Abstract
Purpose
The purpose of this study was to investigate specific risk factors, common fracture locations and possible sex-specific differences in elderly patients with stress fractures.
Methods
This analysis enrolled 105 patients (83 women, 22 men) with stress fractures. For the analysis of possible risk factors related to increasing age, data from 82 patients (67 women, 15 men) aged 40 years and older (mean age of 57.4 ± 11.0 years) were compared with that from a younger control group [23 patients (16 women, seven men), mean age 28.4 ± 6.7 years]. Bone mineral density (BMD) was determined using dual-energy X-ray absorptiometry bone densitometry (DXA) and blood samples were taken.
Results
A total of 211 stress fractures were found. Of these, 177 were found in the study group, of which 90.4 % were located in the lower limb. Lumbar and femoral BMD was significantly lower in elderly patients; however, the BMD of most patients was within the osteopenic or normal range. Within the study group, a total of 83.8 % had a vitamin D insufficiency (<30 μg/l); 75.5 % were not engaged in regular physical activity more than once a week. Overweight patients within the study group had significantly more stress fractures compared to normal weight patients (2.6 ± 1.7 vs. 1.9 ± 1.1, p<0.05).
Conclusions
A similar contribution of risk factors has been found for stress fractures in elderly patients and younger controls of the general population. Stress fracture incidence seems to be rather multifactorial and not based on osteoporotic changes alone. A balanced calcium and vitamin D metabolism seems to be of paramount importance for stress fracture prevention in elderly patients.
Introduction
Stress fractures have been reported to be a common problem in young and active people such as athletes and military recruits [1–5]. Based on their aetiology, stress fractures can be subdivided into either fatigue- or insufficiency-related fractures. While fatigue stress fractures occur due to abnormal and/or repeated stress on normal bone and are regularly seen in athletes [6–11], insufficiency stress fractures are linked to normal stress on impaired bone structure and thus are often observed in postmenopausal osteoporotic women [12, 13]. Whereas in the past, diagnosis of stress fractures was performed by radiography or scintigraphy [14], today magnetic resonance imaging (MRI) is the diagnostic agent of choice due to its higher sensitivity and specificity [15, 16].
The cause of stress fractures is often multifactorial [17, 18] and various modifiable and non-modifiable factors have been proposed to play a role: white race [12], high bone turnover [1], vitamin D insufficiency [19], nicotine and alcohol abuse [5], steroid use [5], low bone density [20], low adult weight [5], anorexia [21], or bisphosphonate therapy [22, 23]. Furthermore, female gender has been identified as an important risk factor and thus sex-specific effects appear to play a role in the pathogenesis of stress fractures [1]. Numerous studies on young female athletes and military recruits have revealed that factors such as poor physical fitness, low BMD, eating disorders, a body mass index (BMI) less than 20 or disturbed menstrual function lead to an increased risk for stress fractures in young women [12, 24, 25]. While almost all of these studies focused on patients in their late second to fourth decade of life, studies on stress fractures in elderly patients and their respective risk factors are rare [26, 27]. Nevertheless, the incidence of stress fractures in elderly patients is an increasingly important issue due to the aging society and the growing interest of elderly people in physical activity, whether to remain fit or as part of therapeutic regimens [26].
Therefore, the objective of this study was to analyse and characterise elderly patients with stress fractures, and to identify specific risk factors, frequent fracture locations and gender-specific differences.
Patients and methods
A retrospective analysis was conducted on 105 patients (82 women, 23 men) with stress fractures. Eighty-two patients above the age of 40 years (with an average age of 57.4 ± 11.0 years) were admitted to our outpatient clinic between April 2008 and January 2012 with MRI diagnosed stress fractures (Table 1 and Fig. 1). To elucidate potential risk factors for elderly patients, 23 patients under the age of 40 years (average age 28.5 ± 6.7 years) with diagnosed stress fractures served as controls (Table 2).
Table 1.
