Abstract
Cellular ferritin is central for iron balance during transfusions therapies; serum ferritin is a small fraction of body ferritin, albeit a convenient reporter. Iron overload induces extra ferritin protein synthesis but the protein is overfilled with the extra iron that damages ferritin, with conversion to toxic hemosiderin. Three new approaches that manipulate ferritin to address excess iron, hemosiderin, and associated oxidative damage in Cooley’s Anemia and other iron overload conditions, are faster removal of ferritin iron with chelators guided to ferritin gated pores by peptides; more ferritin protein synthesis using ferritin mRNA activators, by metal complexes that target mRNA 3D structures; and determining if endocytotic absorption of iron from legumes, which is mostly ferritin, is regulated during iron overload to prevent excess iron entry while providing protein. More of a focus on ferritin features, including protein cage structure, iron mineral, regulatable mRNA, and specific gut absorption properties, will achieve the three novel experimental goals for managing iron homeostasis with transfusion therapies.
Keywords: iron, ferritin, chelator
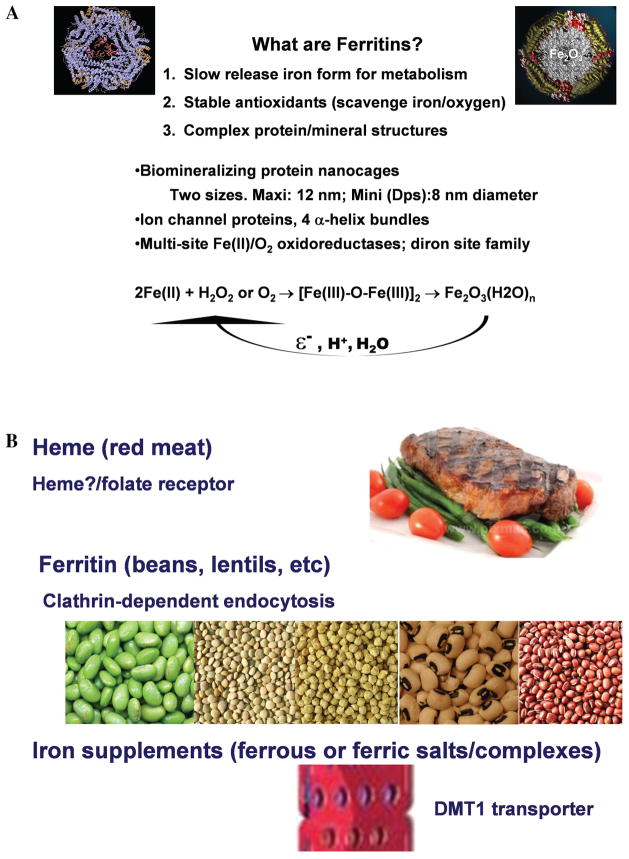
Maintaining the proper amount of iron is a challenge for everyone, but particularly for humans with Cooley’s Anemia. To treat the condition, iron, in the form of new red blood cells must be injected to combat the anemia. In between transfusions, if there is transient anemia, homeostatic mechanisms signal increased absorption of dietary iron that is in iron fortificants such as Fe-EDTA or in meat. The well-known iron overload in Cooley’s Anemia results because humans excrete excess iron poorly, contrasting with other vitamins and minerals. At the center of managing body iron is ferritin, Nature’s antirust protein nanocage (Fig. 1).1 Ferritin is a unique protein found in all organisms, plant, animals, bacteria, and archaea. The amounts of ferritin inside cells vary, depending on the role of the mature cell in the body; serum ferritin represents the iron levels in macrophage cells and is used as a non-invasive marker of iron status. Generally, ferritin is in the cytoplasm of animal cells, except for a small amount in mitochondria. Serum ferritin is a very small fraction of tissue ferritin, but reflects tissue ferritin and iron under normal conditions. Serum ferritin as a reporter for body iron is less accurate during iron overload for two reasons. First, the ferritin content of selected tissues increases. Because the ferritin is not same protein sequence in all tissue and the antibody for the serum ferritin analysis is prepared against ferritin form only a few tissues, a change in the tissue source of the serum ferritin will alter the analytical results for serum ferritin. Second, because the serum ferritin assays measure ferritin protein by an immunologic assay, the actual iron content of the tissue remains only an estimate. When the iron content of tissue ferritin increases, as it does during extreme iron overload, the ferritin protein content will increase much less. Nevertheless, serum ferritin is a reasonably reliable indicator of general increases or decreases in body iron during small changes in iron homeostasis and the most accessible method currently available at many sites around the world.
