Abstract
Objective
To evaluate the clinical utility of tissue Doppler imaging (TDI) in assessment of disease severity and prognostic value in children with idiopathic pulmonary arterial hypertension (PAH).
Study design
A prospective study was performed to evaluate TDI velocities (Sm; systolic velocity, Em; early diastolic velocity, Am; late diastolic velocity), brain natriuretic peptide (BNP), New York Heart association (NYHA) functional class, and hemodynamics in 51 children (mean age;11.6 years) with idiopathic PAH. Fifty-one healthy children with comparable demographics served as controls.
Results
Em, Em/Am ratio, and Sm at mitral annulus, septum, and tricuspid annulus in PAH were significantly reduced compared with controls. Tricuspid Em had significant inverse correlations with plasma BNP levels (r=−0.60, p<0.001), right ventricular end-diastolic pressure (r=−0.79, p<0.001), and mean pulmonary arterial pressure (r=−0.67, p<0.001). Statistically significant differences were observed in tricuspid Em between NYHA functional class II versus combined III and IV (mean and standard deviation; 11.9+/−4.2 cm/s versus 8.2+/−3.6 cm/s, respectively, p=0.002). Cumulative event-free survival rate was significantly lower when tricuspid Em was ≤ 8 cm/s (log-rank test, p<0.001)
Conclusions
Tricuspid Em velocity correlated with NYHA functional class as disease severity and may serve as a useful prognostic marker in children with idiopathic PAH. The present study is the initial report to evaluate TDI velocities against mid-term outcome variables in a relatively large pediatric PAH population.
Keywords: echocardiography, diastolic dysfunction, prognosis, early diastolic velocity at tricuspid annulus
Tissue Doppler imaging (TDI) is a robust and reproducible echocardiographic tool which has been shown to be a quantitative assessment of both global and regional function. TDI is a simple and noninvasive method using two-dimensional Doppler echocardiography. Recently, TDI has emerged as a useful tool for evaluation of both ventricular systolic and diastolic function in various clinical conditions (1-6). The cardiac cycle is represented by 3 waveforms including Sm (systolic myocardial velocity), Em (early diastolic myocardial relaxation velocity), and Am (late diastolic myocardial velocity associated with atrial contraction). Decreased Sm and Em are significantly associated with cardiac mortality among the TDI variables in a large population with various cardiac diseases (7-10).
Idiopathic pulmonary arterial hypertension (PAH) is characterized by the progressive rise in pulmonary artery pressure, leading to right ventricular failure and death (11-14). Recent published studies have demonstrated that peak systolic and diastolic TDI velocities were reduced in adult patients with idiopathic PAH compared with normal controls in both the interventricular septum as well as the tricuspid annulus, whereas no significant differences were noted between idiopathic PAH patients and controls in the mitral annulus velocities (15). In addition, Sm at tricuspid annulus had a positive correlation with pulmonary arterial pressure or pulmonary vascular resistance and significant inverse correlation with cardiac index (16,17). These results suggested that assessment of right ventricular (RV) function is very important in patients with PAH as RV dysfunction leads to adverse outcomes.
The clinical application of TDI has previously been demonstrated in adult patients with idiopathic PAH (15). In children with idiopathic PAH, however, the correlations between TDI velocities and disease severity evaluated by hemodynamic parameters, 6 minutes walk distance, and natriuretic peptide have not been fully investigated. Furthermore, whether TDI measurements can provide prognostic values in pediatric idiopathic PAH is still unknown. We undertook this study to assess the clinical utility of TDI for evaluating disease severity and predicting outcomes in children with idiopathic PAH.
Methods
A prospective study was designed to evaluate the TDI velocities in children who are followed at Toho University Omori medical center (Tokyo, Japan) and University of Colorado School of Medicine, Children’s Hospital Colorado (CO, USA). The diagnosis of idiopathic PAH was established when all other causes of PAH were excluded by echocardiography, blood test including autoantibodies and liver function, pulmonary function tests, pulmonary ventilation–perfusion scans, and catheterization. All children were prospectively enrolled in an IRB approved protocol between February 2007 and August 2011 at Toho University Omori Medical Center and Children’s Hospital Colorado. Children older than 18 years of age were excluded. A healthy control group of children without cardiac disease or abnormal echocardiographic findings was recruited at Children’s Hospital Colorado. Healthy children were enrolled based on age and sex criteria to closely match the distribution of these demographic factors in the PAH group. All healthy children underwent a complete conventional echocardiographic evaluation and TDI study.
