Abstract
The introduction of prostanoid therapy has revolutionized the treatment of pulmonary arterial hypertension (PAH). However, continuous intravenous prostacyclin infusion poses significant risks and challenges, particularly in children. Inhaled treprostinil has been shown to be safe and efficacious in adults. This study describes the safety and efficacy of inhaled treprostinil in children with PAH. A retrospective analysis of 29 children, treated with inhaled treprostinil for ≥ 6 weeks was performed. Effects of inhaled treprostinil on exercise capacity, functional class, echocardiographic, and hemodynamic data were evaluated. Adverse events were documented. Patients received 3 - 9 breaths (6ucg/breath) of inhaled treprostinil 4 times daily. All were receiving background PH therapy; 12 had previously received parenteral prostanoid. Inhaled treprostinil was discontinued in 4 patients because of symptoms including cough and bronchospasm (3) and progression of PAH (1). Mild side effects including cough (9), sore throat(6) did not require discontinuation of therapy. The WHO functional class improved in 19 and was unchanged in 10; exercise capacity significantly improved, with the six minute walk distance (6MWD) improving on follow-up from 455.7+/-71.5 to 498+/-70 meters, (p=0.01) and peak oxygen consumption (pVO2) increasing from 25.5+/-10.2 to 27.4+/-10 (p=0.04). In conclusion, inhaled treprostinil was associated with improvement in exercise capacity and WHO functional class when added to background targeted PAH therapy in children, and had an acceptable safety profile. Based on these early data, further study of inhaled treprostinil appears warranted in pediatric PAH patients.
Keywords: pulmonary arterial hypertension, inhaled treprostinil, pediatric patients, safety and tolerability
Outcomes for children with pulmonary arterial hypertension (PAH) have improved over the past decade since the introduction of advanced PAH therapies. Parenteral prostanoids have increased long-term survival and improved quality of life in PAH.1-7 However, intravenous (IV) and subcutaneous (SQ) prostanoid therapies have significant complications related to the delivery system. Therefore, research has been intensely concentrated on finding other therapeutic targets including endothelin receptor antagonists (ERA), phosphodiesterase-5 (PDE-5) inhibitors, and inhaled prostanoids with more favorable risk-benefit profiles.8-14 Although the use of inhaled iloprost has been reported in children, the delivery system requires 6-9 times per day dosing.15-17 Inhaled treprostinil, a longer acting prostanoid, enabling four times a day dosing, was approved for use in adults in 2010.18 In this descriptive report, we present the early safety and efficacy data of adding inhaled treprostinil to background PAH therapy in children.
Methods
To evaluate the safety, efficacy and tolerability of inhaled treprostinil in children, we retrospectively reviewed the data from all Group 1 PAH patients treated with inhaled treprostinil for at ≥6weeks, at 2 large pediatric pulmonary hypertension Centers, (Columbia University Medical Center and Children's Hospital CO). Indications for initiation of inhaled treprostinil therapy included symptomatic PAH on background therapy, or as a strategy to transition select patients off parenteral prostanoids. Before the initiation of inhaled treprostinil, all children were trained on proper nebulizer technique with the OPTINEB device, (a hand-held ultrasonic, single-breath nebulizer Opti-Neb, Metropolitan Medical, Inc., Winchester, Virginia), and therapy was started at 3 breaths (6 mcg/breath) four times daily. Specialty home nursing was utilized to train the subjects on the technique and monitor therapy. In general, the dose was up-titrated weekly as tolerated as determined by weekly phone call, to a maximum of 9 breaths (54 micrograms/treatment) four times daily (Table 1). Some younger patients were intentionally held at 4- 6 breaths/treatment. Some patients with cough or known asthma were treated with a bronchodilator before treatment. Baseline data collected before initiation of inhaled treprostinil included: demographics, functional class, serum BNP levels, echocardiographic parameters, 6 minute walk (6MWD), cardiopulmonary exercise testing (CPET) and cardiac catheterization with vaso-reactivity testing. Pulmonary function testing (PFT) data included forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, mid-volume forced expiratory flow (FEF 25-75).