Figure 1.
A. Molecular functions of ferritin protein cages and dietary ferritin iron. Functions of the ferritin protein cages in synthesizing and dissolving the iron–oxygen minerals. Ferritin protein cages have catalytic sites that oxidize and couple two iron atoms, channels that facilitate nucleation and movement to cavity entrances,1 and gated pores that control Fe(II) entry for oxidation/mineralization and exit after mineral reduction and dissolution. Dioxygen is the oxidant but the mineral reductant in vivo is unknown; reductants that function in solution to reduce the mineral and allow mineral dissolution (addition of water) are ε− = NADH/FMN (also found in tissues) or diothionite. In vivo the reductant could be FMNH2 but is currently unknown. B. Ferritin is one of three major forms of dietary iron; each has different mechanisms of iron absorption. Nonheme iron has at least two different chemical forms in terms of recognition by the gut during absorption. The classically studied bioavailable form is found in supplements such as ferrous sulfate or Fe-EDTA; small complexes of iron in plants are often poorly absorbed, for example, ferric phytate or tannate. Iron in the ferritin mineral, recently recognized as an abundant form of iron in whole legumes, is readily bioavailable.6
What is the normal function of ferritin? Because iron is so insoluble under physiological conditions, cells have to concentrate iron to have enough for synthesizing iron proteins. First, ferritins concentrate iron in a solid mineral in advance of cell need, and release the iron atoms a few at a time for synthesizing iron proteins in the cell. Iron proteins are catalysts important in critical cell functions such as; making deoxyribose (for DNA), recovering energy from foods (respiration), carrying oxygen (hemoglobin and myoglobin), using solar energy (photosynthesis), making unsaturated fatty acids, and synthesizing steroids. Second, ferritins respond to oxidant damage that releases Fe2+ from iron-damaged proteins to react with O2 and produce free radicals (ROS). Mineralized iron in ferritin cannot react readily with ROS because of the protein cage (Figs. 1 and 2). In addition, the two substrates of ferritin catalytic sites are Fe2+ and O2, which react to form mineral precursors and eventually the ferric oxide mineral itself. By consuming Fe2+ and O2 to make the mineral, ferritin has antioxidant activity. Finally, ferritin is a source of natural, slow release dietary iron that has only been recognized recently.2–5 The iron in many whole legume seed, such as soybeans, lentils, and chickpeas is largely ferritin iron, the natural, protein coated, iron-rich nutrient.6
Figure 2.
Pores in ferritin protein cages control rates of iron chelation. Iron chelation rates increase in solution when ferritin protein cage pores are modified (unfolded/opened). Amino acids substitution by site-directed mutagenesis unfolds ferritin pores without unfolding the protein cage, redrawn from Takagi et al.23 Similar effect can be obtained in solution with physiological concentrations of urea or low heat24 or synthetic peptides from a combinatorial library.8 Rates determined in solution with bipyridyl chelator. Bottom panel. Chemical links between chelators to ferritin pore-modifying peptides increase iron chelation rates. Synthesizing a complex with the chelator covalently linked to desferrioxamine B increases the speed of chelating ferritin iron compared to peptide-desferrioxamine B alone; the rate with the new peptide-chelator is as fast with natural ferritin as with the engineered, unnatural, engineered ferritin. The figure is modified from Takagi et al.;8 and the data are the averages of two to three protein preparations and analyses with the error as the standard deviation. *Significantly different (P < 0.01) from control (no peptide) or peptide mixed with chelator) or peptide DFO, deferroxamine B; Pep1, pore-opening peptide; Pc1-Pep1, deferroxamine B conjugate.