Baseline characteristics of the study group
| Total (n = 82) | Women (n = 67) | Men (n = 15) | |
|---|---|---|---|
| Baseline | |||
| Age (years) | 57.4 (±11.0) | 59.4 (±10.9) | 48.7 (±7.1)* |
| Height (cm) | 168.0 (±9.7) | 165.1 (±7.0) | 180.4 (±10.3)* |
| Weight (kg) | 71.0 (±14.2) | 68.5 (±13.0) | 81.7 (±14.4)* |
| BMI (kg/m2) | 25.2 (±5.1) | 25.2 (±5.4) | 25.0 (±3.6) |
| Total number of fractures | 177 (100 %) | 142 (100 %) | 35 (100 %) |
| Location | |||
| Tarsus | 34 (19.2 %) | 27 (19.0 %) | 7 (20.0 %) |
| Metatarsal | 74 (41.8 %) | 57 (40.1 %) | 17 (48.6 %) |
| Calcaneus | 19 (10.7 %) | 15 (10.6 %) | 4 (11.4 %) |
| Tibia | 21 (11.9 %) | 17 (12.0 %) | 4 (11.4 %) |
| Pelvis | 16 (9.0 %) | 16 (11.3 %) | – |
| Femur | 9 (5.1 %) | 8 (5.6 %) | 1 (2.9 %) |
| Fibula | 3 (1.7 %) | 1 (0.7 %) | 2 (5.7 %) |
| Radius | 1 (0.6 %) | 1 (0.7 %) | – |
| Affected side | |||
| Left | 91 (51.4 %) | 74 (52.1 %) | 17 (48.6 %) |
| Right | 86 (48.6 %) | 68 (47.9 %) | 18 (51.4 %) |
| DXA T-score | |||
| Lumbar spine (L1–L4) | −1,7 (±1.2)a | −1.7 (±1.3) | −1.6 (±1.4) |
| Left femur (Total) | −1,5 (±1.0)a | −1.5 (±1.0) | −1.4 (±1.4) |
| Physical activity level (>30 min) | |||
| <1× per week | 52 (63.4 %) | 43 (64.2 %) | 9 (60.0 %) |
| 1× per week | 10 (12.2 %) | 8 (11.9 %) | 2 (13.3 %) |
| 2× per week | 10 (12.2 %) | 8 (11.9 %) | 2 (13.3 %) |
| 3× or >3 times per week | 10 (12.2 %) | 8 (11.9 %) | 2 (13.3 %) |
Characteristics are presented as mean ± standard deviation
*sex-specific difference, p < 0.05
adifferences between study group and control, p < 0.05
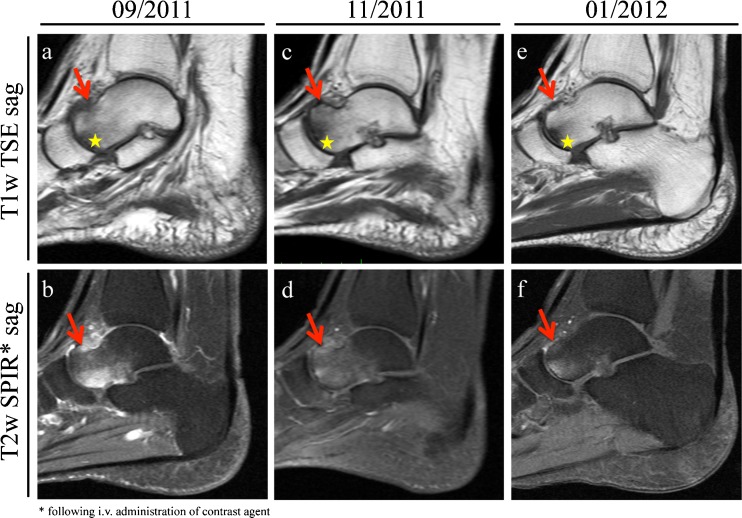
Fig. 1.
A 55-year-old female patient complained of persistent pain in her left foot after a long hike. a, b Subsequent MRI revealed a stress-fracture at the left ventromedial talus (red arrows) accompanied by bone marrow oedema. Furthermore an osteoarthritis of the talonavicular joint with subchondral cyst formation has been noted (yellow asterisks). c, d After pain-adapted weight bearing and correction of vitamin D insufficiency, the MRI at 8-week follow-up showed improvement of the talus. e, f Another 8 weeks later, the patient reported to be free of pain. The follow-up MRI revealed a fully healed stress fracture and an almost complete remission of the bone marrow oedema
Table 2.
