Abstract
Niemann-Pick disease type C, an autosomal recessive lysosomal storage disorder, can present with severe visceral and neurologic involvement and is associated with a significant decrease in life expectancy. As little is known about anesthetic considerations of this disease, we examined the perianesthetic course of patients with Niemann-Pick disease type C. Thirty-two patients with Niemann-Pick disease type C, median age 6.9 years (1.8–33 years), underwent 64 general anesthetics for diagnostic procedures. Perianesthetic morbidity included need for tracheal reintubation, pneumonitis, hypothermia, and seizure. Therefore, Niemann-Pick disease type C–associated neurologic and visceral involvement might have anesthetic implications that neurologists and pediatricians should be aware of and consider discussing with parents, guardians, and the patient’s care team when procedures requiring anesthesia are planned. Furthermore, it is important for delivery of safe anesthesia that there is communication among care team members so that all involved understand the disease manifestation spectrum.
Keywords: neurodegeneration, anesthesia, Niemann-Pick disease type C, cholesterol
Niemann-Pick disease involves a group of autosomal recessive lysosomal storage disorders that includes 4 subgroups: A, B, C, and D. Niemann-Pick disease types A and B are associated with sphingomyelinase deficiency. Conversely, Niemann-Pick disease type C involves alterations in the intracellular transport of endocytosed cholesterol and accumulation of unsterified cholesterol in lysosomes and endosomes due to mutations in either the NPC1 or NPC2 genes (for reviews and clinical recommendations, see references 1–7). Niemann-Pick disease type C has an autosomal recessive pattern of inheritance and an incidence of approximately 1:120,000 live births, and the majority of patients have the NPC1 mutation.2–4
The Niemann-Pick disease type C with NPC1 mutation affects both males and females equally and has been described in all ethnic groups. The age of clinical onset varies widely, and patients with the NPC1 mutation can present with a broad spectrum of signs and symptoms that reflect involvement of the central nervous system and several viscera.2,3 Visceral (liver and spleen) and neurological involvement can manifest at different times and have independent clinical courses. The clinical manifestations can involve a spectrum of neurological deficits including vertical gaze palsy, dystonia, dysphagia, seizures, and progressive dementia. In some patients with NPC1 mutation, because of severe neurological impairment and dysphagia and consequent recurrent aspiration and decreased thoracic muscle strength, lung disease may ensue. With systemic disease, hepatosplenomegaly can be severe but is often well tolerated. Patients with NPC1 mutation usually exhibit normal development for the first few years of life and then develop speech impediment and loss of other neurological skills.2,3,8–11 The disease is associated with significant decrease in life expectancy, and, unfortunately, effective therapy is lacking.12 In severe cases of Niemann-Pick disease type C, early death can be caused by liver failure or severe neurological impairment.
Given the complexity of Niemann-Pick disease type C and its broad spectrum of clinical presentation, it is highly likely that many of these patients will require a number of diagnostic and therapeutic procedures during the course of their disease that will require anesthesia. However, little has been reported on the anesthetic management or the morbidity associated with anesthesia in patients with Niemann-Pick disease type C.13,14 The purpose of this study was to prospectively examine the anesthetic care of a large series of patients with Niemann-Pick disease type C with confirmed NPC1 mutation in order to describe and quantitate the incidence of morbidity during the perianesthetic period.
Methods
The Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development approved the study as part of an investigational protocol of biochemical markers and natural history of Niemann-Pick disease type C. Consent for participation in this research, including anesthetics and procedures, was obtained from parents or guardians. The selection of anesthetic technique used for each patient was made by the primary anesthesiologist at the tertiary clinical research center.
Prospectively, we examined data on patient demographics, primary diagnosis, routine drug regimen, physical findings, diagnostic and therapeutic procedures, anesthetic techniques, drugs used, length of anesthesia, and perianesthetic events from August 2006 to May 2009. When evaluating complications, we focused on anesthetic and procedural complications and noted events and associated clinical findings (seizures, airway complications, decreases in oxygen saturation, postdural puncture headaches) during the postoperative period up to 48 hours after the procedure.
We also reviewed the literature on the clinical characteristics and therapy of Niemann-Pick disease and on anesthetic management of the syndrome. We searched EMBASE (1974 to August 2011) and MED-LINE (1968 to November 2011) and scanned references from all included reports using MeSH headings (carrier proteins, anesthesia, and analgesia), subheadings (diagnosis, classification, etiology), and keywords (Niemann-Pick, anesthesia).