Three approaches, reflecting properties of ferritin during iron overload will be discussed. (1) The ferritin protein cage as a target for novel chelators targeted to the protein (Fig. 2); (2) ferritin mRNA as a target for small molecules to increase ferritin protein synthesis during iron overload, to decrease ferritin iron/ferritin protein and hemosiderin formation (Fig. 4); (3) ferritin found in natural foods is a readily absorbed form of iron that might be more efficaciously regulated during iron overload. While the three topics are particularly important for transfusion-treated patients with both Cooley’s Anemia and sickle cell disease,7 some of the ideas can be useful in hemochromatosis and in iron deficiency anemias.
Figure 4.
Recruiting unused ferritin mRNA for protein synthesis to minimize hemosiderin formation in iron overload: targeting three-dimensional properties of the IRE-RNA. During iron overload, iron increases inside each ferritin protein cage leading to ferritin damage and hemosiderin formation. Some of the mRNA remains bound to repressor14 (top). Approximately 50% ferritin IRE-mRNA is still repressed when iron is in excess. Physical studies of the RNA show the IRE structure is a target that binds metals9 and is recognized by small organic–metal complexes in living cells (bottom right panel). Figure taken from Ke et al.,15 allowing drug design that uses the principles of protein drug discovery to exploit the smaller target size of mRNA and addition information of three-dimensional information, contrasting with the limited two-dimensional structure information of and avoiding cells responses to siRNA. Crystals: structure from reference16 shows exposed IRE-RNA — RNA; — protein; Solution: ■, metal sites from chemical nuclease and NMR analyses.17,19.26
Ferritin protein cages as a target to increase rates of removal of iron
Ferritin protein cages have conserved structures that function as gated pores in ferritin channels between the outside of the cage and the mineral in the cavity. The mineral inside ferritin needs to be protected from reductants in the cytoplasm to prevent rapid turnover/dissolution of the mineral to minimize consumption cell reductants, and to minimize leakage of Fe2+. When the amino acids that hold the pores closed are changed the iron can be reduced and chelated very quickly (Fig. 2). Very likely there are proteins in the cell that regulate ferritin pore opening and are coupled to the use of ferritin iron in the synthesis of iron proteins. The reason iron chelation therapy is so slow in Cooley’s Anemia and other transfusion treated iron overload is that the ferritin pores will be “closed” when iron is in excess.
We identified five synthetic peptides that bound tightly to ferritin from a combinatorial group of 109 heptapeptides. Only one of the five binding peptides increased the rate of iron removal when the peptide was mixed with desferrioxamine B as the chelator (Fig. 2). The pore unfolding amino acid substitutions had a much larger effect, likely because the pores stayed open longer, allowing more iron mineral to be reduced and for the chelator to bind to the emerging Fe2+. We reasoned that if the chelator were bound to the peptide that unfolded the pores, when the ferritin iron was dissolved and Fe2+ approached the exit pores, the chelator attached to the binding peptide would be so close that chelation would occur before the pores had a chance to close and retain the Fe2+. Our hypothesis turned out to be correct.8 When the ferritin pore-opening peptide was linked to the chelator, desferrioxamine B in this example, the rate of chelation of ferritin iron in solution was as high as for the ferritin with an amino acid replacement that forced the pores to be open continuously (Fig. 2, top panel). The experiment proves the importance of targeting chelators directly to ferritin pores. New chelators, developed in the future, could work more effectively, if they are targeted to ferritin, the main site the source of the iron to be removed. The idea is applying the well-known strategy of drug targeting, in this case to ferritin where the extra iron is, to chelators of today and the future for enhanced iron chelation therapy.
Ferritin genes as targets to increase ferritin protein synthesis
During iron overload, ferritin synthesis increases by two mechanisms. Oxidant damage from excess iron that is not stored safely in ferritin will induce ferritin gene transcription mediated by changing the antioxidant response element (ARE) interactions with the DNA transcription regulator bach 1 (Fig. 3). Even before the iron reaches toxic levels and causes oxidative damage and ferritin gene transcription, additional ferritin protein is synthesized by allowing more ferritin mRNA to bind to ribosomes (Fig. 4, top panel); small amounts of free Fe2+ weaken the binding of the IRP protein repressor to the ferritin IRE mRNA structure releasing IRP,9 which is then degraded10,11 as the ferritin mRNA binds to ribosomes. The DNA + RNA regulation of ferritin protein synthesis is unusually complex, indicating the biological importance of ferritin. A significant feature of the regulatory pathway is the preferential targeting of ferritin DNA by oxidant/O2 and of ferritin mRNA by Fe2+, although there is some crosstalk. Because iron and O2 are also the substrate, when the genetic system is upregulated by iron and oxygen signals, ferritin protein synthesis increases to consume the iron and oxygen signals creating a feedback loop with the iron and oxygen signals/substrates.12
Figure 3.