Table 1.
Administration of inhaled treprostinil (dosage and frequency at initiation, 6 months and 1 year follow-up)
| Variable | Initiation (n = 29) | 6 Months (n = 23) | 12 Months (n = 19) |
|---|---|---|---|
| Dose in breaths per treatment (1 breath = 6 microgram) | 1-3 breaths | 8 ± 1.73 breaths | 7.8 ± 1.9 breaths |
| Time to maximum dose | 5.7 ± 4.2 weeks | ||
| Median breaths; (Range) | 8; (3-9) | 9; (3-9) | 9; (3-9) |
| Frequency (treatments/day) | 4 | 4 | 4 |
| Total daily dose (μg) mean ±SD | 24-72 | 192 ± 41.6 | 187.2 ± 45.6 |
Echocardiographic parameters included baseline diagnostic study, tricuspid regurgitation (TR) gradient to estimate right ventricular systolic pressure (mmHg), pulmonary regurgitation (PR) gradient (mmHg) and RV function. Hemodynamic parameters recorded at cardiac catheterization on room air, 100% oxygen and iNO (40-80PPM) included mean right atrial pressures (mRAP), mean pulmonary arterial pressure (mPAP), pulmonary capillary wedge pressure (PCWP), mean arterial pressure (MAP). Oxymetry included mixed venous, pulmonary artery, pulmonary vein (when available) and systemic arterial saturations (SaO2). Parameters calculated were systemic and pulmonary blood flows (Qs and Qp L/min), and indexed resistances (Rs and Rp in wood units*m2). The 6MWD test was performed in children ≥ 6 years old. Borg dyspnea scores were recorded. Heart rate and SaO2 were measured at rest and at 6 minutes and the distance walked was recorded in meters. WHO functional class obtained from the history was included for analysis. BNP (pg/ml) was recorded when available. CPET measurements included peak workload (watts), peak oxygen consumption (pVO2) ml/kg/min, VE/VCO2 and ETCO2.
Patients were followed at 3-6 month intervals and data collected included functional class, echocardiogram, BNP levels, 6MWD, CPET, PFTs, and hemodynamics when available. Adverse events and need to stop or alter the inhaled treprostinil dose were documented.
We compared the 6MWD, WHO class, BNP, CPET and hemodynamics at baseline and follow-up. Because this was a retrospective descriptive analysis, the study was not powered to detect a specific effect. Summary statistics were prepared for numeric results. All values are given as either median with range or mean ± SD. Paired 2-tailed Student t tests were used to compare key variables at baseline and on latest follow-up; p < 0.05 was considered statistically significant.
Results
Twenty-nine consecutive pediatric patients with Group1 PAH who received inhaled treprostinil for ≥ 6 weeks are included. Baseline demographic data are listed in Table 2. There were 14 males and 15 females, mean age 11.3±4.5 years (median 12, range 3.2-19), with IPAH/FPAH (n=19) or APAH (n=10). Average treatment duration was 15.7± 8.2 months; (median 17, range1.5-27.5). All patients were on background therapy. Four patients were on calcium channel blockers, 26 on PDE5 inhibitors, 22 on ERA and 18 on dual therapy. Twelve patients had previously been on parenteral prostanoids for 4.7±3.3 years. Six of these patients were on inhaled iloprost in the interim period between IV/SQ prostanoid and inhaled treprostinil. Twenty patients reached the target dose of 9 breaths/treatment four times daily, and 9 were held at 4-8 breaths due to young age or side effects.
Table 2.
