 cell count.
Results
Studies Included
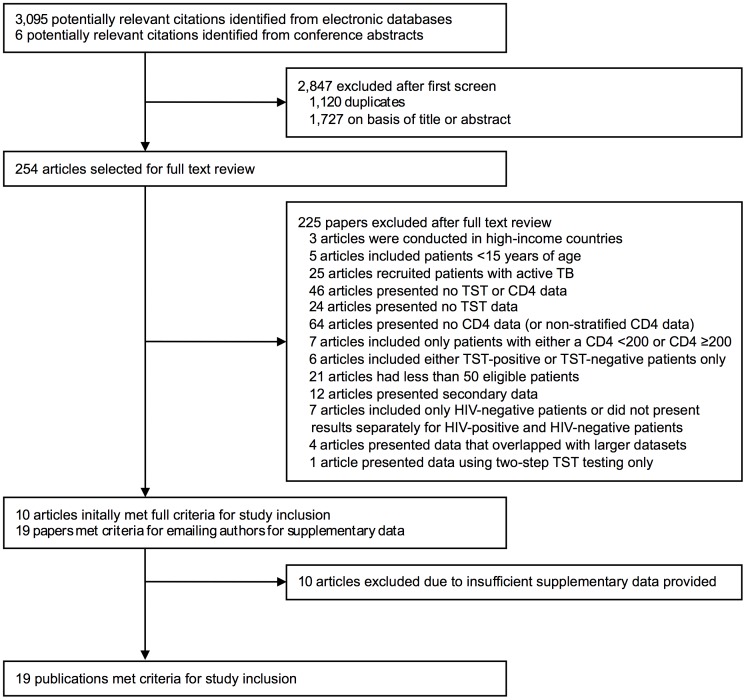
Of 3,095 potentially relevant citations identified, 254 were selected for full-text review (Figure 1 ). Ten publications initially met inclusion criteria and nine others met inclusion criteria after supplementary data were provided by study authors. Ten others remained ineligible despite seeking supplementary data (Table S3). The final group of eligible publications (n = 19) were published between 1997 and 2011.
Figure 1. Selection of eligible studies containing data on the proportion of people living with HIV testing tuberculin skin test (TST)-positive stratified by blood CD4 cell count.
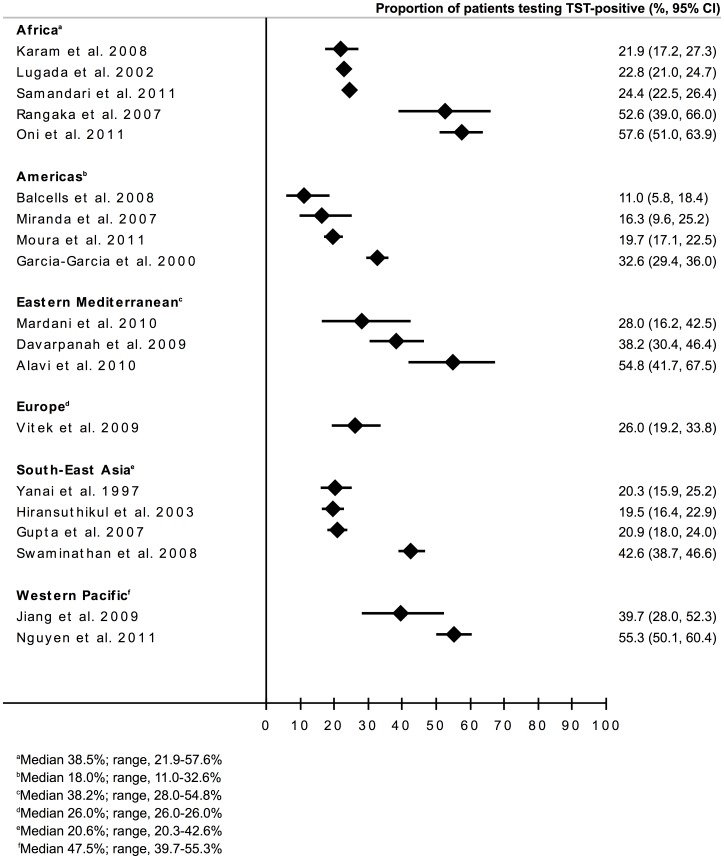
Of the studies included (n = 19), five were from sub-Saharan Africa, four from South-East Asia, four from the Americas, three from the Eastern Mediterranean two from the Western Pacific and one from Europe (Table 1). A majority (n = 14; 74%) were conducted in high-prevalence TB countries (estimated TB prevalence ≥100 cases per 100,000 population). The mean or median age of study subjects ranged between 22 and 40 years, but overall 90% of patients were from studies in which the mean or median age was within the range 29–40 years. The methods used for excluding active TB were reported by 13 (68%) studies but were not standardised. All studies defined TST positivity using a cut-off of ≥5 mm diameter of induration. Study quality was assessed as high in six studies, medium quality in eight and lower quality in five. Risks of bias in reporting the outcome of interest or publication bias were considered to be low.
Table 1. Characteristics of studies presenting data on the proportion of people living with HIV who tested TST-positive stratified by CD4 cell counts.