Standard echocardiography
All children underwent routine two-dimensional, spectral, and color-flow Doppler and M-mode examinations. Standard echocardiographic evaluation included RV diastolic dimension, left ventricular (LV) end-diastolic dimension, LV end-systolic dimension. Mitral and tricuspid inflow Doppler with peak early diastolic velocity (E), peak late diastolic velocity (A), and E/A ratio were also measured. RV systolic pressure was estimated by the maximum velocity of the tricuspid regurgitant jet using the modified Bernoulli equation. Isovolumic contraction time, isovolumic relaxation time, and ejection time were obtained. Myocardial performance index is defined as the sum of the isovolumic contraction time and relaxation time divided by the ejection time.
Tissue Doppler imaging
TDI was recorded using spectral pulsed Doppler at a sweep rate of 100 mm/s. Pulsed Doppler sample volume was placed at the tricuspid annulus, interventricular septum, and lateral mitral annulus in the apical four-chamber orientation. Pulsed-wave TDI was performed to obtain peak systolic (Sm), early (Em), and late (Am) diastolic velocities at the tricuspid annulus, septum, and mitral annulus. The Nyquist limit was set from 10 to 30 cm/s, using minimal filter settings and optimal gains and reducing the sample volume size to less than 5 mm.
Clinical variables
Brain natriuretic peptide (BNP) levels, 6-minute walk distance, and right heart catheterization were included within 5 days of echocardiographic evaluation. New York Heart Association (NYHA) functional class was evaluated at each follow-up clinic visit in children who were over 6 years-old. Blood for measurement of plasma BNP concentrations was collected in ethylenediaminetetraacetic acid tubes. Plasma BNP was assayed on i-STAT® system using the two-site enzyme-linked immunosorbant assay (Abbott Laboratories, IL, USA). Right heart catheterization was performed with a balloon tipped, flow directed Swan-Ganz catheter and systemic arterial line for monitoring. We measured mean right atrial pressure, mean pulmonary arterial pressure, mean systemic blood pressure, RV end-diastolic pressure, and pulmonary capillary wedge pressure. Accordingly, cardiac output was obtained using thermo-dilution and cardiac index was calculated. Pulmonary vascular resistance was calculated by (mean pulmonary artery pressure – mean pulmonary wedge pressure)/pulmonic blood flow. We evaluated pulmonary vascular resistance index as by (mean pulmonary artery pressure – mean pulmonary wedge pressure)/cardiac index.
Statistical Analyses
Descriptive statistics were calculated using means with standard deviations for continuous variables. Static difference in clinical variables between children with idiopathic PAH and control subjects were performed using Student t-tests when comparing data with a normal distribution. If data did not show a normal distribution, we used a non-parametric test for analysis (Mann-Whitney U test). A chi-squared test was used to compare discrete variables such as sex. Mann-Whitney U-test was used for TDI velocities between NYHA functional class II and III or IV. The correlations with 6-minute walk distance, echocardiographic data, BNP levels, and hemodynamic variables were determined using Peason’s correlation coefficient test. A composite outcome was used which included the following adverse events; cardiac mortality, lung transplantation, and hospitalization due to heart failure. Only one adverse event was considered in each patient. The Receiver-Operating Characteristic curve was calculated from a logistic regression to evaluate the predictive ability of TDI for the prediction of the adverse event outcome. To further assess the predictive ability of TDI velocity while accounting for the time until an adverse event, the Kaplan-Meier method was used to compare survival curves for lower and higher TDI velocities which were compared with the log-rank test. The level of statistical significance was defined as p value of 0.01. Analyses were conducted using Statmate III for Windows (Atoms Co., Tokyo, Japan).
Results
Overall, 51 children with idiopathic PAH were enrolled. The mean and standard deviation age was 11.6 +/−4.1 years with 26 males and 25 females. At first echocardiographic evaluation, all patients received vasodilator therapies which included epoprostenol (n=20) or infusion of treprostinil (n=1), inhaled prostanoid (n=2), oral phosphodiesterase type 5 inhibitors (n=39), and endothelin receptor antagonists (n=19).
Clinical and echocardiographic characteristics of idiopathic PAH and controls
Clinical and routine echocardiography variables were compared between children with idiopathic PAH and healthy controls (Tables I and II; available at www.jpeds.com). Control subjects have similar distributions for age and sex (11.7 +/−4.2 years, 24 males and 27 females). In 5 of 51 children with PAH, E and A velocity at tricuspid inflow were not available. The RV dimension in diastole, LV dimension in systole and diastole, LV shortening fraction, RV myocardial performance index, acceleration time/ejection time ratio, E velocity and E/A ratio in mitral and tricuspid inflow, and tricuspid regurgitant velocity between the two groups were significantly different (p<0.001). However, LV myocardial performance index was not different (p=0.50).