Patient characteristics, prior and concomitant targeted therapy and follow-up duration
| Patient | Age (years) | Type of PAH | Gender | Diagnosis | PDE5 | ERA | PGI2 | Previous PGI2 (years) | iTre f-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.2 | APAH | F | CHD | S | B | 11 | ||
| 2 | 4.6 | APAH | M | CDH | S | B | 19.5 | ||
| 3 | 6.7 | APAH | M | CHD | B | 23.6 | |||
| 4 | 10.5 | APAH | F | CHD | S | A | 1.5 | ||
| 5 | 11.8 | APAH | F | CHD | C | B | 19.1 | ||
| 6 | 14.2 | APAH | M | HIV | B | E,I | 4 | 27.3 | |
| 7 | 15 | APAH | F | CHD | C | E,T | 3 | 24.8 | |
| 8 | 15.3 | APAH | M | Down CHD | C | E,T | 11 | 26.1 | |
| 9 | 17.9 | APAH | F | CHD | C | A | E,T,I | 7 | 19.9 |
| 10 | 19.2 | APAH | M | CHD | C | E,T,I | 2 | 17 | |
| 11 | 5.2 | IPAH | F | C | 5.3 | ||||
| 12 | 5.6 | IPAH | M | S | A | 24 | |||
| 13 | 6.2 | IPAH | F | S | A | 19.9 | |||
| 14 | 6.3 | IPAH | M | C | A | 3 | |||
| 15 | 7.1 | IPAH | F | S | A | 7 | |||
| 16 | 7.1 | IPAH | F | S | A | 7.2 | |||
| 17 | 9.3 | IPAH | M | S | B | 13.1 | |||
| 18 | 9.8 | IPAH | F | S | E,T | 2 | 1.9 | ||
| 19 | 9.9 | IPAH | F | S | B | 5 | |||
| 20 | 10.5 | IPAH | F | C | B | E | 8 | 26.5 | |
| 21 | 11.3 | IPAH | M | S | B | 26.2 | |||
| 22 | 12.1 | IPAH | F | C | B | E,I | 9 | 17.3 | |
| 23 | 12.3 | IPAH | F | S | 21 | ||||
| 24 | 12.4 | IPAH | F | S | A | 17 | |||
| 25 | 14 | IPAH | M | S | A | 9.9 | |||
| 26 | 14.2 | IPAH | M | C | B | E | 6 | 10.3 | |
| 27 | 14.8 | IPAH | M | C | B | E | 4 | 16.3 | |
| 28 | 15.3 | IPAH | M | C | A | E,T,I | 5 | 2.3 | |
| 29 | 16.6 | IPAH | M | S | B | E,I | 9 | 13.9 |
Abbreviations: A-Ambrisentan; APAH- Associated pulmonary arterial hypertension, B-Bosentan; C-Cialis (tadalafil); CDH- Congenital diaphragmatic hernia; CHD- Congenital heart defect; E- Epoprostenol; ERA- endothelin receptor antagonist; f-up- follow-up duration (months); I- Iloprost; IPAH- idiopathic pulmonary arterial hypertension; i Tre- inhaled Treprostinil; PDE5- phosphodiesterase inhibitor; PGI2- Prostacyclin; S- Sildenafil; T -parenteral treprostinil.
Four patients discontinued inhaled treprostinil after 4.33± 4 months because of desaturation (1), dyspnea and chest tightness with bronchospasm demonstrated on PFTs (2), and progression of PAH requiring IV prostanoids (1). Children with bronchospasm reported chest tightness after each inhalation. Two patients had their dosage down titrated because of nausea (1) and hypotension (1). Hypotension was suspected as the child developed dizziness and fatigue after each treatment. At follow-up catheterization, inhaled treprostinil caused a 15% fall in arterial pressure and was down titrated from 54 to 36 mcg/treatment. Of the remaining, the most common self-limited and mild side effects were cough (9), sore throat (6), headache and nausea (4). Two patients died. Both had tolerated therapy and titrated up to 54 ucg four times daily without side effects. One, a 10 year old girl with un-operated hemitruncus and a prior history of recurrent hemoptysis died suddenly after a bout of severe hemoptysis after 6 weeks of therapy. Another 11 year old severely depressed child, who had initially improved FC with inhaled treprostinil, stopped all medications, and died shortly after.