Baseline characteristics of the control group
| Total (n = 23) | Women (n = 15) | Men (n = 8) | |
|---|---|---|---|
| Baseline | |||
| Age (years) | 28.5 (±6.7)a | 28.0 (±6.2) | 29.4 (±8.1) |
| Height (cm) | 174.4 (±14.6)a | 168.0 (±7.2) | 185.5 (±17.9) |
| Weight (kg) | 73.8 (±21.3) | 64.8 (±13.8) | 89.6 (±23.6) |
| BMI (kg/m2) | 23.9 (±4.3) | 22.8 (±4.2) | 25.8 (±4.1) |
| Total number of fractures | 34 (100 %) | 23 (100 %) | 11 (100 %) |
| Location | |||
| Tarsus | 4 (11.8 %) | 3 (13.0 %) | 1 (9.1 %) |
| Metatarsal | 13 (38.2 %) | 9 (39.1 %) | 4 (36.4 %) |
| Calcaneus | 3 (8.8 %) | 2 (8.7 %) | 1 (9.1 %) |
| Tibia | 7 (20.6 %) | 4 (17.4 %) | 3 (27.3 %) |
| Pelvis | 6 (17.7 %) | 4 (17.4 %) | 2 (18.2 %) |
| Femur | 1 (2.9 %) | 1 (4.3 %) | – |
| Fibula | – | – | – |
| Radius | – | – | – |
| Affected side | |||
| Left | 14 (41.2 %) | 10 (43.5 %) | 4 (36.4 %) |
| Right | 20 (58.2 %) | 13 (56.5 %) | 7 (63.6 %) |
| DXA T-score | |||
| Lumbar spine (L1–L4) | −0.8 (±1.4)a | −0.8 (±1.5) | −0.8 (±1.3) |
| Left femur (Total) | −0.8 (±1.3)a | −0.9 (±1.2) | −0.6 (±1.3) |
| Physical activity level (>30 min) | |||
| <1× per week | 12 (52.2 %) | 10 (66.7 %) | 2 (25.0 %) |
| 1× per week | 1 (4.3 %) | 1 (6.7 %) | – |
| 2× per week | 4 (17.4 %) | 1 (6.7 %) | 3 (37.5 %) |
| 3× or >3 times per week | 6 (26.1 %) | 3 (20.0 %) | 3 (37.5 %) |
Characteristics are presented as mean ± standard deviation
A detailed medical history (including age, sex, height, weight, BMI, medication, affected location, possible history of stress fracture) was taken from every patient with special emphasis on possible risk factors for bone diseases and stress fractures such as vitamin D insufficiency, proton pump inhibitor (PPI) medication, immunosuppressive medication, lactose intolerance, type 2 diabetes, alcohol and nicotine abuse, cortisone therapy, chemotherapy, positive family history of bone diseases, anorexia, and rheumatoid arthritis (Tables 3 and 4). In addition, blood samples were drawn from the patients at their first appointment to determine the calcium and vitamin D serum level. As vitamin D serum levels of at least 30 ng/ml or 75 nmol/l, have been considered to be of paramount importance for skeletal health [28], we considered a level below 30 ng/ml or 75 nmol/l to indicate vitamin D insufficiency. Following the guidelines of the German Society of Osteology [29] a real BMD was determined using DXA (Lunar Prodigy, Lunar Corporation, Madison, WI, USA) for the lumbar spine and both proximal femurs. Thus, we were able to categorise the patients’ BMD according to WHO criteria [30] into a) normal bone mass (t-score > −1.0), b) osteopenia (t-score of −2.5 to −1.0) and c) osteoporosis (t-score < −2.5). Furthermore, all patients were asked whether and how often per week they regularly participated in sports or fitness activities such as jogging, cycling, fitness or Nordic walking. Possible answers were: a) no regular physical activity, b) >30 min, 1× per week, c) >30 min, 2× per week or d) >30 min, ≥3× per week. In addition, informed consent to participate in the study was obtained from every patient.
Table 3.
Distribution of potential risk factors in the study group, stratified by sex
| Risk factor | Total (n = 82) | Women (n = 67) | Men (n = 15) |
|---|---|---|---|
| Vitamin D insufficiency | 60/72 (83.3 %) | 49/58 (84.5 %) | 11/14 (78.6 %) |
| Family history | 25/80 (31.3 %)a | 23/65 (35.8 %) | 2/15 (13.3 %) |
| Nicotine abuse | 21/80 (26.3 %) | 14/65 (22.4 %) | 7/15 (46.7 %)* |
| PPI medication | 18/80 (22.5 %) | 13/65 (22.4 %) | 5/15 (33.3 %) |
| Cortisone therapy | 14/80 (17.5 %) | 12/65 (17.9 %) | 2/15 (13.3 %) |
| Immunosuppressives | 11/80 (13.8 %) | 10/65 (14.9 %) | 1/15 (6.7 %) |
| Alcohol abuse | 10/80 (12.5 %) | 7/65 (10.4 %) | 3/15 (20.0 %) |
| Type 2 diabetes | 4/80 (5.0 %) | 4/65 (6.0 %) | – |
| Chemotherapy | 4/80 (5.0 %) | 4/65 (6.0 %) | – |
| Lactose intolerance | 4/80 (5.0 %) | 2/65 (3.0 %) | – |
| Anorexia nervosa | 1/80 (1.3 %)a | 1/65 (1.5 %) | – |
*sex-specific differences, p < 0.05
agroup-specific differences, p < 0.05
Table 4.