Descriptive results are presented as means, medians, and mean ± standard deviation as indicated.
Results
Patients
We enrolled 32 patients with Niemann-Pick disease type C and NPC1 mutation who underwent 64 general anesthetics for diagnostic procedures. Every effort was made to combine 2 or more procedures during the same anesthesia in order to minimize the number of anesthesia episodes. Results of biochemical, metabolic, and clinical progression studies associated with Niemann-Pick disease type C that included patients enrolled in the present study have been previously reported.6,15,16
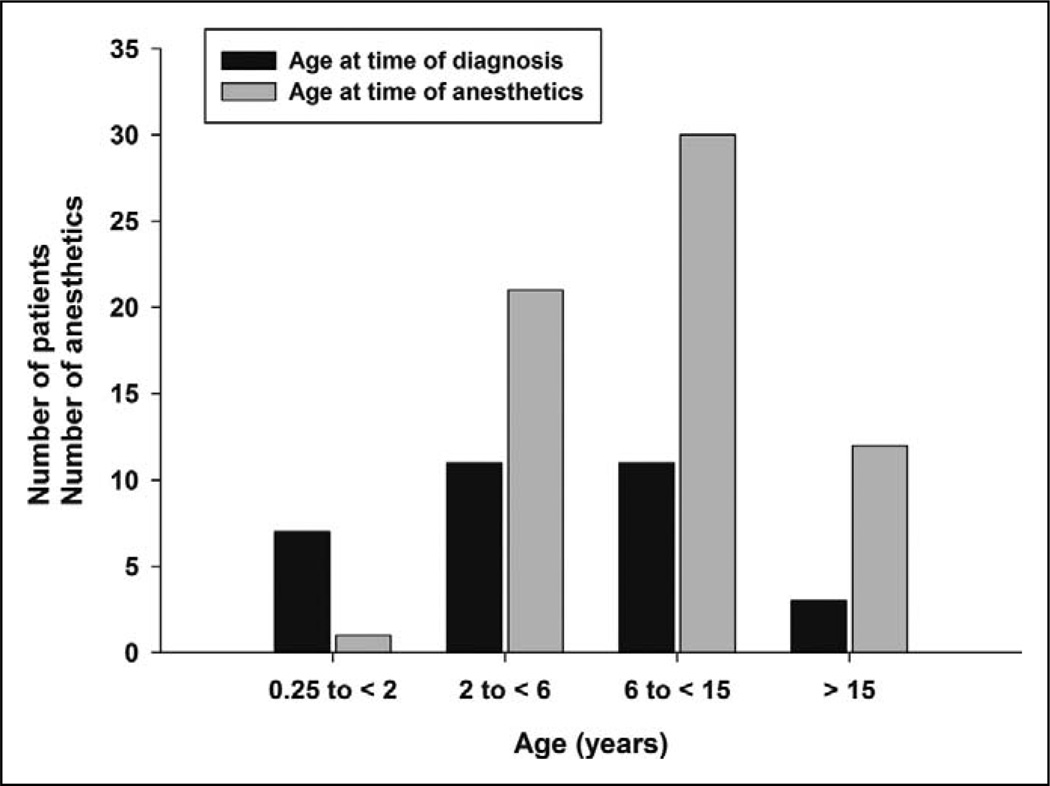
Table 1 lists demographic characteristics and major clinical findings of all patients enrolled and Table 2 their preoperative drug regimen at time of anesthesia. Among 32 patients, 29 were children and adolescents who were diagnosed at ages ranging from 3 months to 15 years. Specifically, 7 patients were diagnosed in the early-infantile period (3 months to <2 years), 11 in the late-infantile period (2 to <6 years), and 11 in the juvenile period (6–15 years); 3 patients were diagnosed with Niemann-Pick disease type C at the age of 21, 24, and 33 years.2 Figure 1 shows the number of patients and inductions of anesthesia according to age at times of diagnosis and time of anesthesia conducted for diagnostic procedures.
Table 1.