Twelve patients had received prostanoids in the past. In 3 patients, IV prostanoids were weaned to 15 ±5 ng/kg/min and inhaled treprostinil was initiated during the last 3 weeks of wean before discontinuation of intravenous therapy. 19 Six were switched from iloprost to inhaled treprostinil for ease of administration, one of whom preferred iloprost and switched back. Three other patients had IV prostanoids weaned off to oral medications 4.6 ± 2.5 years prior to adding inhaled treprostinil to optimize vasodilator therapy. The baseline profile of patients who were prostanoid naïve and those who were on prior prostanoids is shown on Table 3. The patients who were previously on prostanoids had better hemodynamics as evidenced by lower Rp and Rp/Rs suggesting that they had improved enough on the prior IV prostanoids to have been considered optimal candidates to be weaned off parenteral prostanoids.
Table 3.
Baseline Profile of patients who were prostanoid naive vs those transitioned from prior prostanoids
| Prior prostanoid | Age | APAH/IPAH | F/M | Dose i-Tre | 6MWD | Rp* | Rp/Rs# |
|---|---|---|---|---|---|---|---|
| Naïve (N=17) | 10.2±4.6 | 6/11 | 9/8 | 6.6±3.2 | 477±149 | 14.5±6 | 1 ± 0.5 |
| Transitioned (N=12) | 13.8±3.2 | 5/7 | 6/6 | 7±3.5 | 491±128 | 9.6±4.6 | 0.6 ± 0.3 |
Naïve (no prior prostanoid); Transitioned (previous prostanoid); i-Tre; final inhaled treprostinil dose in inhalations; 6MWD- 6 minute walk distance; Rp- pulmonary vascular resistance index in wood units.m^2; Rp/Rs pulmonary to systemic resistance ratio at baseline catheterization.
P<0.03
p<0.01
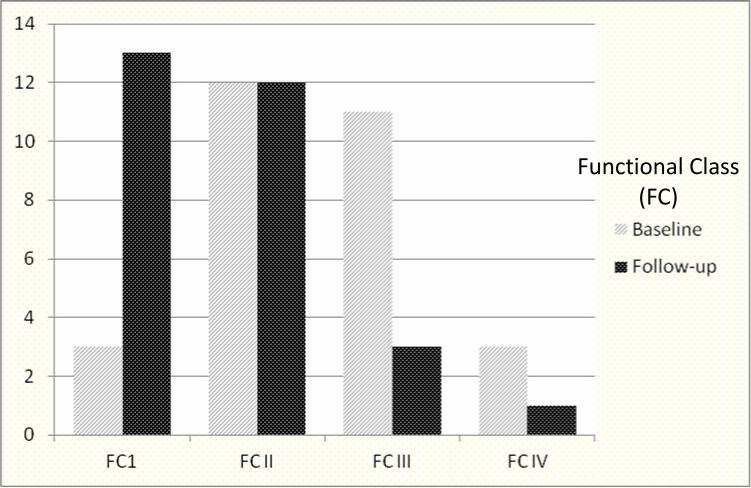
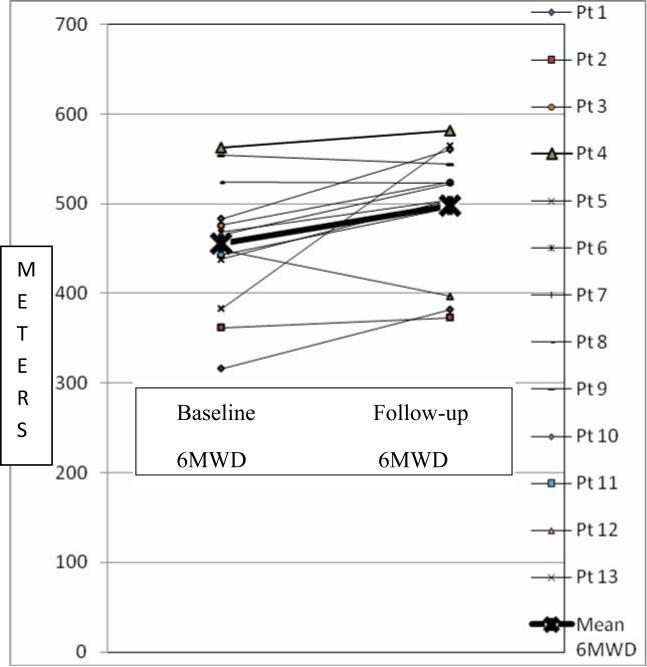
Figures 1 and Table 4 depict the improvement in exercise capacity from baseline compared to the most recent follow up in patients who had these data available. Baseline WHO functional class (FC) (FCI = 3, FCII = 12, FCIII = 11, FCIV = 3) was compared to follow up (FC I = 13, FCII = 12, FCIII = 3, FCIV = 1) and demonstrated improved WHO FC in 19 and unchanged FC in 10. The 3 patients with baseline FC I symptoms had echocardiographic and catheterization evidence of increasing PAH despite being asymptomatic, and hence were started on the inhaled therapy, and remained FC I. Figure 2 demonstrates the improvement in 6MWD from baseline to follow-up.