Distribution of potential risk factors in the control group, stratified by sex
| Risk factor | Controls (n = 23) | Women (n = 15) | Men (n = 8) |
|---|---|---|---|
| Vitamin D insufficiency | 21/23 (91.3 %) | 13/15 (86.7 %) | 8/8 (100.0 %) |
| Family history | 1/22 (4.5 %) | – | 1/8 (13.3 %) |
| Nicotine abuse | 3/22 (13.6 %) | 3/14 (22.4 %) | – |
| PPI medication | 6/22 (27.3 %) | 6/14 (42.9 %) | – |
| Cortisone therapy | 1/22 (4.5 %) | – | 1/8 (13.3 %) |
| Immunosuppression | 1/22 (4.5 %) | 1/14 (14.9 %) | – |
| Alcohol abuse | 2/22 (9.1 %) | 2/14 (10.4 %) | – |
| Type 2 diabetes | 1/22 (4.5 %) | 1/14 (6.0 %) | – |
| Chemotherapy | 3/22 (13.6 %) | 3/14 (6.0 %) | – |
| Lactose intolerance | 1/22 (4.5 %) | – | 1/8 (13.3 %) |
| Anorexia nervosa | 3/23 (13.0 %) | 3/15 (20.0 %) | – |
Statistical analysis
Statistical analysis was carried out using SPSS 14.0 (SPSS Inc, Chicago, IL). Mean values ± standard deviations (SD) are reported for all parameters. Student’s t-test was used to report mean differences. Since fracture location and affected sides clearly deviated from a normal distribution non-parametric test methods (Mann–Whitney-U) were applied. Chi-square tests were performed to test univariate relations between categorical variables. All tests were two-sided and a p-value of less than 0.05 was considered significant.
Results
A total of 211 stress fractures were found in 105 patients. Of these, 177 were found in the study group (Table 1) while 34 were found in the younger controls (Table 2). In the study group, eight out of 15 men (53.3 %) and 25 out of 67 women (37.3 %) reported a history of stress fractures. None of the included patients had a history of bone or bone marrow malignancy.
Within the study cohort we found significant sex-specific differences, as the men were significantly younger, heavier and larger than the women (p < 0.05) (Table 1). The study group presented with a normal to slightly overweight BMI (25.2 ± 5.1 kg/m2). In detail, 40.4 % of the patients were found to be overweight with a BMI over 25 kg/m2. Overweight patients suffered significantly more stress fractures compared to normal weight (BMI of 18.5–25 kg/m2) patients: 2.6 ± 1.7 vs. 1.9 ± 1.1, p < 0.05.
The lumbar as well as the femoral BMD was significantly lower in the study group compared to controls (p < 0.05) (Tables 1 and 2). A detailed BMD analysis of the study group revealed that 75.3 % of the stress fractures occurred in patients with a lumbar BMD within the osteopenic or normal range. This observation was even more pronounced for the femoral BMD, as 83.1 % of the stress fractures were found in patients with an osteopenic or normal t-score.
Within the study group, 90.4 % of the stress fractures were located in the lower limb. The most common locations were the metatarsals (41.8 %), the tarsus (19.2 %), the tibia (11.9 %) and the calcaneus (10.7 %) (Table 1). There were no significant differences compared with controls (Tables 1 and 2). The distribution of affected sides in the study group was even and comparable for both sexes (women: 52.1 % left and 47.9 % right; men: 48.6 % left and 52.1 % right) with no differences to controls (Tables 1 and 2).
The analysis of physical activity levels revealed that 63.4 % of the patients within the study group were not engaged in any regular physical activity. Overall, more than three quarters of patients (75.5 %) within the study group did not exercise for more than 30 min once a week (Table 1). In contrast, 43.5 % of the patients within the younger control group did exercise at least twice a week or more (Table 2).
The risk factor analysis revealed significant differences between the study group and controls. Elderly patients reported a positive family history more frequently (31.3 % vs. 4.5 %, p < 0.05), while younger controls were more likely to present with a history of anorexia (13 % vs. 1.3 %, p < 0.05) (Tables 3 and 4). The sex-specific analysis of the study group revealed significantly higher nicotine abuse in affected men than women (46.7 % vs. 22.4 %, p < 0.05). Other risk factors such as alcohol abuse and PPI medication occurred more frequently in men, while factors such as family history or immunosuppression were more common in women (Table 3). However, these differences were not significant.