| Study | Country | Study years | Five year average TB prevalence rate (per 100,000) | Study setting | Eligible patients (N) | Mean age (years) | Study population | Special populations | Methods used to exclude active TB among study subjects | TST methods | Patients on ART at enrolment (%) | Patients with at least one BCG scar (%) | Included in meta-analysis |
| AFRICA | |||||||||||||
| Lugada et al (2002) [44] | Uganda (Entebbe) | 1995–1999 | 227.0 | Semi-urban | 2001 | 32.3 | Individuals living within 15 km of community HIV care clinics | No WHO stage 4 patients were included | Symptom screen, clinical examination, chest X-ray | 5-TU PPD | 0 | 50.7 | Yes |
| Rangaka et al (2007) [45] | South Africa (Khayelitsha) | … | 654.5 | Urban | 57 | 31 | Individuals attending an integrated HIV-TB clinic for HIV testing | … | Symptom screen, clinical examination, Karnofsky score <60 | 2-TU PPD RT23 | 0 | 51.0 | No |
| Karam et al (2008) [46] | Senegal (Dakar) | 2003–2005 | 462.0 | Urban | 274 | 37* | Individuals attending an infectious disease department or ambulatory care centre of a hospital | Only patients with an HIV diagnosis in the previous three months | Clinical examination, chest X-ray or microbiological evidence or Karnofsky score <80 | 2-TU PPD RT23 and read after 48–72 hours | 0 | 72.6 | Yes |
| Oni et al (2011) [47] | South Africa (Khayelitsha) | 2008–2010 | 785.0 | Urban | 238 | 30* | Individuals attending an integrated HIV-TB clinic pre ART initiation | … | Symptom screen, chest X-ray (if TST+) and sputum smear and culture | 2-TU PPD RT23 and read after 48–72 hours | 0 | 54.0 | No |
| Samandari et al (2011) [21] | Botswana (Gaborone and Francistown) | 2004–2009 | 750.0 | Urban | 1891 | 35 | Individuals attending government clinics providing ART and IPT services | … | Symptom screen and chest X-ray | 5-TU PPD RT23 and read after 48–72 hours | 2.0 | 78.0 | Yes |
| AMERICAS | |||||||||||||
| Garcia-Garcia et al (2000) [48] | Mexico (Mexico City) | 1992–1993 | 123.0 | Urban | 801 | 31.0 | Individuals requesting an HIV test at an HIV testing centre | 72.9% of patients were MSM and 1.1% were IDU | Clinical screen, chest X-ray, sputum smear and culture | 5-TU PPD RT23 and read after 48–72 hours | 12.4 | 81.5 | Yes |
| Miranda et al (2007) [49] | Brazil (7 states) | 1995–2001 | 104.0 | … | 98 | 34* | Individuals attending a public HIV treatment facility | 59% of patients were MSM, 13% had a history IDU and 7% had a history of incarceration | Methods unclear | … | 84.0 | … | No |
| Balcells et al (2008) [50] | Chile (Santiago) | 2006–2007 | 16.5 | Urban | 109 | 38.8* | Individuals attending HIV outpatient clinics | Only patients with CD4 count >100 were included | Symptom screen | 2-TU PPD RT23 and read after 48–72 hours | 57.8 | 83.5 | No |
| Moura et al (2011) [51] | Brazil (Recife) | 2007–2010 | 59.0 | Urban | 864 | 40.4 | Individuals in outpatient hospital services that serve as HIV/AIDS referral services | … | … | 0.1 ml PPD RT23 and read after 72 hours | 77.7 | … | No |
| EASTERN MEDITERRANEAN | |||||||||||||
| Davarpanah et al (2009) [52] | Iran (Shiraz) | 2008 | 36.0 | Urban | 152 | 38* | Individuals attending an infectious disease clinic | … | … | 5-TU PPD and read after 48–72 hours | … | … | No |
| Alavi et al (2010) [53] | Iran (Ahvaz) | 2008 | 36.0 | Urban | 62 | 30.5 and 34.3 | Randomly selected individuals in prison and attending drug addiction centres | Only drug addicted individuals were included | Methods unclear | 0.1 ml 5-TU PPD (Razi Institute) and read after 48–72 hours | … | … | No |
| Mardani et al (2010) [54] | Iran (Tehran) | 2007 | 37.7 | Urban | 50 | 39 | Individuals attending an HIV clinic | … | Methods unclear | 0.1 ml PPD and read after 48 hours | 24.0 | 100 | No |
| EUROPE | |||||||||||||
| Vitek et al (2009) [55] | Russia (Orel Oblast) | 2004 | 178.0 | … | 150 | 28 | Individuals adherent to previous follow-up visits at an AIDS centre | 61% of patients were IDU and 17.3% had a history of imprisonment | … | 2-TU Russian tuberculin (Research Institute of Vaccines and Antitoxins) and read after 48–72 hours | … | 78.0 | No |
| SOUTH-EAST ASIA | |||||||||||||
| Yanai et al (1997) [56] | Thailand (Chiang Rai) | 1992–1994 | 208 | Urban | 85 | 28.7 and 22.3 | Individuals attending a blood bank and female sex workers attending STD clinic | 62.7% of patients were blood donors and were 37.3% female sex workers | … | 5-TU Tubersol PPD (Connaught Laboratories) and read after 48–72 hours, but up to 5 days | … | 72.4 and 62.0 | No |
| Hiransuthikul et al (2003) [57] | Thailand (Bangkok) | 1994–1996 | 201.0 | Urban | 160 | 29* | Individuals attending an AIDS clinic | Only asymptomatic and early symptomatic patients were included. 37.5% of patients were homosexual or bisexual and 19.3% were commercial sex workers | Symptom screen, chest X-ray and sputum microscopy or Karnofsky score <70 | 5-TU PPD-S and read after 48–72 hours | … | … | Yes |
| Gupta et al (2007) [58] | India (Pune) | 2002–2005 | 284.0 | Urban | 752 | 23* | Pregnant women attending a public hospital | Only pregnant women were included | Symptom screening: if positive, chest x-ray, sputum smear and culture | 5-TU PPD and read after 48–72 hours | … | … | No |
| Swaminathan et al (2008) [59] | India (Chennai and Madurai) | 2000–2005 | 248.0 | Urban | 158 | 31 | Individuals attending government-funded TB clinics | … | Symptom screen, chest X-ray and 3 sputum cultures | 1-TU PPD RT23 and read after 48–72 hours | … | 48.3 | No |
| WESTERN PACIFIC | |||||||||||||
| Jiang et al (2009) [60] | China (Yunnan Province) | … | 146.7 | … | 68 | 33.9 and 33.7 | Individuals from within a province | … | Chest X-ray, sputum microscopy and sputum culture | 5-TU PPD RT23 and read after 48–72 hours | 8.8 | 100 | No |
| Nguyen et al (2011) [61] | Viet Nam (Ho Chi Minh City) | 2009–2010 | 334.4 | Urban | 369 | 30* | Individuals attending a public clinic that offers HIV services | 62.9% of patients were IDU, 15.5% had a history of incarceration, 44.2% have a history of TB | Symptom screen, chest X-ray, sputum smear and culture | 5-TU PPD and read after 48–72 hours | 58.3 | 55.6 | No |
Denotes median age.