Figure 1.
Comparison of exercise capacity -WHO functional class (FC) at baseline and at latest follow-up on inhaled treprostinil. The X axis represents Functional class at baseline and follow-up and Y axis represents number of patients in each group.
Table 4.
Exercise capacity and BNP values at baseline and on follow-up
| Variable | N | Baseline | Follow-up on iTre | P Value |
|---|---|---|---|---|
| 6MWD | 13 | 456±72 | 498±70 | 0.017 |
| Watts | 9 | 54.3±29 | 67.7±29.1 | 0.0002 |
| pVO2 | 11 | 25.5±10 | 27.4±10.2 | 0.04 |
| BNP (pg/ml) | 13 | 93+77 | 58+62 | 0.003 |
BNP- Brain natriuretic peptide. N= number of patients with both baseline and follow-up data available 6MWD= 6 minute walk distance; pVO2= peak oxygen consumption; Watts- exercise capacity
Figure 2.
Change in 6 minute Walk Distance (6MWD) from baseline to latest follow-up. The thick line represents the mean values for the group. The X axis depicts baseline and follow-up values for each patient. The Y axis represents the meters walked in 6 minutes.
On echocardiography, mean TR gradient reduced from 76 ± 23mmHg to 52 ± 12mmHg at 9-12 month follow up; (n=15; P<0.05) and further reduced at 18 months to 48± 22 (n=6). Baseline hemodynamic data (n=26) showed mRAP of 7.2±3; mPAP 56.8±22; mSAP 69.7±20; Rp 13.1± 12.2; Rp/Rs 0.9±0.7. Ten patients were vasodilator responsive. Follow up cardiac catheterization was available in 8 patients and right heart parameters were improved, however the numbers were too small for meaningful statistics.
Data on acute testing of PFTs pre and post inhaled treprostinil inhalation was available in 6 patients. Seven had a previous history of bronchodilator therapy and 3 received pretreatment bronchodilator for cough. Inhaled treprostinil induced bronchospasm was seen on PFTs in 2 patients with chest tightness, who were eventually weaned off to other medications.
Discussion
Inhaled iloprost was initially studied in children with PAH in 22 pediatric patients and was found to have acceptable safety and tolerability.20 Iloprost therapy requires treatments every 2-4 hours, with each treatment taking 5-20 minutes. The delivery of inhaled iloprost can be difficult for young children due to the need for actuated breaths with the iNeb device. The availability of a longer acting inhaled prostanoid, inhaled treprostinil has enabled patients to transition from IV/SQ prostanoid or iloprost while maintaining improvements in clinical parameters. Treprostinil is a thermo stable tricyclic benzidene prostacyclin analog with an elimination half-life of 4.6 hours, allowing a 4 times a day therapeutic regimen.21 Several adult studies, including the placebo controlled TRIUMPH study have demonstrated safety and improved exercise capacity in adults with inhaled treprostinil added to background therapy.17,22,23 In this retrospective observational study, we studied the early safety and tolerability profile of inhaled treprostinil added to background targeted PAH therapy in children and found that the medication is well tolerated in most children, with an acceptable side effect profile.