Laboratory findings revealed vitamin D insufficiency (vitamin D serum level <30 ng/ml) in 60 out of 72 patients within the study group (49 of 58 women (84.5 %) and 11 of 14 men (78.6 %)) (Table 3). A similar situation was observed in the control group, in which overall 91.3 % presented with a vitamin D insufficiency (Table 4). However, there were no significant differences in stress fracture frequency in patients with vitamin D insufficiency compared to patients with sufficient vitamin D (2.2 ± 1.5 vs. 1.92 ± 1.3, p = 0.5).
A quantitative analysis of cumulative risk factors revealed, that only six cases presented without any risk factor at all (7.3 %). The majority (85.4 %) presented with one to three risk factors. In detail, we found an almost even distribution of cases with one (28.1 %), two (29.3 %) or three (28.1 %) risk factors. However, only six cases (7.3 %) had more than three risk factors. The most common singular risk factor for all risk factor combinations was vitamin D insufficiency.
Discussion
This retrospective analysis was conducted to analyse the incidence of stress fractures in an elderly cohort. While osteoporosis is certainly one of the leading risk factors for stress fractures, especially in elderly patients, only 24.7 % of the patients within our study cohort actually had a t-score assessed by DXA within the osteoporotic range. Overall, the mean t-score for both the lumbar spine and the femur was within the osteopenic range. These findings may either indicate that stress fracture risk in the elderly already increases in the osteopenic t-score range or that impaired bone microarchitecture leading to stress fracture occurrence cannot be fully assessed according to the BMD of the proximal femur and lumbar spine as measured by DXA.
To gain further insight, we analysed additional risk factors that could contribute to the incidence of stress fractures. Particular attention was given to calcium homeostasis and vitamin D status, both of which are known to be of paramount importance for bone and skeletal health [28, 31, 32]. For instance, vitamin D insufficiency leads to a lower serum calcium concentration, which in turn results in secondary hyperparathyroidism [33]. Moreover, the demand for calcium further increases in physically active people, as bone formation is stimulated and microfractures require repair [34]. As previously shown, vitamin D serum levels of at least 30 ng/ml or 75 nmol/l are considered to be necessary for skeletal health [28, 35]. However, the majority of elderly patients in our study group as well as the younger controls presented with insufficient vitamin D levels. These observations are consistent with other studies, which found that vitamin D insufficiency significantly increases the risk for stress fractures in young and active patients. In a study on Israeli soldiers, Givon et al. found that vitamin D levels were lower in patients with high grade stress fractures compared to controls [36]. Ruohola et al. prospectively followed 800 randomly chosen male Finnish recruits and reported vitamin D serum levels below the median of 75.8 nmol/l to be a significant risk factor for the incidence of stress fractures [33]. Moreover, Lappe and colleagues analysed 5201 female Navy recruits in a randomised, double-blind, placebo-controlled study and were able to show a 20 % reduction in stress fracture incidence in a cohort with daily supplementation of 2,000 mg calcium and 800 IE vitamin D [34]. A case–control study by Burgi et al. analysed 600 female Navy recruits (mean age 19.5 years) with stress fractures of the tibia or fibula and found a two-fold higher risk of stress fractures of tibia and fibula in women with a vitamin D level of less than 20 ng/ml compared to women with concentrations of over 40 ng/ml [3]. They concluded that a target for stress fracture prevention would be a vitamin D serum level of 40 ng/ml or greater and underlined the importance of a balanced calcium and vitamin D metabolism in the prevention of stress fractures. Overall, this study on elderly patients from the general population supports the aforementioned findings on young and active people regarding a possible relationship between vitamin D insufficiency and an increased risk of stress fracture.
In addition, the widespread use of PPI among elderly patients has recently been linked to increased fracture risk and disrupted calcium absorption [37, 38]. Yang and co-workers found an association between long-term PPI therapy and an increased risk of hip fracture, which led us to investigate this issue within our study cohort. Although PPI therapy was reported in 22.5 % of affected patients within the study group, there was no significant difference in use of PPIs in comparison with younger controls. Previous studies further indicated an increased stress fracture risk for patients suffering from anorexia nervosa or other eating disorders such as lactose intolerance that can result in an insufficient calcium supply [1, 12]. However, in our study group only one elderly woman reported suffering from anorexia nervosa and only four women were lactose intolerant. In addition, anorexia nervosa was observed significantly more often in younger controls, indicating that lactose intolerance as well as anorexia nervosa are not typical risk factors for the occurrence of stress fractures in elderly patients.