DNA: Ferritin transcription is coordinately regulated with antioxidant response (Phase II) proteins. Regulation of the ferritin promoter, the upstream, ARE (antioxidant response element) linked to a Luc reporter studies in HepG2 cells is similar to other ARE protein such as thioredoxin reductase and NADPH quinone oxdioreductase and shows greater sensitivity to oxidants or antioxidant inducers than to iron, contrasting with ferritin mRNA regulation which is more sensitive to iron. The figure is modified from Hintze et al.,25 and represents at least three independent cultures and transfections. * *P < 0.01; *P < 0.05.
Transfusion iron overload apparently pushes safe limits to beyond homeostatic mechanisms because the iron content of each ferritin protein cage increases abnormally.12,13 The extra iron entering ferritin minerals damages the ferritin protein cage because hemosiderin form (damaged ferritin) during iron overload; hemosiderin is a toxic form of iron in the cell contrasting with the intact protein cage of ferritin. Not even the sophistication of ferritin DNA + mRNA regulatory targets is enough. However, for reasons that are unknown, even during iron overload some of the ferritin mRNA remains bound to the IRP repressor (Fig. 4, top panel).14
The protein repressor (IRP) binding site on ferritin mRNA is a folded, three-dimensional RNA structure, much like a folded, three-dimensional protein structure (Fig. 4, bottom left panel). The folded, IRE-RNA structure has metal binding sites, as do proteins. In a “proof of principle experiment,” using a metal complex, Cu(phen)2, which binds to two or three sites on the ferritin IRE-RNA as a reporter, we observed that Cu(phen)2, cleaved ferritin mRNA in HeLa cells at the same sites as in solution.15,16 The results proved that small, RNA-binding molecules can enter living cells and that the structure of the ferritin IRE-RNA studied in solution (NMR, nuclease probing, X-ray crystallography)17–19 folded in the same way in living cells. Thus, a search for selectively binding molecules that can enter cells should be able to recruit any unused ferritin mRNA and decrease the hemosiderin content of cells during iron overload.
Ferritin iron in foods as a dietary source resistant to iron absorption changes
Current molecular information about the absorption of iron is incomplete (Fig. 1, bottom panel). Of the three chemical forms of iron known that are recognized by the gut, heme (iron-protoporphyrin), ferritin (iron-oxy mineral), and iron ions, only the transporter for ferrous ions DMT1 (also called DCT1 or nRAMP2) is extensively studied.20,21 Mutations in DMT1, expressed both in intestine and red blood cells are associated with anemia. Before the availability of processed, iron-fortified foods in the twentieth century, little iron would have been available from natural foods as ferrous ion. Thus, transporters/receptors for other chemical forms of nonheme iron must exist.
The two natural forms of iron in the diet, consumed for millennia before food processing are heme (meat) and ferritin (whole legumes). Currently, the only heme transporter known is also a folate transporter, SLC46A1.20,21 Given the selectivity of known carriers of heme beyond the gut22 and the importance of heme iron in early humans, it seems unlikely that the only heme transporter is one shared with folate. Information about ferritin iron absorption is different than for heme, but is also incomplete. The presence of a high affinity receptor that binds the ferritin nanocage, followed by clathrin-dependent endocytosis of the intact protein and mineral is known; after entering the cell, the iron is released from the ferritin mineral and the protein cages is degraded.4,22 However, the intracellular iron carriers, and the receptor itself remain unidentified.