Demographic Characteristics and Major Clinical Findings in 32 Patients With Niemann-Pick Disease Type C
| Variable | |
|---|---|
| Sex, n (%) | |
| Male | 17 (53) |
| Female | 15 (47) |
| Age at diagnosis, y | |
| Median | 3.7 |
| Range | 0.25–33 |
| Age at first anesthetic, y | |
| Median | 6.9 |
| Range | 1.8–33 |
| Weight, kg | |
| Mean | 30.4 |
| Range | 9–142 |
| ASA physical status, n (%) | |
| II | 3 (9) |
| III | 29 (91) |
| Clinical and laboratory findings, n (%) | |
| Hepatosplenomegaly | 31 (97) |
| Elevated transaminases | 31 (97) |
| Thrombocytopenia | 16 (50) |
| Pulmonary disease | 10 (31) |
| Cognitive impairment | 20 (63) |
| Ataxia | 26 (81) |
| Vertical gaze palsy | 26 (81) |
| Hearing problem | 22 (69) |
| Speech delay | 27 (84) |
| Dysphagia | 20 (63) |
| Behavior problems | 13 (41) |
| Seizure | 12 (38) |
ASA, American Society of Anesthesiologists. Pulmonary disease included chronic cough and/or history of aspiration pneumonia.
Table 2.
Preanesthetic Drug Regimen in 32 Patients With Niemann-Pick Type C Disease
| Drug Category | No. of Patients (%) |
|---|---|
| Enzyme inhibitors | |
| Miglustat | 17 (53) |
| Anticonvulsants | |
| Valproate semisodium | 2 (6) |
| Carbamazepine | 1 (3) |
| Phenytoin | 2 (6) |
| Levetiracetam | 2 (6) |
| Diazepam | 2 (6) |
| Oxcarbazepine | 2 (6) |
| Antidepressants | |
| Venlafaxine | 1 (3) |
| Imipramine | 1 (3) |
| Fluoxetine | 2 (6) |
| Psychostimulants | |
| Methylphenidate | 3 (9) |
| Bronchodilators | |
| Albuterol | 2 (6) |
| Levalbuterol | 1 (3) |
| Sodium cromoglycate | 2 (6) |
| Fluticasone and salmeterol | 2 (6) |
| Muscle relaxants | |
| Baclofen | 2 (6) |
| Antihistaminic | |
| Cetirizine | 2 (6) |
| Fexofenadine | 2 (6) |
In this series, efforts were made to maintain the routine drug administration regimen during the perianesthesia period.
Figure 1.
Number of patients with Niemann-Pick type C disease and corresponding number of anesthesia episodes for each age group. The black bars indicate the number of patients diagnosed at given age ranges and the gray bars the number of patients anesthetized in each age range.
Almost all patients had mild to moderate hepatosplenomegaly and elevation of transaminases (97%), and 50% had thrombocytopenia. Thirty-one percent of patients had pulmonary disease manifested by chronic cough and prior episodes of aspiration (Table 1). Most patients had varying degrees of neurological deficits including cognitive impairment, ataxia, impaired eye movement, hearing deficit, speech delay, and swallowing abnormalities. Given the severity of their disease, 29 (91%) patients were classified as American Society of Anesthesiologist category III, defined as patients with severe systemic disease and definite functional impairment (Table 1).
Procedures and Anesthetic Management
We conducted 64 imaging studies, 62 lumbar punctures, 52 auditory brainstem response evaluations, and 20 skin biopsies according to requirements of the investigational protocol (Table 3). A few patients needed to be transported between locations in the hospital for the conduct of different procedures. Given the experimental protocol requirements, brain magnetic resonance imaging (MRI) and spectroscopy were conducted in the radiology department and lasted 2.5 to 3 hours. Auditory brain response, skin biopsy, and lumbar puncture were performed in the operating room or a procedure room (adjacent to radiology), which combined lasted approximately about 1.5 to 2 hours. The skin biopsy and lumbar puncture were done in the lateral decubitus position concomitantly with the auditory brain response. In 1 patient, a lumbar puncture was deferred because coagulation studies were abnormal.
Table 3.
Perianesthetic Course of 64 Anesthetics for Diagnostic Procedures in 32 Patients With Niemann-Pick Type C Disease
| Variable | No. (%) or Amount Mean ± SD |
|---|---|
| Diagnostic procedure | |
| MRI/MRS | 64 |
| Lumbar puncture | 62 |
| Auditory brainstem response | 52 |
| Skin biopsy | 20 |
| Anesthetic technique | |
| General | 64 |
| Anesthetic induction | |
| Inhalational agent (sevoflurane, nitrous oxide) | 12 (19) |
| Intravenous agent (propofol) | 52 (81) |
| Anesthetic maintenance | |
| Propofol (mg) | 1391 ± 754 |
| Monitoring | |
| Standard | 64 |
| Airway management | |
| Endotracheal tube | 3 |
| Laryngeal mask airway | 0 |
| Nasal cannula | 61 |
| Other drugs administered during procedure | |
| Muscle relaxant | 1 |
| Opioid | 0 |
| Benzodiazepine | 19 |
| Anesthesia time, min | 251 ± 46 |
| Recovery time, min | 66 ± 53 |
| Intravenous fluid, mL | 431 ± 125 |
MRI/MRS, magnetic resonance imaging and spectroscopy.