“…” denotes information not stated.
TU = tuberculin units; PPD = purified protein derivative; STD = sexually transmitted disease; MSM = men who have sex with men;
IDU = people who inject drugs; ART = antiretroviral therapy; IPT = isoniazid preventive therapy.
The proportions of PLWH receiving ART at the time of TST assessment was reported by ten studies and two other studies pre-dated national ART implementation and these were therefore assumed to be ART-naïve populations. Among these twelve studies, the proportion of patients receiving ART ranged from 0 to 84% with a median of 5.4% (IQR, 0–32.5%).
The studies included a total of 9,478 subjects and 2,820 (29.8%) of these had CD4 cell counts <200 cells/µL and 6,658 (70.3%) had counts of >200 cells/µL. In 16 studies the data were further stratified, providing data from 4,034 subjects with CD4 counts of 200–499 cells/µL and 2,231 with counts of ≥500 cells/µL.
Proportions Testing TST-positive
The numbers and proportions of PLWH testing TST-positive for each of the 19 studies are summarized (Table 2 ). The overall median proportion testing TST positive was 26.0% (IQR, 20.6–41.2%; range 11.0–57.6%). The proportions testing positive stratified according to geographical region (Figure 2A) and country TB prevalence (Figure 2B) were displayed in forest-plots. The data were found to be heterogeneous and no clear associations with either of these two variables were evident.
Table 2. The proportion of people living with HIV who tested TST-positive stratified by CD4 cell count categories <200, ≥200, 200–499 and ≥500 cells/µL.
| Study | All study subjects | CD4 count <200 cells/µL | CD4 count ≥200 cells/µL | CD4 count 200–499 cells/µL | CD4 count ≥500 cells/µL | |||||
| No. with TST results | Positive TST n (%) | No. with TST results | Positive TST n (%) | No. with TST results | Positive TST n (%) | No. with TST results | Positive TST n (%) | No. with TST results | Positive TST n (%) | |
| AFRICA | ||||||||||
| Lugada et al (2002) [44] | 2001 | 457 (22.8) | 931 | 115 (12.4) | 1070 | 342 (32.0) | 647 | 184 (28.4) | 423 | 158 (37.4) |
| Rangaka et al (2007) [45] | 57 | 30 (52.6) | 8 | 2 (25.0) | 49 | 28 (57.1) | 31 | 17 (54.8) | 18 | 11 (61.1) |
| Karam et al (2008) [46] | 274 | 60 (21.9) | 146 | 12 (8.2) | 128 | 48 (37.5) | 85 | 25 (29.4) | 43 | 23 (53.5) |
| Oni et al (2011) [47] | 238 | 137 (57.6) | 51 | 25 (49.0) | 187 | 112 (59.9) | 137 | 79 (57.7) | 50 | 33 (66.0) |
| Samandari et al (2011) [21] | 1891 | 462 (24.4) | 575 | 88 (15.3) | 1316 | 374 (28.4) | 952 | 250 (26.3) | 364 | 124 (34.1) |
| AMERICAS | ||||||||||
| Garcia-Garcia et al (2000) [48] | 801 | 261 (32.6) | 309 | 47 (15.2) | 492 | 214 (43.5) | 325 | 120 (36.9) | 167 | 94 (56.3) |
| Miranda et al (2007) [49] | 98 | 16 (16.3) | 35 | 3 (8.6) | 63 | 13 (20.6) | 35 | 5 (14.3) | 28 | 8 (28.6) |
| Balcells et al (2008) [50] | 109 | 12 (11.0) | 13 | 1 (7.7) | 96 | 11 (11.5) | 73 | 4 (5.5) | 23 | 7 (30.4) |
| Moura et al (2011) [51] | 864 | 170 (19.7) | 137 | 13 (9.5) | 727 | 157 (21.6) | 386 | 70 (18.1) | 341 | 87 (25.5) |
| EASTERN-MEDITERRANEAN | ||||||||||
| Davarpanah et al (2009) [52] | 152 | 58 (38.2) | 35 | 8 (22.9) | 117 | 50 (42.7) | ||||
| Alavi et al (2010) [53] | 62 | 34 (54.8) | 12 | 6 (50.0) | 50 | 28 (56.0) | ||||
| Mardani et al (2010) [54] | 50 | 14 (28.0) | 14 | 2 (14.3) | 36 | 12 (33.3) | 33 | 10 (30.3) | 3 | 2 (66.7) |
| EUROPE | ||||||||||
| Vitek et al (2009) [55] | 150 | 39 (26.0) | 10 | 2 (20.0) | 140 | 37 (26.4) | 79 | 15 (19.0) | 61 | 22 (36.1) |
| SOUTH & SOUTH-EAST ASIA | ||||||||||
| Yanai et al (1997) [56] | 311 | 63 (20.3) | 85 | 14 (16.5) | 226 | 49 (21.7) | ||||
| Hiransuthikul et al (2003) [57] | 600 | 117 (19.5) | 160 | 18 (11.3) | 440 | 99 (22.5) | 344 | 69 (20.1) | 96 | 30 (31.3) |
| Gupta et al (2007) [58] | 752 | 157 (20.9) | 57 | 11 (19.3) | 695 | 146 (21.0) | 360 | 77 (21.4) | 335 | 69 (20.6) |
| Swaminathan et al (2008) [59] | 631 | 269 (42.6) | 158 | 64 (40.5) | 473 | 205 (43.3) | 313 | 138 (44.1) | 160 | 67 (41.9) |
| WESTERN PACIFIC | ||||||||||
| Jiang et al (2009) [60] | 68 | 27 (39.7) | 9 | 1 (11.1) | 59 | 26 (44.1) | 28 | 10 (35.7) | 31 | 16 (51.6) |
| Nguyen et al (2011) [61] | 369 | 204 (55.3) | 75 | 31 (41.3) | 294 | 173 (58.8) | 206 | 116 (56.3) | 88 | 57 (64.8) |
Figure 2. Forest plot showing the proportions (%, 95%CI) of people living with HIV testing TST-positive in the all studies (n = 19) with data grouped according to: a) geographical region; b) country TB prevalence (<100, 100–249 and ≥250 cases per 100,000 population) and c) CD4 cell count (<200 or ≥200 cells/µL).