Table 1. Clinical characteristics of children with idiopathic PAH and healthy controls.
| Variables | Idiopathic PAH | Healthy control | |
|---|---|---|---|
| (n=51) | (n=51) | p value | |
| mean +/− SD | mean +/− SD | ||
| Age (years) | 11.7 +/−4.2 | 11.6 +/−4.1 | 0.90 |
| Gender (male/female) | 26/25 | 24/27 | 0.69 |
| Body surface area (m2) | 1.2+/−0.3 | 1.4 +/−0.4 | 0.02 |
| Blood pressure (systolic) (mmHg) | 102.5+/−10.4 | 104.0+/−9.8 | 0.43 |
| Blood pressure (diastolic) (mmHg) | 57.0+/−7.4 | 59.4+/−9.3 | 0.17 |
| Heat rate at rest (/min) | 82.2+/−17.6 | 80.6+/−13.8 | 0.62 |
| Oxygen saturation at rest (%) | 97.3+/−1.7 | 98.2+/−2.6 | 0.01 |
SD; standard deviations
Table 2. Conventional echocardiographic characteristics of children with idiopathic PAH and healthy controls.
| Variables | Idiopathic PAH | Healthy control | |
|---|---|---|---|
| (n=51) | (n=51) | p value | |
| mean +/− SD | mean +/− SD | ||
| RV internal dimension in diastole (mm) | 23.5+/−6.0 | 18.1+/−4.9 | < 0.001 |
| LV internal dimension in diastole (mm) | 37.7+/−5.8 | 43.6+/−6.8 | < 0.001 |
| LV internal dimension in systole (mm) | 20.2+/−5.5 | 27.1+/−4.8 | < 0.001 |
| LV fractional shortening (%) | 47.1+/−10.9 | 37.4+/−4.3 | < 0.001 |
| RV myocardial performance index | 0.63+/−0.30 | 0.21+/−0.10 | < 0.001 |
| LV myocardial performance index | 0.43+/−0.16 | 0.36+/−1.69 | 0.50 |
| Acceleration time/ejection time (RV outflow) | 0.3+/−0.1 | 0.4+/−0.1 | < 0.001 |
| LV E velocity (cm/s) | 77.7+/−19.8 | 97.0+/−17.9 | < 0.001 |
| LV E/A ratio | 1.4+/−0.5 | 2.4+/−0.9 | < 0.001 |
| RV E velocity (cm/s)* | 53.6+/−12.9 | 63.1+/−15.9 | < 0.001 |
| RV E/A ratio* | 1.1+/−0.5 | 2.2+/−0.6 | < 0.001 |
| Tricuspid regurgitant velocity (m/s)¶ | 4.1+/−0.8 | 2.2+/−0.2 | < 0.001 |
LV; left ventricular, PAH; pulmonary arterial hypertension, RV; right ventricular, SD; standard deviations
46 cases (RV E and A velocity were not available in 5 children with PAH)
27aaees (TR eeloctty was not aaailable in 24 heal thy contols)
Comparison of TDI velocities in idiopathic PAH and controls
TDI measurements between idiopathic PAH and controls are displayed in Table III. In children with idiopathic PAH, Em, Em/Am ratio, and Sm at mitral annulus, septum, and tricuspid annulus are significantly decreased compared with controls. Am velocity was not different at mitral annulus and septum between idiopathic PAH and controls, but tricuspid Am velocity in PAH was higher than controls. In addition, E/Em ratio was significantly increased in idiopathic PAH compared with controls at mitral annulus, septum, and tricuspid annulus.
Table 3. Tissue Doppler velocity in children with idiopathic PAH and healthy controls.