It has been suggested that prior parenteral epoprostenol may serve as an “induction” therapy leading to favorable outcome in patients with severe PAH.18,19 Thus inhaled treprostinil may be an option to be used either as a therapeutic strategy to wean from intravenous prostanoid therapy, or as “optimizing” additional therapy when oral agents alone are perceived to be inadequate in controlling the PAH, as was done in several of our patients. It must be emphasized that patient selection is very important in the decision to wean from IV epoprostenol, which currently still remains the choice of therapy in very sick patients with severe PAH and/ or heart failure. However, in patients in whom maintaining an intravenous catheter becomes problematic because of infections or line breakages, transitioning to inhaled prostanoids is a viable option. In patients who are stable on dual oral therapy, addition of inhaled treprostinil is an attractive alternative to placing an indwelling line and starting parenteral prostanoid therapy to optimize vasodilator therapy and improve functional capability. An interesting effect described by several patients, is an “energy boost” after taking the inhaled treprostinil inhalation, which is sustained for 4-6 hours, suggesting indirectly, acute improvement in pulmonary hemodynamics. The improved functional class noted on follow-up possibly reflects the sustained vasodilatory effect of prostanoids as a group.4,5 Since the inhalation equipment is small and portable, all the children continue to attend regular school, and school nurses are trained to supervise the inhalations, ensuring improved patient compliance.
Our results support that inhaled treprostinil is well tolerated in the pediatric age group and is easy to administer even in young children. The group included one 5 year old with Down syndrome, who could be trained to self-administer the inhalation. Though treatment goal was 9 breaths (54 mcg) of inhaled treprostinil, some younger patients were held at lower doses, and still demonstrated significant improvement in exercise tolerance and WHO functional class. Though the TRIUMPH trial used a dose of 54 mcg in adults, no pediatric dosage has been studied. Hemodynamic side effects were rarely noted with inhaled treprostinil in our study (as well as in adult studies), suggesting that the effect on the pulmonary vasculature is mainly local.21,23 The usual systemic effects of intravenous epoprostenol, including flushing, diarrhea and jaw pain were rare in the patients studied. Compliance with inhaled therapy was good with 23/29 patients continuing therapy without difficulty. Throat irritation and cough is an important side effect, which seemed to improve with continued usage but at times required topical throat anesthetics to control. Proper technique was reinforced to maximize delivery into the trachea and not the mouth, by placing the Opti-neb further back in the mouth to minimize deposition of aerosolized drug in the throat. Chest tightness and cough was the cause for stopping medication in 2 patients, with acute bronchospasm demonstrated on PFT testing. However, there was also a history of asthma in 10 patients, who were on a combination of inhaled steroids and bronchodilators at baseline, and tolerated the inhaled treprostinil without worsening of symptoms. Pulmonary function testing before and after inhalation of inhaled treprostinil may be a valuable method to select patients prior to initiation of inhaled treprostinil therapy, and should be considered in those patients with cough or chest discomfort during and after inhalation and those with pre-existing asthma. Pre-treatment with bronchodilators and steroids was successful in preventing bronchospasm in some patients. Patients with very severe reactive airway disease may not be candidates for inhaled treprostinil therapy or may need additional therapy for their asthma consistent with previous reports by Ivy et al with inhaled iloprost. 20
Over a follow-up of 3-18 months, the majority of patients had an improvement in WHO functional class, as well as improved 6MWD and peak VO2. This was similar to results from the TRIUMPH trial as well as the initial pilot study by Channick et al, where inhaled treprostinil was added to background bosentan therapy. 23 Echocardiographic as well as hemodynamic parameters of PAH showed improvement in this group. An interesting finding was improvement in BNP levels in the patients, even in those whose levels were < 100 (suggesting improvement in RV myocardial function). These data collected on 29 children treated with inhaled treprostinil in addition to background therapy suggest that inhaled treprostinil is safe, and leads to improvement in exercise capacity, hemodynamics, and WHO functional class.