We next summarized the data stratified according to CD4 cell count strata (Table 2). These data stratified by CD4 cell counts of <200 cells/µL and ≥200 cells/µL were available for all 19 studies included and are displayed in Figure 2C. Although there was heterogeneity within these groups, the proportions testing TST-positive tended to be higher among PLWH with CD4 cell counts of ≥200 cells/µL (median 33.3%; IQR, 22.1–43.8%; range, 11.5–59.9%) compared to those with CD4 cell counts <200 cells/µL (median 15.3%; IQR, 11.2–23.9%; range, 7.7–50.0%).
Sub-analysis of Studies
Of the 16 studies that provided TST data stratified by three CD4 cell count strata (<200, 200–499 and ≥500 cells/µL), 5 also fulfilled the additional criteria for inclusion in the meta-analysis (Table 1). Four studies were scored as high quality and one medium quality and they included 5,567 (58.7%) of the overall 9,478 PLWH in the review. The studies were from Senegal, Uganda, Botswana, Mexico and Thailand and the average five-year national TB prevalence estimates for these countries ranged between 123 and 750 cases per 100,000 population. Mean age ranged between 29 and 37 years. The proportion of PLWH receiving ART at the time of TST assessment was low (range, 0–12.4%) and most had evidence of BCG vaccination (Table 1). Among subjects (n = 5,567) included in the analysis, 2,121 (38.1%) had a CD4 cell count <200 cells/µL, 2,353 (42.3%) a CD4 cell count 200–499 cells/µL, and 1,093 (19.6%) a CD4 cell count ≥500 cells/µL.
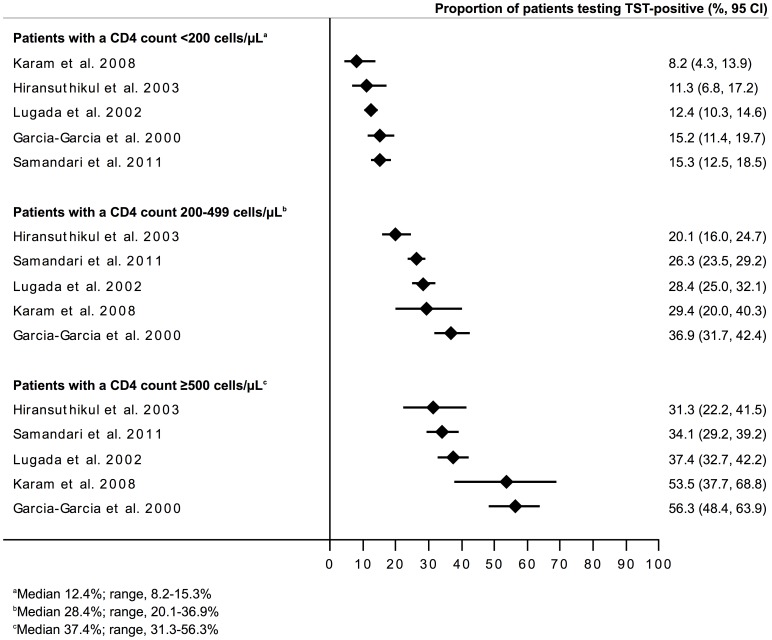
The median proportion testing TST-positive in these 5 studies was 22.8% (range, 19.5–32.6%). These data are shown in a forest plot stratified by CD4 cell count <200 cells/µL, 200–499 cells/µL and ≥500 cells/µL (Figure 3). The proportions testing TST-positive differed between the three groups, with medians of 12.4% (range, 8.2–15.3%), 28.4% (range, 20.1–36.9%) and 37.4% (range, 31.3–56.3%), respectively. I-squared statistics showed significant heterogeneity within each stratum, however, precluding the calculation of pooled summary estimates.
Figure 3. Forest plot showing the proportions (%, 95%CI) of people living with HIV testing TST-positive in studies (n = 5) included in the meta-analysis with data grouped according to CD4 cell count strata (<200, 200–499 and ≥500 cells/µL).
The median proportions testing positive in these three groups were 12.4% (range, 8.2–15.3%), 28.4% (range, 20.1–36.9%) and 37.4% (range, 31.3–56.3%). The I2 statistics for these three groups of data were 54.4% (P = 0.067), 84.2% (P<0.001) and 86.8% (P<0.001), respectively.
Discussion
In this systematic review, we summarized data from 19 studies that included 9,478 PLWH from low- and middle-income countries in sub-Saharan Africa, Asia and Central and South America. The median proportion of PLWH testing TST-positive was 26.0% overall but with substantial heterogeneity (range, 11.0–57.6%). Five of these studies (5,567 subjects) were selected for inclusion in a sub-analysis on the basis of high country TB prevalence, adequate study size and quality and inclusion of PLWH without TB attending HIV care and treatment settings. Among these studies, the median proportions of patients with CD4 cell count <200 cells/µL, 200–499 cells/µL and ≥500 cells/µL testing TST-positive were 12.4% (range, 8.2–15.3%), 28.4% (range, 20.1–36.9%) and 37.4% (range, 31.3–56.3%), respectively. Data heterogeneity, however, precluded calculation of pooled summary estimates. These data indicate that in most settings, implementation of IPT among PLWH without assessment of TST status is likely to benefit a minority of patients across all CD4 count categories.