| Variables | Idiopathic PAH (n=51) mean +/− SD |
Healthy control (n=51) mean +/− SD |
p value |
|---|---|---|---|
| Mitral | |||
| Em velocity (cm/s) | 12.7+/−5.5 | 20.5+/−3.4 | < 0.001 |
| Am velocity (cm/s) | 6.9+/−2.7 | 6.9+/−2.3 | 0.85 |
| Em/Am ratio | 1.8+/−0.2 | 3.3+/−1.2 | < 0.001 |
| E/Em ratio | 6.7+/−2.3 | 4.8+/−1.0 | < 0.001 |
| Sm velocity (cm/s) | 8.3+/−2.8 | 10.1+/−2.5 | < 0.001 |
| Septal | |||
| Em velocity (cm/s) | 8.1+/−3.7 | 15.5+/−3.0 | < 0.001 |
| Am velocity (cm/s) | 6.7+/−2.4 | 6.8+/−1.8 | 0.85 |
| Em/Am ratio | 1.3+/−0.6 | 2.4+/−0.8 | < 0.001 |
| E/Em ratio | 10.3+/−3.6 | 6.4+/−1.6 | < 0.001 |
| Sm velocity (cm/s) | 6.7+/−1.8 | 8.6+/−1.2 | < 0.001 |
| Tricuspid | |||
| Em velocity (cm/s) | 10.2+/−4.3 | 17.3+/−3.1 | < 0.001 |
| Am velocity (cm/s) | 11.4+/−3.2 | 9.5+/−3.3 | 0.01 |
| Em/Am ratio | 0.9+/−0.4 | 2.0+/−0.9 | < 0.001 |
| E/Em ratio* | 5.9+/−2.6 | 3.8+/−1.1 | < 0.001 |
| Sm velocity (cm/s) | 11.3+/−2.4 | 13.6+/−2.8 | < 0.001 |
NS; not significant, PAH; pulmonary arterial hypertension
46 cases (RV E velocity was not available in 5 cases)
Correlation with plasma brain natriuretic peptide and 6-minute walk distance
All PAH had plasma BNP levels performed and 6-minute walk distance was measured in 46 of 51 patients (Table IV; available at www.jpeds.com). Five children were not evaluated for 6-minute walk distance because of severe PAH symptoms or age less than 6 years-old. There was no significant correlation between BNP levels and Em at mitral annulus or septum, however tricuspid Em and E/Em ratio had significant correlations with plasma BNP levels (r=−0.60, p<0.001, r=0.48, p<0.01, respectively). Sm at mitral annulus, septum, and tricuspid annulus had no correlation with BNP levels. Although there was significant, but weak, correlation between mitral Sm correlation with 6-minute walk distance (r=0.50, p=0.001), neither septum nor tricuspid Sm correlated with 6-minute walk distance.
Table 4. BNP level, 6-minutes walk distance, and hemodynamic data in children with idiopathic PAH.
| Variables | Idiopathic PAH mean +/− SD |
|---|---|
|
| |
| BNP (pg/ml) (n=51) | 133.4+/−261.5 |
|
| |
| 6-minute walk distance (m) (n=46) | 473.0+/−113.5 |
|
| |
| Right heart catheterization (n=45) | |
| Mean right atrial pressure (mmHg) | 6.1+/−3.0 |
| Mean pulmonary arterial pressure (mmHg) | 59.9+/−20.2 |
| Pulmonary vascular resistance index (unitsxm2) | 15.7+/−8.2 |
| Pulmonary/systemic vascular resistance index ratio | 0.8+/−0.3 |
| RV end-diastolic pressure (mmHg) | 11.6+/−4.8 |
| Pulmonary wedge pressure (mmHg) | 8.8+/−2.3 |
| Cardiac index (l/min/m2) | 3.4+/−0.8 |
BNP; brain natriuretic peptide, PAH; pulmonary arterial hypertension, RV; right ventricular, SD, standard deviation
Correlation with hemodynamics
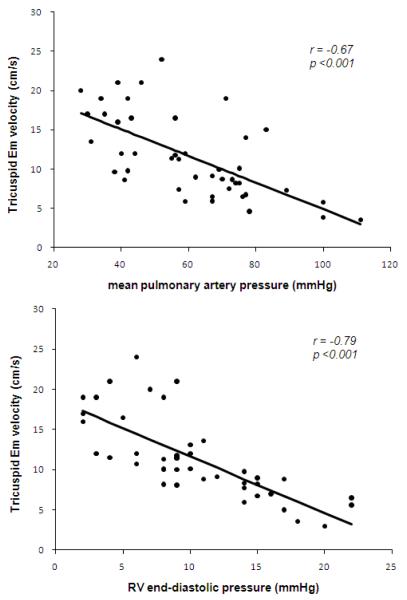
Forty-five (88%) of the PAH children had catheterization within 5 days of echocardiographic evaluation. Tricuspid Em demonstrated significant correlation with mean pulmonary artery pressure (r=−0.67, p<0.001), pulmonary vascular resistance index (r=−0.57, p<0.001), pulmonary/systemic vascular resistance index ratio (r=−0.49, p<0.001), cardiac index (r=0.35, p<0.01), and RV end-diastolic pressure (r=−0.79, p<0.001) (Figure 1). Similarly, tricuspid Sm was correlated with mean pulmonary artery pressure, but the correlation coefficient of Sm was lower than that of Em (r=−0.41, p<0.01). Neither Em nor Sm at the mitral annulus were correlated with any of the hemodynamic variables, although mitral E/Em ratio was significantly correlated with pulmonary wedge pressure (r=0.47, p<0.001).
Figure 1. Linear correlation between tricuspid Em velocity and mean pulmonary artery pressure, RV end-diastolic pressure in 45 children with idiopathic PAH.