One limitation to this study is the small number of patients and short follow-up duration. Since this study represents use of inhaled treprostinil as an “add on” therapy, it does not reflect the efficacy of the medication as a single drug in treatment naïve patients. A longer term, large multicenter placebo controlled double blinded study is necessary to validate the results of this current pilot report. Inhaled treprostinil may be a viable treatment option for symptomatic PAH children who are not optimized on their background oral targeted PAH therapy, or those who do not tolerate SQ or IV prostanoids.
Acknowledgments
Funding source : Institution a: None.
Institution b: This study was funded by National Institutes of Health (NIH) Specialized Centers of Clinically Oriented Research grant HL-084923-02, NIH/NCRR Colorado CTSI grant number UL1 RR025780, the Jayden DeLuca Foundation and the Leah Bult Pulmonary Hypertension Research Fund.
This study was funded by National Institutes of Health (NIH) Specialized Centers of Clinically Oriented Research grant HL-084923-02, NIH/NCRR Colorado CTSI grant number UL1RR025780, the Jayden DeLuca Foundation and the Leah Bult PH Research Fund. Columbia University and University of Colorado receive consultant fees from Actelion, Gilead, Pfizer and United Therapeutics.
The study was approved by Columbia University IRB # IRB-AAAF3463
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All children from the University of Colorado were enrolled and consented to this study as part of the PEACH protocol COMIRB (05-0551, Prospective Evaluation of Children with Pulmonary Hypertension)
References
- 1.Barst RJ, Rubin LJ, McGoon MD, Caldwell EJ, Long WA, Levy PS. Survival in primary pulmonary hypertension with long-term continuous intravenous prostacyclin. Ann Intern Med. 1994;121:409–415. doi: 10.7326/0003-4819-121-6-199409150-00003. [DOI] [PubMed] [Google Scholar]
- 2.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, Levy PS, Pietra GG, Reid LM, Reeves JT, Rich S, Vreim CE, Williams GW, Wu M. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 3.Berger RM, Beghetti M, Humpl T, Raskob GE, Ivy DD, Jing ZC, Bonnet D, Schulze-Neick I, Barst RJ. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet. 2012;379:537–546. doi: 10.1016/S0140-6736(11)61621-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barst RJ, McGoon MD, Elliott CG, Foreman AJ, Miller DP, Ivy DD. Survival in childhood pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation. 2012;125:113–122. doi: 10.1161/CIRCULATIONAHA.111.026591. [DOI] [PubMed] [Google Scholar]
- 5.Delcroix M, Spaas K, Quarck R. Long-term outcome in pulmonary arterial hypertension: a plea for earlier parenteral prostacyclin therapy. Eur Respir Rev. 2009;18:253–259. doi: 10.1183/09059180.00003109. [DOI] [PubMed] [Google Scholar]
- 6.Yung D, Widlitz AC, Rosenzweig EB, Kerstein D, Maislin G, Barst RJ. Outcomes in children with idiopathic pulmonary arterial hypertension. Circulation. 2004;110:660–665. doi: 10.1161/01.CIR.0000138104.83366.E9. [DOI] [PubMed] [Google Scholar]
- 7.Moledina S, Hislop AA, Foster H, Schulze-Neick I, Haworth SG. Childhood idiopathic pulmonary arterial hypertension: a national cohort study. Heart. 2010;96:1401–1406. doi: 10.1136/hrt.2009.182378. [DOI] [PubMed] [Google Scholar]
- 8.Hislop AA, Moledina S, Foster H, Schulze-Neick I, Haworth SG. Long-term efficacy of bosentan in treatment of pulmonary arterial hypertension in children. Eur Respir J. 2011;38:70–77. doi: 10.1183/09031936.00053510. [DOI] [PubMed] [Google Scholar]