In resource-limited settings, assessment of TST status is the only recommended means of identifying patients who are likely to benefit from IPT [10]. However, this unfortunately presents a substantial operational barrier to implementation. The test requires a refrigerated supply of purified protein derivative and sterile supplies. Health care workers must be trained to administer the test and accurately read the result. In order to reduce loss to follow-up after TST placement [17] such novel interventions as training of community outreach auxiliaries in the reading of the induration must be studied. Although interferon-γ-release assays give fewer false-positive results, there is no evidence that these are any more predictive of IPT benefit and these tests are not recommended for use in resource-limited settings [10], [18].
Removal of the requirement for TST assessment simplifies implementation of IPT and may thereby help catalyze scale-up of IPT. However, this study suggests that overall only approximately 1 in 4 PLWH stand to benefit from IPT if implemented in an untargeted way. Although this would save resources needed to assess TSTs, this would be wasteful of both patient and scarce health care resources used in treating and following-up large numbers of PLWH who would derive no benefit. Cost-effectiveness analyses suggest that use of IPT either in a targeted or untargeted way is cost-effective compared to not implementing IPT at all [19], [20]. In South Africa, both strategies were estimated to be equally cost-effective [20] whereas in Uganda targeting IPT by TST status was more favourable in terms of incremental cost per QALY gained [19]. Both studies used optimistic assumptions about the duration of the benefit of IPT. As ART is increasingly available in resource-constrained settings, cost effectiveness analyses of IPT – including long-term IPT – in combination with ART are urgently needed to better assess the potential public health utility of these interventions.
Data from southern Africa increasingly suggest that because of high prevailing rates of community TB transmission, benefit from IPT is largely limited to the period within which it is taken [21]–[23]. Thus, long-term IPT is now an option recommended by WHO [10]. If IPT is to be increasingly used on a long-term basis, TST assessment may be even more important as suggested by a cost-effectiveness analysis from Botswana. In this study, provision of IPT for 36 months to TST-positive individuals only was more cost-effective than providing IPT without TST assessment or providing IPT for 6 months only [24].
Concerns have been expressed about the risks of widespread use of isoniazid monotherapy fuelling development of drug resistance. There is no evidence of such an effect from data generated during carefully conducted randomized controlled trials [25], and yet it is unknown what risk would be associated with widespread availability of isoniazid monotherapy within communities outside the context of intervention trials. Since development of antimicrobial resistance is directly related to how much a drug is used, limiting drug exposure to the minority of PLWH who are likely to benefit would be prudent.
Isoniazid is well known to be associated with low rates of hepatotoxicity and occasional deaths [26]. Consistent with previous studies, rates in two large IPT trials in southern Africa have recently reported rates of hepatotoxicity of approximately 0.1–1.0% [27], [28]. Peripheral neuropathy may also be exacerbated by isoniazid and can be disabling and significantly reduce the quality of life. Although overall rates of toxicity are low and mortality rare, these events cannot be ignored and they provide an important argument against use of IPT in those who are TST-negative.
There was great heterogeneity between studies in the proportions testing TST positive. This observation suggests that knowledge of TST responses in specific countries or settings may help local decisions on the pros and cons of targeted or untargeted IPT delivery. This heterogeneity may reflect both the local TB epidemiology as well as study-related factors. The latter may include study design, methodology used for TST assessment (including dose of PPD), the accuracy with which this was done, potential biases associated with non-return of patients for TST assessment, the rigour with which TB was excluded and the characteristics of the patients tested. While we have attempted to broadly summarize these variables, it was not possible to precisely define the role of each. Importantly, however, the mean or median ages of PLWH included in the meta-analysis all fell within a narrow range (29–37 years). It was notable that country TB prevalence did not appear from the forest plots to be a more dominant factor. This may be related to imprecision of TB prevalence estimates, differences between national estimates and prevalence in the specific communities studied or the fact that TB exposure is related to cumulative exposure over the life-time of the individual rather than current prevalence.
The proportions of positive TST results stratified by CD4 cell count observed among the total group of 19 studies were very similar to those found in the 5 studies that were selected for further analysis, providing reassurance that study selection did not introduce substantial bias. Moreover, many of the other studies that were excluded from the systematic review also report that between 22–25% of PLWH tested TST-positive [29]–[33] (Table S3). Although we were unable to include the unpublished data from the THRio study in Brazil, an earlier publication from this study reported that 24.8% of 5,492 PLWH tested TST positive [34]. The proportions testing positive may be higher in South Africa where rates of TB transmission are very high [11]. However, although the two studies identified from South Africa were not eligible for inclusion in the meta-analysis, data from 1,891 PLWH in neighbouring Botswana were included (Table 2).
These data help to rationalize prevention strategies that need to be tailored according to subjects’ CD4 cell counts. As CD4 cell counts decrease during the natural history of HIV infection, risk of TB progressively increases [14]. However, at the same time, the proportion of individuals who are TST-positive and who might benefit from IPT paradoxically decreases. Thus, at low CD4 cell counts, ART is the key intervention needed to reduce the risk of death and to serve as the principal TB preventive intervention. This decreases TB risk by 67% (95%CI, 61–73%) [35], provides benefit regardless of TST status [21] and can be started without the need for careful exclusion of active TB [36]. There is also growing evidence of the TB preventive effect of ART at higher CD4 cell counts >350 cells/µL [37]. During long-term ART, however, TB incidence rates remain several times higher than rates in the community despite good CD4 cell count recovery [38], [39]. Observational data suggest there is an additive effect by using ART and IPT concurrently [21], [34], [35] and this has now been confirmed by data from a randomised controlled trial [40]. ART induces recovery of TST responses [41], [42] and so a greater proportion of PLWH during long-term ART may benefit from IPT compared to the proportion at baseline. Few PLWH in this meta-analysis, however, were receiving ART and so this could not be assessed. Studies defining the impact of IPT according to TST status in patients receiving ART are needed as this may not necessarily be the same as that observed in the pre-ART era [8].