Linear correlation between tricuspid Em velocity and mean pulmonary artery pressure, RV end-diastolic pressure in 45 children with idiopathic PAH
To investigate the potential for confounding, a multivariate regression was performed to investigate the association between tricuspid Em velocity and mean pulmonary artery pressure after adjusting for heart rate and body surface area. The association was robust after accounting for any contribution related to heart rate or body size (p < 0.001).
NYHA functional class
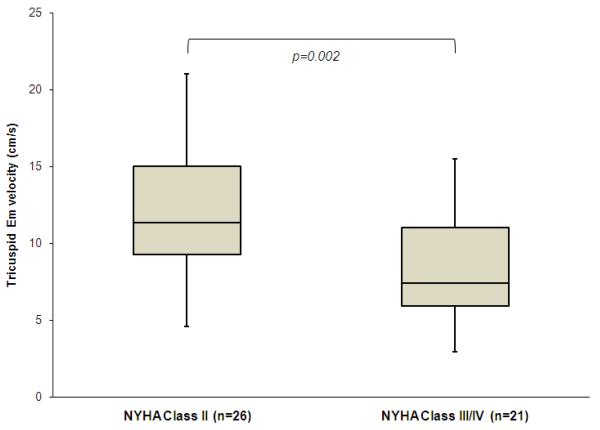
Overall, 47 children could be assessed by NYHA functional class and the remaining 4 children could not be evaluated due to age less than 6 years-old. Twenty-six children were in NYHA functional class II, 15 were in NYHA class III, and 6 were in NYHA class IV. Statistically significant differences were observed in tricuspid Em between NYHA functional class II versus III combined with IV (mean and standard deviation; 11.9+/-4.2 cm/s versus 8.2+/−3.6 cm/s, respectively, p=0.002) (Figure 2).
Figure 2. Comparison of tricuspid Em velocity in children with NYHA functional class II and class III or IV.
Forty-seven children who were ≥ 6 years-old could be evaluated. Tricuspid Em of the children with New York Heart Association (NYHA) functional class II (n=26) was significantly higher compared with those of class III or IV (n=21) by the Mann-Whitney U test.
Outcomes
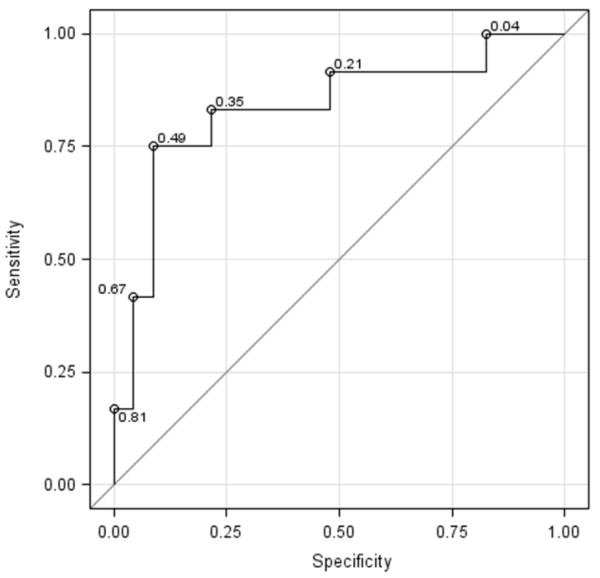
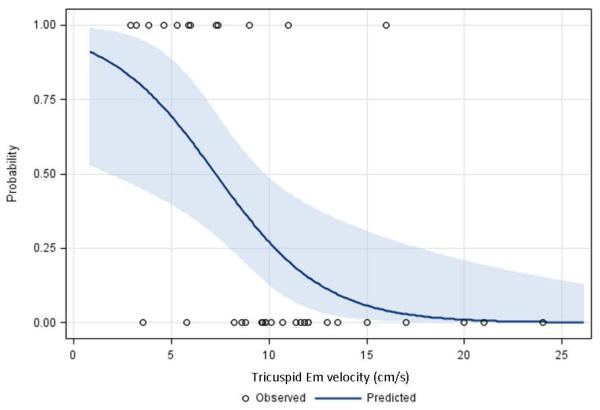
The median follow-up period for the PAH patients was 20 months (range; 3-51 months). Thirty-five (67%) children had follow-up data. Although no patient died during follow-up, there were 12 of 35 (34%) children who had an adverse event including initiation of hospitalization due to heart failure (n=11) and lung transplantation (n=1). An initial tricuspid Em cutoff value of 8 cm/s for prediction of the adverse event was evaluated. Eleven of 35 children with lower Em (< 8cm/s) had 9 events (82%) and the remaining 24 children with higher Em had only 3 events (13%) during follow-up. Receiver-Operating Characteristic curve corresponded to a c-index (area under the Receiver-Operating Characteristic curve) of 0.83. The cutoff value of 8 cm/s corresponds to a sensitivity of 75% and a specificity of 87% (Figure 3, A; available at www.jpeds.com). In addition, the relationship between the continuous predictor and the probability of the adverse event was investigated. The risk of having an event decreases as the value for tricuspid Em velocity increases and using a cutoff value of 8 cm/s provided desired sensitivity and specificity compared with other cutoffs (Figure 3, B).