We also considered stress fracture locations. Studies on young military recruits and athletes found that the tibia was the most common stress fracture location [24]. In contrast, in our elderly cohort from the general population the metatarsals were the most frequent stress fracture location. One reason for this observation might be that the physical stress leading to the incidence of stress fractures in elderly patients is different and often lower than that in professional athletes or military personnel. This was reflected by the fact that elderly patients often reported that long hikes or recently started endurance training had led to the stress fracture. On the other hand, 75.5 % of patients in the study cohort were not engaged in regular physical activity more than once a week. Thus, their bone microarchitecture could not adequately adapt to the sudden increased stress level that occurred during long mountain hikes or endurance sports. This is in accordance with Shaffer and colleagues who analysed 152 recruits with stress fractures and identified low aerobic fitness as the only modifiable risk factor for stress fractures within their study [24]. The authors further pointed out that training intensity should be increased gradually before exposure to larger loads to prevent stress fractures. Although all these studies were performed on young female military recruits, the principle may also apply to elderly patients. Thus elderly patients should be encouraged to increase their physical activity and improve their aerobic fitness. However, the intensity of physical exertion should not be increased too quickly.
Law and colleagues reported an increased risk for hip fracture in postmenopausal smokers over the age of 50 years [39]. In addition, Lappe et al. analysed 3,758 female recruits and found that after eight weeks of basic training stress fractures were more likely to be reported by recruits with a current or past history of smoking or regular consumption of alcohol [5]. We also analysed lifestyle risk factors and found nicotine abuse in 46.7 % of male patients within the study group and thus significantly more often compared to female patients. Overall, the incidence of smoking was more than twice that of daily alcohol consumption (26.3 % vs. 12.5 %) among study group patients. Thus our findings indicate that modifiable risk factors such as nicotine or alcohol abuse may also increase the risk of stress fracture incidence in the elderly as reported for young military recruits.
There are some limitations to this study. Firstly, this was a retrospective analysis with no control group of elderly patients without stress fractures was available. However, we included a control group of younger patients with stress fractures to look for possible risk factors, especially those related to increasing age. Second, the sample size in this study was relatively small compared to the large and prospective studies on stress fractures conducted on military recruits and athletes. However, comparably large and prospective studies on stress fracture incidence in the elderly general population are difficult to perform.
Conclusions
In conclusion, the results of this retrospective observational study indicate a similar contribution of stress fracture risk factors in elderly patients and younger controls. As more than 75 % of the analysed elderly patients presented with a DXA t-score within the osteopenic or even normal range, our data further suggests that stress fracture incidence in the elderly seems to be rather multifactorial and not based on osteoporotic changes alone. It is thus important to examine the patient’s personal risk factors and to minimise them wherever possible. However, larger and prospective studies are needed to confirm our findings and to investigate the potential for stress fracture prevention in the elderly by ensuring a balanced calcium and vitamin D metabolism and minimising further possible risk factors.
Acknowledgments
Conflict of interest
The authors state that there is no conflict of interest.
Footnotes
Stefan Breer and Matthias Krause contributed equally to this work and therefore share first authorship.