A feature of ferritin absorption, which is different from heme or fortificant iron, is the absorption efficiency. For each single heme or iron atom that crosses the membrane, for example, on DMT1 or SLC46A transporter, more than 1,000 iron atoms will enter from ferritin. Iron excess depresses the DMT1 transport of nonheme iron salts, but whether the repression is more or less efficient for ferritin or nonheme iron is unknown. If absorption of ferritin iron via the endocytic receptor were regulated more efficiently than ferrous iron absorption via DMT1, or heme iron, dietary recommendations in transfusion-associated iron overload could be modified to increase the consumption of nonheme iron from ferritin-rich foods and eliminate the extra iron entering the body from food. The research to study iron regulation of gut receptors for iron from ferritin, especially during transfusion iron overload, remain to be performed. Because the mechanism of ferritin absorption is different than heme or ferrous salts, and more iron enters/absorption event, regulation of ferritin iron absorption might be more sensitive to body iron than heme or ferrous salts. If so, legumes rich in protein and ferritin might be more efficacious food than meat.
Summary and conclusions
Ferritin mineralized protein cages are the sites of much of the extra body iron introduced by lifetime infusions of red bloods cells to correct Cooley’s Anemia. The finite capacity of the body to synthesize ferritin and the toxic effects hemosiderin, which is ferritin damaged by too much iron, require iron removal by chelators. Three novel, complementary approaches are outlined. (1) Targeting iron chelators (current or in the future) to ferritin protein pores by linking to a synthetic peptide known to modify the pores and increase iron removal and chelation ex vivo; (2) recruiting the ferritin mRNA unused during iron overload, with small, IRE-inactivating molecules to synthesize more ferritin protein and minimize hemosiderin; (3) Explore the regulation of ferritin iron absorption by the gut during iron overload with the goal of minimizing dietary iron absorption by increasing the intake of foods such as legumes that are rich in ferritin iron.
Acknowledgments
NIH (DK20251); Condycet (Project number 1050068), CHORI and Cooley’s Anemia Foundations. The author gratefully acknowledges the creative contributions and thoughtful discussion made by members of the Theil Group and our many colleagues.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest; NIH funded.
References
- 1.Liu X, Theil EC. Ferritin: dynamic management of biological iron and oxygen chemistry. Acc Chem Res. 2005;38:167–175. doi: 10.1021/ar0302336. [DOI] [PubMed] [Google Scholar]
- 2.Murray-Kolb LE, Welch R, Theil EC, Beard JL. Women with low iron stores absorb iron from soybeans. Am J Clin Nutr. 2003;77:180–184. doi: 10.1093/ajcn/77.1.180. [DOI] [PubMed] [Google Scholar]
- 3.Davila-Hicks P, Theil EC, Lonnerdal B. Iron in ferritin or in salts (ferrous sulfate) is equally bioavailable in nonanemic women. Am J Clin Nutr. 2004;80:936–940. doi: 10.1093/ajcn/80.4.936. [DOI] [PubMed] [Google Scholar]
- 4.San Martin CD, Garri C, Pizarro F, et al. Caco-2 intestinal epithelial cells absorb soybean ferritin by mu2 (AP2)-dependent endocytosis. J Nutr. 2008;138:659–666. doi: 10.1093/jn/138.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalgaonkar S, Lonnerdal B. Effects of dietary factors on iron uptake from ferritin by Caco-2 cells. J Nutr Biochem. 2008;19:33–39. doi: 10.1016/j.jnutbio.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theil EC. Iron, ferritin and nutrition. Annu Rev Nutr. 2004;24:327–343. doi: 10.1146/annurev.nutr.24.012003.132212. [DOI] [PubMed] [Google Scholar]
- 7.Walter PB, Harmatz P, Vichinsky E. Iron metabolism and iron chelation in sickle cell disease. Acta Haematol. 2009;122:174–183. doi: 10.1159/000243802. [DOI] [PubMed] [Google Scholar]
- 8.Liu XS, Patterson LD, Miller MJ, Theil EC. Peptides selected for the protein nanocage pores change rates of iron recovery from the ferritin mineral. J Biol Chem. 2007;282:31821–31825. doi: 10.1074/jbc.C700153200. [DOI] [PubMed] [Google Scholar]