Figure 3.
a Receiver-Operating Characteristic curve
b Predictive probabilities for adverse event
A, Logistic regression was used to evaluate the cutoff at 8 cm/s of initial tricuspid Em velocity for prediction of adverse events including death, hospitalization, and lung transplantation. This Receiver-Operating Characteristic curve corresponded to a c-index (area under the curve) of 0.83. The cutoff value of 8 cm/s corresponds to a sensitivity of 75% and a specificity of 87%. B, Plot of the relationship between the continuous predictor and the probability of the adverse event is displayed. The circles correspond to the observed outcomes (0 - did not have an adverse event, 1 - adverse event). As the value for tricuspid Em velocity increases, the risk of having an event decreases.
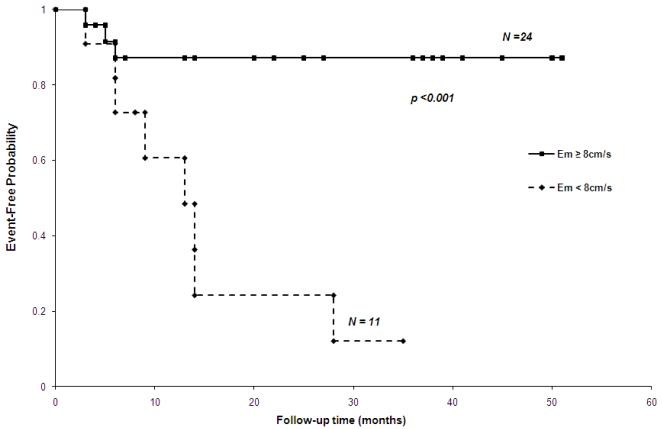
The impact of tricuspid Em < 8 cm/s on observed event-free probability was assessed by Kaplan-Meier analysis. The Cumulative event-free rate was significantly lower when the initial tricuspid Em was lower than 8 cm/s (log-rank test, p<0.001) (Figure 4).
Figure 4. Kaplan-Meier curve according to Em velocity at tricuspid annulus in 35 children with idiopathic PAH.
Kaplan-Meier curve. Tricuspid Em < 8 cm/s was associated with time until an adverse outcome in 35 children with idiopathic PAH. After 12 months, the adverse event rate in the patients classified in the < 8 cm/s group was 39% compared with 13% in the > 8 cm/s group.
Discussion
Several studies have demonstrated the correlation between hemodynamics and systolic or diastolic function using TDI or strain imaging in the PAH population. Most of these studies showed that tricuspid Sm had significant correlation with invasively determined mean pulmonary artery pressure in adult patients with various etiologies of PAH (17-19). Our results differ in that we found tricuspid Em had higher correlation coefficient with BNP levels and hemodynamics than those of tricuspid Sm. In the present study, our patients had high RV end-diastolic pressure (mean+/−standard deviation; 11.6+/−4.8 mmHg) which was significantly correlated with tricuspid Em. This correlation can explain the usefulness of tricuspid Em as a marker of detecting RV diastolic dysfunction. In addition, we found that tricuspid Em in NYHA functional class III or IV was significantly lower than those in class II.
This study highlights a clinically relevant application of TDI in the detection of RV diastolic dysfunction which may provide useful information of predicting outcomes in children with idiopathic PAH. Previous studies demonstrated that mitral Em is the best marker for predicting cardiac mortality among TDI variables in patients with LV dysfunction (9,20-22). However, there are no studies describing TDI velocities for predicting outcome in PAH. Most importantly, we found that tricuspid Em cutoff value of 8 cm/s predicted adverse event in children with idiopathic PAH.
We found diastolic and systolic dysfunction in children with idiopathic PAH as evaluated by lower TDI velocities in diastole and systole compared with healthy children. Interestingly, our results suggested systolic and diastolic dysfunction even in the LV because reduced Em and Sm at mitral annulus reflect the possibility of LV diastolic and systolic dysfunction. The mechanism of LV dysfunction noted in patients with idiopathic PAH is not clear, but LV systolic and diastolic performance may be secondarily influenced by mechanical interactions via the shared ventricular septum between both ventricles and LV dysfunction may develop during the progression of PAH (23-26).