References
- 1.Mattila VM, Niva M, Kiuru M, Pihlajamaki H. Risk factors for bone stress injuries: a follow-up study of 102,515 person-years. Med Sci Sports Exerc. 2007;39:1061–1066. doi: 10.1249/01.mss.0b013e318053721d. [DOI] [PubMed] [Google Scholar]
- 2.Maquirriain J, Ghisi JP. The incidence and distribution of stress fractures in elite tennis players. Br J Sports Med. 2006;40:454–459. doi: 10.1136/bjsm.2005.023465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgi AA, Gorham ED, Garland CF, Mohr SB, Garland FC, Zeng K, Thompson K, Lappe JM. High serum 25-hydroxyvitamin D is associated with a low incidence of stress fractures. J Bone Miner Res. 2011;26:2371–2377. doi: 10.1002/jbmr.451. [DOI] [PubMed] [Google Scholar]
- 4.Valimaki VV, Alfthan H, Lehmuskallio E, Loyttyniemi E, Sahi T, Suominen H, Valimaki MJ. Risk factors for clinical stress fractures in male military recruits: a prospective cohort study. Bone. 2005;37:267–273. doi: 10.1016/j.bone.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Lappe JM, Stegman MR, Recker RR. The impact of lifestyle factors on stress fractures in female Army recruits. Osteoporos Int. 2001;12:35–42. doi: 10.1007/s001980170155. [DOI] [PubMed] [Google Scholar]
- 6.Kiuru MJ, Pihlajamaki HK, Ahovuo JA. Fatigue stress injuries of the pelvic bones and proximal femur: evaluation with MR imaging. Eur Radiol. 2003;13:605–611. doi: 10.1007/s00330-002-1562-4. [DOI] [PubMed] [Google Scholar]
- 7.Albisetti W, Perugia D, Bartolomeo O, Tagliabue L, Camerucci E, Calori GM. Stress fractures of the base of the metatarsal bones in young trainee ballet dancers. Int Orthop. 2010;34:51–55. doi: 10.1007/s00264-009-0784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pecina M, Bojanic I, Dubravcic S. Stress fractures in figure skaters. Am J Sports Med. 1990;18:277–279. doi: 10.1177/036354659001800310. [DOI] [PubMed] [Google Scholar]
- 9.Ivkovic A, Franic M, Bojanic I, Pecina M. Overuse injuries in female athletes. Croat Med J. 2007;48:767–778. doi: 10.3325/cmj.2007.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivkovic A, Bojanic I, Pecina M. Stress fractures of the femoral shaft in athletes: a new treatment algorithm. Br J Sports Med. 2006;40:518–520. doi: 10.1136/bjsm.2005.023655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pecina M, Bojanic I, Smoljanovic T, Ivkovic A, Mirkovic M, Jelic M. Surgical treatment of diaphyseal stress fractures of the fifth metatarsal in competitive athletes: long-term follow-up and computerized pedobarographic analysis. J Am Podiatr Med Assoc. 2011;101:517–522. doi: 10.7547/1010517. [DOI] [PubMed] [Google Scholar]
- 12.Pegrum J, Crisp T, Padhiar N. Diagnosis and management of bone stress injuries of the lower limb in athletes. BMJ. 2012;344:e2511. doi: 10.1136/bmj.e2511. [DOI] [PubMed] [Google Scholar]
- 13.Daffner RH, Pavlov H. Stress fractures: current concepts. Am J Roentgenol. 1992;159:245–252. doi: 10.2214/ajr.159.2.1632335. [DOI] [PubMed] [Google Scholar]
- 14.Anderson MW, Greenspan A. Stress fractures. Radiology. 1996;199:1–12. doi: 10.1148/radiology.199.1.8633129. [DOI] [PubMed] [Google Scholar]
- 15.Fayad LM, Kawamoto S, Kamel IR, Bluemke DA, Eng J, Frassica FJ, Fishman EK. Distinction of long bone stress fractures from pathologic fractures on cross-sectional imaging: how successful are we? AJR Am J Roentgenol. 2005;185:915–924. doi: 10.2214/AJR.04.0950. [DOI] [PubMed] [Google Scholar]
- 16.Gaeta M, Minutoli F, Scribano E, Ascenti G, Vinci S, Bruschetta D, Magaudda L, Blandino A. CT and MR imaging findings in athletes with early tibial stress injuries: comparison with bone scintigraphy findings and emphasis on cortical abnormalities. Radiology. 2005;235:553–561. doi: 10.1148/radiol.2352040406. [DOI] [PubMed] [Google Scholar]
- 17.Pepper M, Akuthota V, McCarty EC. The pathophysiology of stress fractures. Clin Sports Med. 2006;25:1–16, vii. doi: 10.1016/j.csm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Bennell KL, Malcolm SA, Thomas SA, Reid SJ, Brukner PD, Ebeling PR, Wark JD. Risk factors for stress fractures in track and field athletes. A twelve-month prospective study. Am J Sports Med. 1996;24:810–818. doi: 10.1177/036354659602400617. [DOI] [PubMed] [Google Scholar]
- 19.McClellan JWr, Vernon BA, White MA, Stamm S and Ryschon KL (2011) Should 25-Hydroxyvitamin D and bone density using DXA be tested in adolescents with lumbar stress fractures of the Pars Interarticularis? J Spinal Disord Tech. doi:10.1097/BSD.0b013e31823f324f [DOI] [PubMed]