Although it is being recommended that ART be started at increasingly high CD4 cell counts, IPT might be prioritized for PLWH prior to ART eligibility. Ideally HIV would be identified early in the course of disease and all those testing TST-positive would receive a course of IPT prior to becoming eligible for ART. However, even in those with CD4 cell counts of ≥500 cells/µL, less than two in five PLWH (37.4%) tested TST-positive. Use of TST assessment (or a new alternative test) in such patients would permit appropriate targeting of therapy and could substantially reduce the numbers unnecessarily treated.
Strengths of this study include a comprehensive search strategy which identified studies from diverse geographic regions with a range of TB prevalence estimates. Large numbers of PLWH overall and with a broad range of CD4 cell counts were included. Weaknesses include the paucity of data from PLWH receiving ART, the lack of availability of data from other large as yet unpublished studies and the inability to precisely define the impact of other potential variables. In addition, the reliability of TST assessment could not be assessed and the methods for excluding TB were not standardized. Heterogeneity in the data precluded calculation of pooled summary estimates.
In conclusion, we found that a minority PLWH were TST-positive, even in the highest TB prevalence settings, and that the proportion testing positive was strongly associated with CD4 cell counts. This factor may undermine the cost-effectiveness of use of untargeted IPT. Local knowledge of TST response rates may help inform policies. Operational research to improve TST implementation as well as development of new, simple means to identify those who will benefit from IPT are urgently needed [43].
Supporting Information
Overview of search strategy.
(DOCX)
Graded quality assessment checklist.
(DOCX)
Summary of studies meeting criteria for contacting authors for further data but for which insufficient secondary data was provided for study inclusion.
(DOCX)
Acknowledgments
We would like to thank the following authors for the contribution of their original study data: Maria Elvira Balcells, Amita Gupta, Christian Lienhardt, Abraham G. Miranda, Líbia Cristina Rocha Vilela Moura, Duc Tan Minh Nguyen, Tolullah Oni, Payam Tabarsi and Robert J. Wilkinson. We would also like to thank Darshini Govindasamy for allowing us to adapt the quality assessment checklist that she developed. Finally, we are grateful to Alexandra W. Gomes for her assistance in defining search terms and guidance in conducting the search strategy. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the U.S. Centers for Disease Control and Prevention.
Funding Statement
SDL and KK were funded by the Wellcome Trust. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lawn SD, Zumla AI (2011) Tuberculosis. Lancet 378: 57–72. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (2011) Global tuberculosis control 2011. Available: http://www.who.int/tb/publications/global_report/en/index.html. Accessed 2011 Dec 21.
- 3.World Health Organization (2010) The Global Plan to STOP TB 2011–2015. World Health Organization, Geneva. [DOI] [PMC free article] [PubMed]
- 4. Lawn SD, Kranzer K, Wood R (2009) Antiretroviral Therapy for Control of the HIV-associated Tuberculosis Epidemic in Resource-Limited Settings. Clin Chest Med 30: 685–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (2008) WHO three I’s meeting. Report of a joint WHO HIV/AIDS and TB Department Meeting. WHO, Geneva. Available: http://www.who.int/hiv/pub/meetingreports/WHO_3Is_meeting_report.pdf.
- 6. Harries AD, Zachariah R, Corbett EL, Lawn SD, Santos-Filho ET, et al. (2010) The HIV-associated tuberculosis epidemic–when will we act? Lancet 375: 1906–19. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization (2010) Global tuberculosis control 2010. Geneva: World Health Organization. Available: http://www.who.int/tb/publications/global_report/2010/en/index.html.
- 8.Akolo C, Adetifa I, Shepperd S, Volmink J (2010) Treatment of latent tuberculosis infection in HIV-infected persons. Cochrane Database of Systematic Reviews, Issue 1. Art. No.: CD000171. DOI: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed]
- 9.World Health Organization (1998) Policy statement on preventive therapy against tuberculosis in people living with HIV.WHO/TB/98.255 UNAIDS/98.34. Available: http://whqlibdoc.who.int/hq/1998/WHO_TB_98.255.pdf.
- 10.World Health Organization (2011) Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. World Health Organization, Geneva, Switzerland. Available: http://whqlibdoc.who.int/publications/2011/9789241500708_eng.pdf. Accessed 2011 Jan 30.
- 11. Wood R, Liang H, Wu H, Middelkoop K, Oni T, et al. (2010) Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis 14: 406–12. [PMC free article] [PubMed] [Google Scholar]
- 12. Lawn SD, Butera ST, Shinnick TM (2002) Tuberculosis unleashed: the impact of human immunodeficiency virus infection on the host granulomatous response to Mycobacterium tuberculosis. Microbes Infect 4: 635–46. [DOI] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lawn SD, Churchyard G (2009) Epidemiology of HIV-associated tuberculosis. Curr Opin HIV AIDS 4: 325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Bank website. (2011) Available: http://data.worldbank.org/about/country-classifications/country-and-lending-groups.