- 9.Khan MA, Walden WE, Goss DJ, Theil EC. Direct Fe2+ sensing by iron-responsive messenger RNA × repressor complexes weakens binding. J Biol Chem. 2009;284:30122–30128. doi: 10.1074/jbc.M109.041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallander ML, Leibold EA, Eisenstein RS. Molecular control of vertebrate iron homeostasis by iron regulatory proteins. Biochim Biophys Acta. 2006;1763:668–689. doi: 10.1016/j.bbamcr.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol. 2006;2:406–414. doi: 10.1038/nchembio807. [DOI] [PubMed] [Google Scholar]
- 12.Theil EC, Goss DJ. Living with iron (and oxygen): questions and answers about iron homeostasis. Chem Rev. 2009;109:4568–4579. doi: 10.1021/cr900052g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treffry A, Lee PJ, Harrison PM. Iron-induced changes in rat liver isoferritins. Biochem J. 1984;220:717–722. doi: 10.1042/bj2200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melefors O, Goossen B, Johansson HE, et al. Translational control of 5-aminolevulinate synthase mRNA by iron-responsive elements in erythroid cells. J Biol Chem. 1993;268:5974–5978. [PubMed] [Google Scholar]
- 15.Ke Y, Theil EC. An mRNA loop/bulge in the ferritin iron responsive element (IRE) formed in vivo, and detected by radical probing with Cu-phen and protein (IRP) footprinting. J Biol Chem. 2002;277:2373–2376. doi: 10.1074/jbc.C100614200. [DOI] [PubMed] [Google Scholar]
- 16.Walden WE, Selezneva AI, Dupuy J, et al. Structure of dual function iron regulatory protein 1 complexed with ferritin IRE-RNA. Science. 2006;314:1903–1908. doi: 10.1126/science.1133116. [DOI] [PubMed] [Google Scholar]
- 17.Wang YH, Sczekan SR, Theil EC. Structure of the 5′ untranslated regulatory region of ferritin mRNA studied in solution. Nucleic Acids Res. 1990;18:4463–4468. doi: 10.1093/nar/18.15.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Addess KJ, Basilion JP, Klausner RD, et al. Structure and dynamics of the iron responsive element RNA: implications for binding of the RNA by iron regulatory binding proteins. J Mol Biol. 1997;274:72–83. doi: 10.1006/jmbi.1997.1377. [DOI] [PubMed] [Google Scholar]
- 19.Gdaniec Z, Sierzputowska-Gracz H, Theil EC. Iron regulatory element and internal loop/bulge structure for ferritin mRNA studied by cobalt(iii) hexamine binding, molecular modeling, and NMR spectroscopy. Biochemistry. 1998;37:1505–1512. doi: 10.1021/bi9719814. [DOI] [PubMed] [Google Scholar]
- 20.Laftah AH, Latunde-Dada GO, Fakih S, et al. Haem and folate transport by proton-coupled folate transporter/haem carrier protein 1 (slc46a1) Br J Nutr. 2009;101:1150–1156. doi: 10.1017/S0007114508066762. [DOI] [PubMed] [Google Scholar]
- 21.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- 22.Severance S, Hamza I. Trafficking of heme and porphyrins in metazoa. Chem Rev. 2009;109:4596–4616. doi: 10.1021/cr9001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takagi H, Shi D, Hall Y, et al. Localized unfolding at the junction of three ferritin subunits. A mechanism for iron release? J Biol Chem. 1998;273:18685–18688. doi: 10.1074/jbc.273.30.18685. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Jin W, Theil EC. Opening protein pores with chaotropes enhances Fe reduction and chelation of Fe from the ferritin biomineral. Proc Natl Acad Sci USA. 2003;100:3653–3658. doi: 10.1073/pnas.0636928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hintze KJ, Katoh Y, Igarashi K, Theil EC. Bach1 repression of ferritin and thioredoxin reductase1 is heme-sensitive in cells and in vitro, and coordinates expression with heme oxygenase1, beta-globin and NADP(h) quinone (oxido)reductase1. J Biol Chem. 2007;282:34365–34371. doi: 10.1074/jbc.M700254200. [DOI] [PubMed] [Google Scholar]
- 26.Ke Y, Sierzputowska-Gracz H, Gdaniec Z, Theil EC. Internal loop/bulge and hairpin loop of the iron-responsive element of ferritin mRNA contribute to maximal iron regulatory protein 2 binding and translational regulation in the iso-iron-responsive element/iso-iron regulatory protein family. Biochemistry. 2000;39:6235–6242. doi: 10.1021/bi9924765. [DOI] [PubMed] [Google Scholar]