Sixty-three of the 64 MRI studies were conducted in a 3-T magnet. All procedures required general anesthesia, all patients were continuously monitored according to the American Society of Anesthesiologists monitoring standards, and no invasive monitors were used. Nineteen patients received preoperative sedation with midazolam (0.1 mg/kg up to 2 mg). When no intravenous access was available, anesthesia was induced with inhalation of sevoflurane and 70% nitrous oxide in oxygen (12 anesthetics in 12 patients), and when patients had an intravenous catheter in place (52 anesthetics for 20 patients), anesthesia was induced with propofol. Three patients who had a history of recurrent aspiration and chronic cough underwent trachea intubation after induction of anesthesia. For the duration of studies, anesthesia was maintained with intravenous propofol (total mean propofol administered 1391 ± 754 mg; range, 278–3756 mg). Mean anesthesia and recovery times were 251 minutes (range, 170–360 minutes) and 66 minutes (range, 30–360 minutes), respectively. The administration of intravenous fluid was determined according to the patient’s age, weight, and duration of anesthesia (431 ± 125 mL, mean ± standard deviation; range 200–1100 mL).
Table 4 lists the perianesthetic events observed in this series. Twelve patients with ages ranging from 1.8 to 11 years had profuse sweating without significant elevation of temperature while in the 3-T magnet during MRI. Four older patients became mildly hypothermic with temperatures decreasing to levels between 34.4°C and 35.2°C. A 32-year-old patient with severe neurological impairment had bradycardia (44 beats per minute) associated with moderate hypothermia (temperature 34.4°C). Three patients who had preexisting cough, dysphagia, and history of aspiration had intraprocedural episodes of decreases in oxygen saturation, tachypnea and tachycardia, and persistent oxygen requirement in the recovery room. These patients were then admitted to an intensive care unit for monitoring and observation. Two of these 3 patients required reintubation of the trachea for a short period of time to secure airway, facilitate tracheal suctioning, and improve oxygenation. The third patient remained in the intensive care unit for 3 days because a chest radiograph showed a new left lower lobe infiltrate compatible with pneumonitis. One patient displayed short-lived and spontaneously resolving myoclonic jerks during induction of anesthesia with sevoflurane. Another patient with a known history of seizure was found to have seizure activity in an electroencephalogram after extubation and required midazolam and additional doses of anticonvulsants.
Table 4.
Perianesthetic Events During Anesthesia for Diagnostic Procedures in Patients With Niemann-Pick Type C Disease
| Event | No. | Patient Age Range, y |
|---|---|---|
| Profuse sweating during MRI | 16 | 1.8–11 |
| Hypothermia during MRI | 4 | 12–32 |
| Hypoxia | 4 | 5–25 |
| Seizure activity | 3 | 4–15 |
| Bradycardia | 1 | 32 |
| Endotracheal intubation | 2 | 5–11 |
| Admission to intensive care unit | 3 | 5–22 |
MRI, magnetic resonance imaging. Patient age range indicates that in which the events were observed. Hypothermia indicates temperature below 36°C, and hypoxia indicates oxygen saturation below 90%.