- 16. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–58. [DOI] [PubMed] [Google Scholar]
- 17. Mugisha B, Bock N, Mermin J, Odeke RM, Miller B, et al. (2006) Tuberculosis case finding and preventive therapy in an HIV voluntary counseling and testing center in Uganda. Int J Tuberc Lung Dis 10: 761–7. [PubMed] [Google Scholar]
- 18. Rangaka MX, Wilkinson KA, Glynn JR, Ling D, Menzies D, et al. (2012) Predictive value of interferon-gamma release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 12: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shrestha RK, Mugisha B, Bunnell R, Mermin J, Odeke R, et al. (2007) Cost-utility of tuberculosis prevention among HIV-infected adults in Kampala, Uganda. Int J Tuberc Lung Dis 11: 747–54. [PubMed] [Google Scholar]
- 20. Hausler HP, Sinanovic E, Kumaranayake L, Naidoo P, Schoeman H, et al. (2006) Costs of measures to control tuberculosis/HIV in public primary care facilities in Cape Town, South Africa. Bull World Health Organ 84: 528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Samandari T, Agizew TB, Nyirenda S, Tedla Z, Sibanda T, et al. (2011) 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet 377: 1588–98. [DOI] [PubMed] [Google Scholar]
- 22.Samandari T, Agizew T, Nyirenda S, Tedla Z, Sibanda T, et al.. (2012) TB incidence increase after cessation of 36 months isoniazid prophylaxis in HIV+ adults in Botswana. Abstracts of the 19th Conference on Retroviruses and Opportunistic Infections. Seattle, WA, USA. Abstract #147.
- 23. Martinson NA, Barnes GL, Moulton LH, Msandiwa R, Hausler H, et al. (2011) New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med 365: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith T, Samandari T, Abimola T, Marston B, Sangrujee N (2011) Cost-effectiveness of alternative policies to reduce TB disease and death in HIV-infected persons in Botswana. Am J Respir Crit Care Med 183: A5310. [Google Scholar]
- 25. Balcells ME, Thomas SL, Godfrey-Faussett P, Grant AD (2006) Isoniazid preventive therapy and risk for resistant tuberculosis. Emerg Infect Dis 12: 744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Centers for Disease Control and Prevention (2010) Severe isoniazid-associated liver injuries among persons being treated for latent tuberculosis infection - United States, 2004–2008. MMWR Morb Mortal Wkly Rep 59: 224–9. [PubMed] [Google Scholar]
- 27. Tedla Z, Nyirenda S, Peeler C, Agizew T, Sibanda T, et al. (2010) Isoniazid-associated hepatitis and antiretroviral drugs during tuberculosis prophylaxis in HIV-infected adults in Botswana. Am J Respir Crit Care Med 182: 278–85. [DOI] [PubMed] [Google Scholar]
- 28. Grant AD, Mngadi KT, van Halsema CL, Luttig MM, Fielding KL, et al. (2010) Adverse events with isoniazid preventive therapy: experience from a large trial. AIDS 24 Suppl 5S29–S36. [DOI] [PubMed] [Google Scholar]
- 29. Corbett EL, Bandason T, Cheung YB, Munyati S, Godfrey-Faussett P, et al. (2007) Epidemiology of Tuberculosis in a High HIV Prevalence Population Provided with Enhanced Diagnosis of Symptomatic Disease. PLoS Med 4: e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Quigley MA, Mwinga A, Hosp M, Lisse I, Fuchs D, et al. (2001) Long-term effect of preventive therapy for tuberculosis in a cohort of HIV-infected Zambian adults. AIDS 15: 215–22. [DOI] [PubMed] [Google Scholar]
- 31. Rodriguez JI, Arias M, Paris SC, Arbelaez MP, Betancur J, et al. (1997) Tuberculin skin test and CD4+/CD8+ T cell counts in adults infected with the human immunodeficiency virus in Medellin, Colombia. Mem Inst Oswaldo Cruz 92: 245–50. [DOI] [PubMed] [Google Scholar]
- 32. Hawken MP, Meme HK, Elliott LC, Chakaya JM, Morris JS, et al. (1997) Isoniazid preventive therapy for tuberculosis in HIV-1-infected adults: results of a randomized controlled trial. AIDS 11: 875–82. [DOI] [PubMed] [Google Scholar]
- 33. Pistone T, Kony S, Faye-Niang MA, Ndour CT, Gueye PM, et al. (2002) A simple clinical and paraclinical score predictive of CD4 cells counts below 400/mm3 in HIV-infected adults in Dakar University Hospital, Senegal. Trans R Soc Trop Med Hyg 96: 167–72. [DOI] [PubMed] [Google Scholar]
- 34. Golub JE, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH, et al. (2007) The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS 21: 1441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lawn SD, Wood R, De Cock KM, Kranzer K, Lewis JJ, et al. (2010) Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources. Lancet Infect Dis 10: 489–98. [DOI] [PubMed] [Google Scholar]
- 36. Kerkhoff AD, Wood R, Lawn SD (2011) Optimum time to start antiretroviral therapy in patients with HIV-associated tuberculosis: before or after tuberculosis diagnosis? AIDS 25: 1003–6. [DOI] [PubMed] [Google Scholar]
- 37. Suthar AB, Lawn SD, Del Amo J, Getahun H, Dye C, et al. (2012) Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLoS Med 9: e1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lawn SD, Myer L, Bekker LG, Wood R (2006) Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS 20: 1605–12. [DOI] [PubMed] [Google Scholar]
- 39. Gupta A, Wood R, Kaplan R, Bekker LG, Lawn SD (2012) Tuberculosis Incidence Rates during 8 Years of Follow-Up of an Antiretroviral Treatment Cohort in South Africa: Comparison with Rates in the Community. PLoS One 7: e34156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rangaka MX, Boulle A, Wilkinson RJ, van Cutsem G, Cohen K, et al.. (2012) Randomized controlled trial of isoniazid preventive therapy in HIV-infected persons on antiretroviral therapy. Programme and Abstracts of the XIX International AIDS Conference. International AIDS Society. Washington DC, USA. Abstract THLBB03.