Recently, strain and strain rate derived by TDI has been evaluated in patients with PAH (17,21,27). However, strain and strain rate as new methods have been unacceptable for routine clinical use due to the challenging techniques. In contrast, TDI is available in most echocardiographic machines during routine clinic visits.
Several limitations should be mentioned. Our study was limited by relatively small numbers. Therefore, a larger study involving children with idiopathic PAH is needed to determine whether our results can be generalized to the larger population. During follow-up, all patients survived owing to various vasodilator therapies. Thus, we could not determine whether TDI velocity was predictive of mortality in the present study. Furthermore, all patients were treated with pulmonary vasodilator therapies at the initial evaluation. We could not assess difference in TDI velocities before and after initiation of vasodilator therapies. Finally, echocardiography and right heart catheterization were not performed simultaneously. Despite these limitations, this prospective study demonstrated that TDI velocities can be noninvasively assessed for the evaluation of disease severity and prognosis in children with idiopathic PAH as a homogeneous group. We concluded that tricuspid Em velocity, a parameter of right ventricular diastolic dysfunction, correlated with NYHA functional class as disease severity and may serve as a useful prognostic marker in children with idiopathic PAH. Our study is the initial report to evaluate TDI velocities against mid-term outcome variables in a relatively large pediatric PAH population.
Acknowledgments
Supported by the Jayden DeLuca Foundation, the Leah Bult Foundation, Colorado Clinical Translational Science Institute (UL1 RR025780), National Center for Research Resources, and National Institutes of Health. D.I. serves as a member of the Gilead Sciences Research Scholars Program, and is a consultant for Actelion, Gilead, Pfizer, and United Therapeutics.
Abbreviations List
- Am
late diastolic myocardial velocity associated with atrial contraction
- BNP
brain natriuretic peptide
- Em
early diastolic myocardial relaxation velocity
- LV
left ventricle
- NYHA
New York Heart Association
- PAH
pulmonary arterial hypertension
- RV
right ventricular
- Sm
systolic myocardial velocity
- TDI
Tissue Doppler imaging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors declare no conflicts of interest.
References
- 1.Gorcsan J, 3rd, Gulati VK, Mandarino WA, Katz WE. Color-coded measures of myocardial velocity throughout the cardiac cycle by tissue Doppler imaging to quantify regional left ventricular function. Am Heart J. 1996;131:1203–13. doi: 10.1016/s0002-8703(96)90097-6. [DOI] [PubMed] [Google Scholar]
- 2.Gulati VK, Katz WE, Follansbee WP, Gorcsan J., 3rd Mitral annular descent velocity by tissue Doppler echocardiography as an index of global left ventricular function. Am J Cardiol. 1996;77:979–84. doi: 10.1016/s0002-9149(96)00033-1. [DOI] [PubMed] [Google Scholar]
- 3.Oki T, Tabata T, Yamada H, Wakatsuki T, Shinohara H, Nishikado A, et al. Clinical application of pulsed Doppler tissue imaging for assessing abnormal left ventricular relaxation. Am J Cardiol. 1997;79:921–8. doi: 10.1016/s0002-9149(97)00015-5. [DOI] [PubMed] [Google Scholar]
- 4.Alam M, Wardell J, Andersson E, Samad BA, Nordlander R. Characteristics of mitral and tricuspid annular velocities determined by pulsed wave Doppler tissue imaging in healthy subjects. J Am Soc Echocardiogr. 1999;12:618–28. doi: 10.1053/je.1999.v12.a99246. [DOI] [PubMed] [Google Scholar]
- 5.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–94. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 6.Yu CM, Lin H, Ho PC, Yang H. Assessment of left and right ventricular systolic and diastolic synchronicity in normal subjects by tissue Doppler echocardiography and the effects of age and heart rate. Echocardiography. 2003;20:19–27. doi: 10.1046/j.1540-8175.2003.00003.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Yip GW, Wang AY, Zhang Y, Ho PY, Tse MK, et al. Peak early diastolic mitral annulus velocity by tissue Doppler imaging adds independent and incremental prognostic value. J Am Coll Cardiol. 2003;41:820–6. doi: 10.1016/s0735-1097(02)02921-2. [DOI] [PubMed] [Google Scholar]