- 20.Marx RG, Saint-Phard D, Callahan LR, Chu J, Hannafin JA. Stress fracture sites related to underlying bone health in athletic females. Clin J Sport Med. 2001;11:73–76. doi: 10.1097/00042752-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Teng K. Premenopausal osteoporosis, an overlooked consequence of anorexia nervosa. Cleve Clin J Med. 2011;78:50–58. doi: 10.3949/ccjm.78a.10023. [DOI] [PubMed] [Google Scholar]
- 22.Koh JS, Goh SK, Png MA, Kwek EB, Howe TS. Femoral cortical stress lesions in long-term bisphosphonate therapy: a herald of impending fracture? J Orthop Trauma. 2010;24:75–81. doi: 10.1097/BOT.0b013e3181b6499b. [DOI] [PubMed] [Google Scholar]
- 23.Isaacs JD, Shidiak L, Harris IA, Szomor ZL. Femoral insufficiency fractures associated with prolonged bisphosphonate therapy. Clin Orthop Relat Res. 2010;468:3384–3392. doi: 10.1007/s11999-010-1535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaffer RA, Rauh MJ, Brodine SK, Trone DW, Macera CA. Predictors of stress fracture susceptibility in young female recruits. Am J Sports Med. 2006;34:108–115. doi: 10.1177/0363546505278703. [DOI] [PubMed] [Google Scholar]
- 25.Barrow GW, Saha S. Menstrual irregularity and stress fractures in collegiate female distance runners. Am J Sports Med. 1988;16:209–216. doi: 10.1177/036354658801600302. [DOI] [PubMed] [Google Scholar]
- 26.Carpintero P, Berral FJ, Baena P, Garcia-Frasquet A, Lancho JL. Delayed diagnosis of fatigue fractures in the elderly. Am J Sports Med. 1997;25:659–662. doi: 10.1177/036354659702500512. [DOI] [PubMed] [Google Scholar]
- 27.Kaye RA (1998) Insufficiency stress fractures of the foot and ankle in postmenopausal women. Foot & ankle international/American Orthopaedic Foot and Ankle Society [and] Swiss Foot and Ankle Society 19:221 [DOI] [PubMed]
- 28.Priemel M, Domarus C, Klatte TO, Kessler S, Schlie J, Meier S, Proksch N, Pastor F, Netter C, Streichert T, Puschel K, Amling M. Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res. 2010;25:305–312. doi: 10.1359/jbmr.090728. [DOI] [PubMed] [Google Scholar]
- 29.DVO Guideline 2009 for prevention, diagnosis and therapy of osteoporosis in adults. Osteologie. 2011;20:55–74. [Google Scholar]
- 30.WHO Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 31.Tenforde AS, Sayres LC, Sainani KL, Fredericson M. Evaluating the relationship of calcium and vitamin D in the prevention of stress fracture injuries in the young athlete: a review of the literature. PM&R. 2010;2:945–949. doi: 10.1016/j.pmrj.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Gennari C. Calcium and vitamin D nutrition and bone disease of the elderly. Public Health Nutr. 2001;4:547–560. doi: 10.1079/PHN2001140. [DOI] [PubMed] [Google Scholar]
- 33.Ruohola JP, Laaksi I, Ylikomi T, Haataja R, Mattila VM, Sahi T, Tuohimaa P, Pihlajamäki H. Association between serum 25 (OH) D concentrations and bone stress fractures in Finnish young men. J Bone Miner Res. 2006;21:1483–1488. doi: 10.1359/jbmr.060607. [DOI] [PubMed] [Google Scholar]
- 34.Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin d supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23:741–749. doi: 10.1359/jbmr.080102. [DOI] [PubMed] [Google Scholar]
- 35.Bischoff-Ferrari H. Vitamin D: what is an adequate vitamin D level and how much supplementation is necessary? Best Pract Res Clin Rheumatol. 2009;23:789–795. doi: 10.1016/j.berh.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Givon U, Friedman E, Reiner A, Vered I, Finestone A and Shemer J (2000) Stress fractures in the Israeli defense forces from 1995 to 1996. Clin Orthop Relat Res 373:227–232 [DOI] [PubMed]
- 37.Schinke T, Schilling AF, Baranowsky A, Seitz S, Marshall RP, Linn T, Blaeker M, Huebner AK, Schulz A, Simon R, Gebauer M, Priemel M, Kornak U, Perkovic S, Barvencik F, Beil FT, Fattore A, Frattini A, Streichert T, Pueschel K, Villa A, Debatin KM, Rueger JM, Teti A, Zustin J, Sauter G, Amling M. Impaired gastric acidification negatively affects calcium homeostasis and bone mass. Nat Med. 2009;15:674–681. doi: 10.1038/nm.1963. [DOI] [PubMed] [Google Scholar]
- 38.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947–2953. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 39.Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ. 1997;315:841–846. doi: 10.1136/bmj.315.7112.841. [DOI] [PMC free article] [PubMed] [Google Scholar]