- 9.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 10.Oudiz RJ, Galiè N, Olschewski H, Torres F, Frost A, Ghofrani HA, Badesch DB, McGoon MD, McLaughlin VV, Roecker EB, Harrison BC, Despain D, Dufton C, Rubin LJ, ARIES Study Group Long-term ambrisentan therapy for the treatment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:1971–1981. doi: 10.1016/j.jacc.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 11.Ghofrani HA, Voswinckel R, Reichenberger F, Olschewski H, Haredza P, Karadaş B, Schermuly RT, Weissmann N, Seeger W, Grimminger F. Differences in hemodynamic and oxygenation responses to three different phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension: a randomized prospective study. J Am Coll Cardiol. 2004;44:1488–1496. doi: 10.1016/j.jacc.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 12.Badesch DB, Abman SH, Simonneau G, Rubin LJ, McLaughlin VV. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest. 2007;131:1917–1928. doi: 10.1378/chest.06-2674. [DOI] [PubMed] [Google Scholar]
- 13.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–788. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106:1477–1482. doi: 10.1161/01.cir.0000029100.82385.58. [DOI] [PubMed] [Google Scholar]
- 15.Olschewski H, Simonneau G, Galie N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347:322–329. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 16.Ivy DD. Prostacyclin in the intensive care setting. Pediatr Crit Care Med. 2010;11(2 Suppl):S41–45. doi: 10.1097/PCC.0b013e3181d10845. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLaughlin VV, Benza RL, Rubin LJ, Channick RN, Voswinckel R, Tapson VF, Robbins IM, Olschewski H, Rubenfire M, Seeger W. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol. 2010;55:1915–1922. doi: 10.1016/j.jacc.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 18.Barst R. How has epoprostenol changed the outcome for patients with pulmonary arterial hypertension? Int J Clin Pract. Suppl. 2010;168:23–32. doi: 10.1111/j.1742-1241.2010.02525.x. [DOI] [PubMed] [Google Scholar]
- 19.Melnick L, Barst RJ, Rowan CA, Kerstein D, Rosenzweig EB. Effectiveness of transition from intravenous epoprostenol to oral/inhaled targeted pulmonary arterial hypertension therapy in pediatric idiopathic and familial pulmonary arterial hypertension. Am J Cardiol. 2010;105:1485–1489. doi: 10.1016/j.amjcard.2009.12.075. [DOI] [PubMed] [Google Scholar]
- 20.Ivy DD, Doran AK, Smith KJ, Mallory GB, Jr, Beghetti M, Barst RJ, Brady D, Law Y, Parker D, Claussen L, Abman SH. Short and long-term effects of inhaled iloprost therapy in children with pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51:161–169. doi: 10.1016/j.jacc.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laliberte K, Arneson C, Jeffs R, Hunt T, Wade M. Pharmacokinetics and steady-state bioequivalence of treprostinil sodium (Remodulin) administered by the intravenous and subcutaneous route to normal volunteers. J Cardiovasc Pharmacol. 2004;44:209–214. doi: 10.1097/00005344-200408000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Benza RL, Seeger W, McLaughlin VV, Channick RN, Voswinckel R, Tapson VF, Robbins IM, Olschewski H, Rubin LJ. Long-term effects of inhaled treprostinil in patients with pulmonary arterial hypertension: the Treprostinil Sodium Inhalation Used in the Management of Pulmonary Arterial Hypertension (TRIUMPH) study open-label extension. J Heart Lung Transplant. 2011;30:1327–1333. doi: 10.1016/j.healun.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Channick RN, Olschewski H, Seeger W, Staub T, Voswinckel R, Rubin LJ. Safety and efficacy of inhaled treprostinil as add-on therapy to bosentan in pulmonary arterial hypertension. J Am Coll Cardiol. 2006;48:1433–1437. doi: 10.1016/j.jacc.2006.05.070. [DOI] [PubMed] [Google Scholar]