- 8.Agricola E, Galderisi M, Oppizzi M, Schinkel AF, Maisano F, De Bonis M, et al. Pulsed tissue Doppler imaging detects early myocardial dysfunction in asymptomatic patients with severe mitral regurgitation. Heart. 2004;90:406–10. doi: 10.1136/hrt.2002.009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Yip G, Yu CM, Zhang Q, Zhang Y, Tse D, et al. Independent and incremental prognostic value of early mitral annulus velocity in patients with impaired left ventricular systolic function. J Am Coll Cardiol. 2005;45:272–7. doi: 10.1016/j.jacc.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 10.Nikitin NP, Loh PH, Silva R, Ghosh J, Khaleva OY, Goode K, et al. Prognostic value of systolic mitral annular velocity measured with Doppler tissue imaging in patients with chronic heart failure caused by left ventricular systolic dysfunction. Heart. 2006;92:775–9. doi: 10.1136/hrt.2005.067140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrar JF. Idiopathic Pulmonary Hypertension. Am Heart J. 1963;66:128–35. doi: 10.1016/0002-8703(63)90079-6. [DOI] [PubMed] [Google Scholar]
- 12.Rubin LJ. Primary pulmonary hypertension. Chest. 1993;104:236–50. doi: 10.1378/chest.104.1.236. [DOI] [PubMed] [Google Scholar]
- 13.Rubin LJ. Primary pulmonary hypertension. N Engl J Med. 1997;336:111–7. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- 14.Galie N, Manes A, Uguccioni L, Serafini F, De Rosa M, Branzi A, et al. Primary pulmonary hypertension: insights into pathogenesis from epidemiology. Chest. 1998;114:184S–94S. doi: 10.1378/chest.114.3_supplement.184s. [DOI] [PubMed] [Google Scholar]
- 15.Ruan Q, Nagueh SF. Clinical application of tissue Doppler imaging in patients with idiopathic pulmonary hypertension. Chest. 2007;131:395–401. doi: 10.1378/chest.06-1556. [DOI] [PubMed] [Google Scholar]
- 16.Chang SM, Lin CC, Hsiao SH, Lee CY, Yang SH, Lin SK, et al. Pulmonary hypertension and left heart function: insights from tissue Doppler imaging and myocardial performance index. Echocardiography. 2007;24:366–73. doi: 10.1111/j.1540-8175.2007.00405.x. [DOI] [PubMed] [Google Scholar]
- 17.Rajagopalan N, Simon MA, Mathier MA, Lopez-Candales A. Identifying right ventricular dysfunction with tissue Doppler imaging in pulmonary hypertension. Int J Cardiol. 2008;128:359–63. doi: 10.1016/j.ijcard.2007.06.094. [DOI] [PubMed] [Google Scholar]
- 18.Huez S, Vachiery JL, Unger P, Brimioulle S, Naeije R. Tissue Doppler imaging evaluation of cardiac adaptation to severe pulmonary hypertension. Am J Cardiol. 2007;100:1473–8. doi: 10.1016/j.amjcard.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 19.Gurudevan SV, Malouf PJ, Kahn AM, Auger WR, Waltman TJ, Madani M, et al. Noninvasive assessment of pulmonary vascular resistance using Doppler tissue imaging of the tricuspid annulus. J Am Soc Echocardiogr. 2007;20:1167–71. doi: 10.1016/j.echo.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–80. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 21.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quinones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–33. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 22.Richartz BM, Werner GS, Ferrari M, Figulla HR. Comparison of left ventricular systolic and diastolic function in patients with idiopathic dilated cardiomyopathy and mild heart failure versus those with severe heart failure. Am J Cardiol. 2002;90:390–4. doi: 10.1016/s0002-9149(02)02495-5. [DOI] [PubMed] [Google Scholar]
- 23.Bove AA, Santamore WP. Ventricular interdependence. Prog Cardiovasc Dis. 1981;23:365–88. doi: 10.1016/0033-0620(81)90022-0. [DOI] [PubMed] [Google Scholar]
- 24.Jardin F. Ventricular interdependence: how does it impact on hemodynamic evaluation in clinical practice? Intensive Care Med. 2003;29:361–3. doi: 10.1007/s00134-003-1643-0. [DOI] [PubMed] [Google Scholar]
- 25.Walker RE, Moran AM, Gauvreau K, Colan SD. Evidence of adverse ventricular interdependence in patients with atrial septal defects. Am J Cardiol. 2004;93:1374–7. A6. doi: 10.1016/j.amjcard.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 26.Rajdev S, Nanda NC, Patel V, Singh A, Mehmood F, Vengala S, et al. Tissue Doppler assessment of longitudinal right and left ventricular strain and strain rate in pulmonary artery hypertension. Echocardiography. 2006;23:872–9. doi: 10.1111/j.1540-8175.2006.00337.x. [DOI] [PubMed] [Google Scholar]
- 27.Lumens J, Blanchard DG, Arts T, Mahmud E, Delhaas T. Left ventricular underfilling and not septal bulging dominates abnormal left ventricular filling hemodynamics in chronic thromboembolic pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2010;299:H1083–91. doi: 10.1152/ajpheart.00607.2010. [DOI] [PubMed] [Google Scholar]