- 41. Girardi E, Palmieri F, Zaccarelli M, Tozzi V, Trotta MP, et al. (2002) High incidence of tuberculin skin test conversion among HIV-infected individuals who have a favourable immunological response to highly active antiretroviral therapy. AIDS 16: 1976–9. [DOI] [PubMed] [Google Scholar]
- 42. Lawn SD, Bekker LG, Wood R (2005) How effectively does HAART restore immune responses to Mycobacterium tuberculosis? Implications for tuberculosis control. AIDS 19: 1113–24. [DOI] [PubMed] [Google Scholar]
- 43. Kasprowicz VO, Churchyard G, Lawn SD, Squire SB, Lalvani A (2011) Diagnosing latent tuberculosis in high-risk individuals: rising to the challenge in high-burden areas. J Infect Dis 204 Suppl 4S1168–S1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lugada ES, Watera C, Nakiyingi J, Elliott A, Brink A, et al. (2002) Operational assessment of isoniazid prophylaxis in a community AIDS service organisation in Uganda. Int J Tuberc Lung Dis 6: 326–31. [PubMed] [Google Scholar]
- 45. Rangaka MX, Wilkinson KA, Seldon R, Van CG, Meintjes GA, et al. (2007) Effect of HIV-1 Infection on T-Cell-based and Skin Test Detection of Tuberculosis Infection. Am J Respir Crit Care Med 175: 514–20. [DOI] [PubMed] [Google Scholar]
- 46. Karam F, Mbow F, Fletcher H, Senghor CS, Coulibaly KD, et al. (2008) Sensitivity of IFN-gamma release assay to detect latent tuberculosis infection is retained in HIV-infected patients but dependent on HIV/AIDS progression. PLoS One 3: e1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oni T, Burke R, Tsekela R, Bangani N, Seldon R, et al. (2011) High prevalence of subclinical tuberculosis in HIV-1-infected persons without advanced immunodeficiency: implications for TB screening. Thorax 66: 669–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Garcia-Garcia ML, Valdespino-Gomez JL, Garcia-Sancho C, Mayar-Maya ME, Palacios-Martinez M, et al. (2000) Underestimation of Mycobacterium tuberculosis infection in HIV-infected subjects using reactivity to tuberculin and anergy panel. Int J Epidemiol 29: 369–75. [DOI] [PubMed] [Google Scholar]
- 49. Miranda A, Morgan M, Jamal L, Laserson K, Barreira D, et al. (2007) Impact of antiretroviral therapy on the incidence of tuberculosis: the Brazilian experience, 1995–2001. PLoS ONE 2: e826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Balcells ME, Perez CM, Chanqueo L, Lasso M, Villanueva M, et al. (2008) A comparative study of two different methods for the detection of latent tuberculosis in HIV-positive individuals in Chile. Int J Infect Dis 12: 645–52. [DOI] [PubMed] [Google Scholar]
- 51. Moura LC, Ximenes RA, Ramos HL, Miranda Filho DB, Freitas CD, et al. (2011) An evaluation of factors associated with taking and responding positive to the tuberculin skin test in individuals with HIV/AIDS. BMC Public Health 11: 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Davarpanah MA, Rasti M, Mehrabani SS, Allahyari R, Neirami R, et al. (2009) Association between PPD and QuantiFERON Gold TB test in TB infection and disease among HIV-infected individuals in southern Iran. Iranian Red Crescent Med J 11: 71–75. [Google Scholar]
- 53. Alavi SM, Nadimi M, Shokri S, Zamani GA (2010) Latent tuberculosis infection in individuals with human immunodeficiency virus infection: comparison of tuberculin skin test to the anti TB-IgM antibodies.Pakistan J Med Sci. 26: 11–14. [Google Scholar]
- 54. Mardani M, Tabarsi P, Mohammadtaheri Z, Chitsaz E, Farokhzad B, et al. (2010) Performance of QuantiFERON-TB Gold test compared to tuberculin skin test in detecting latent tuberculosis infection in HIV- positive individuals in Iran. Ann Thorac Med 5: 43–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vitek E, Gusseinova N, Laricheva N, Vasiliev S, Molotilov V, et al. (2009) Factors associated with positive tuberculin skin test results among HIV-infected persons in Orel Oblast, Russia. Int J Tuberc Lung Dis 13: 829–35. [PubMed] [Google Scholar]
- 56. Yanai H, Uthaivoravit W, Mastro TD, Limpakarnjanarat K, Sawanpanyalert P, et al. (1997) Utility of tuberculin and anergy skin testing in predicting tuberculosis infection in human immunodeficiency virus-infected persons in Thailand. Int J Tuberc Lung Dis 1: 427–34. [PubMed] [Google Scholar]
- 57. Hiransuthikul N, Hanvanich M, Dore GJ, Mokkhawes T, Li Y, et al. (2003) Factors associated with tuberculin skin test reactivity among HIV-infected people in Bangkok. Southeast Asian J Trop Med Public Health 34: 804–9. [PubMed] [Google Scholar]
- 58. Gupta A, Nayak U, Ram M, Bhosale R, Patil S, et al. (2007) Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002–2005. Clin Infect Dis 45: 241–9. [DOI] [PubMed] [Google Scholar]
- 59. Swaminathan S, Subbaraman R, Venkatesan P, Subramanyam S, Kumar SR, et al. (2008) Tuberculin skin test results in HIV-infected patients in India: implications for latent tuberculosis treatment. Int J Tuberc Lung Dis 12: 168–73. [PubMed] [Google Scholar]
- 60. Jiang W, Shao L, Zhang Y, Zhang S, Meng C, et al. (2009) High-sensitive and rapid detection of Mycobacterium tuberculosis infection by IFN-gamma release assay among HIV-infected individuals in BCG-vaccinated area. BMC Immunol 10: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen DT, Hung NQ, Giang LT, Dung NH, Lan NT, et al.. (2011) Improving the diagnosis of pulmonary tuberculosis in HIV-infected individuals in Ho Chi Minh City, Viet Nam. Int J Tuberc Lung Dis 15: 1528–34, i. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Overview of search strategy.
(DOCX)
Graded quality assessment checklist.
(DOCX)
Summary of studies meeting criteria for contacting authors for further data but for which insufficient secondary data was provided for study inclusion.
(DOCX)