Review of the Literature
We found only 1 report addressing the implications of anesthesia or its management in patients with Niemann-Pick disease type C. Although not specifically stated in the article, it is highly probable that all of the patients from that report had Niemann-Pick disease type C with NPC1 mutation given the clinical presentation described.17 That article reported the use of sedation with chlorpromazine, meperidine, and pentobarbital (a sedation regimen that is seldom used in current practice) for the conduct of liver biopsies in 14 patients with Niemann-Pick disease type C.17 Two other reports referred to the anesthetic management of patients with Niemann-Pick disease type A and type B, which are very distinct diseases from Niemann-Pick disease type C. One reports a 2-year-old boy with Niemann-Pick disease type A who underwent an emergent splenectomy14 and the other a 56-year-old male patient with Niemann-Pick disease type B who underwent elective coronary artery bypass graft.13
Discussion
In patients with Niemann-Pick disease type C, given the involvement of multiple viscera and of the central nervous system, a multitude of diagnostic and therapeutic procedures will likely be required for diagnosis and monitoring of response to therapy. Because many of these procedures are done in young children and cognitively impaired adolescents and adults, general anesthesia is likely to be needed even for noninvasive procedures. Although much has been learned about the genetic, molecular, and diagnostic features of the disease, we found only 1 report addressing the anesthetic management of Niemann-Pick disease type C. In this investigation, we report a series of 32 patients with Niemann-Pick disease type C with NPC1 mutation who underwent a total of 64 general anesthetics for diagnostic procedures in a tertiary care research center. To our knowledge, this is the largest series of anesthetic procedures in children with confirmed Niemann-Pick disease type C with NPC1 mutation. The data reported here likely are of interest to parents, guardians, pediatricians, internists, and neurologists alike who might prepare and counsel patients who will undergo procedures that require anesthesia. It is imperative that information related to a patient’s diagnosis, natural history, and drug regimen be conveyed to anesthesiologists caring for the patient in the perioperative period so that all anesthetic implications can be considered and discussed.
Our findings suggest that a number of issues related to the visceral and central nervous system involvement in Niemann-Pick disease type C can have implications for anesthesia. One such issue is the presence of hepatosplenomegaly with associated transaminase elevation, which in our series was present in 97% of the patients. Hepatosplenomegaly in patients with Niemann-Pick disease type C can be severe and associated with liver function deficits and ascites. In addition, splenomegaly may be associated with thrombocytopenia, which was observed in 50% of our patients. Therefore, when anesthetizing children with Niemann-Pick disease type C, the issues of increased intra-abdominal pressure and decreased functional residual capacity need to be considered. Although we do not advocate routine evaluation of liver function tests prior to elective procedures, one must be keenly aware of the possibility that they can be altered. Nevertheless, the visceral involvement in patients with Niemann-Pick disease type C can be severe, can have implications in the perioperative period, and may require additional preoperative investigations.
In our series, most patients had a spectrum of neurological deficits that included cognitive impairment, ataxia and/or clumsiness, vertical supranuclear gaze palsy, dysarthria, dysphagia, hearing impairment, and seizure. The presence of such deficits can make the approach to the patient and the patient’s separation from the parents more challenging. Preoperative sedation should be considered to facilitate the acceptance of inhalation induction or insertion of intravenous catheter. Many patients have difficulties swallowing secretions, which might increase the risk of aspiration; therefore, administration of anticholinergic agents should be considered during anesthesia.
One of our patients, a 4-year-old child without a history of seizures, developed a short episode of generalized tonic–clonic seizure-like activity during mask induction of anesthesia with sevoflurane. As this episode was short-lived and spontaneously resolved, the anesthetic was continued and the planned procedures performed. Postoperatively, the patient was found to have seizure-like spike discharges on electroencephalogram. Another patient had a seizure postoperatively that was thought to be related to a missed dose of his anticonvulsant. Researchers have shown that with sevoflurane, a faster anesthetic induction, higher concentrations (>2.0 minimum alveolar concentration), and hyperventilation can be associated with epileptiform activity on electroencephalogram.18–20 These authors suggested that sevoflurane concentrations used for inhaled induction in pediatric anesthesia should be reduced and hyperventilation by controlled ventilation should be avoided. Therefore, our findings further demonstrate that the routine schedule of preoperative medications, especially all anticonvulsants, should be maintained. In addition, to avoid the possibility of sevoflurane-associated seizures, intravenous induction of anesthesia might be preferable to avoid the use of sevoflurane.
Pulmonary involvement and lung disease in patients with Niemann-Pick disease type C should be considered when planning the anesthetic for these patients. It is noteworthy that contrary to what is seen in patients with Niemann-Pick disease type C with NPC2 mutation who might have primary lung disease,21 in patients with NPC1 mutation, pulmonary involvement is predominantly secondary to profound neurological impairment and consequent recurrent aspiration and muscle weakness. In our series, one fourth of the patients had a history of chronic cough and/or history of aspiration pneumonia. Following our institutional practice,22 in the present study, unless contraindicated, we maintained patients anesthetized without airway instrumentation for diagnostic procedures in the 3-T MRI suite and main operating room. With this regimen, the conduct of all scheduled studies was possible. Most patients tolerated the anesthesia and procedures well and were discharged from the postanesthesia care unit after a 1-hour stay (average) without anesthetic complications. Therefore, for elective noninvasive procedures, patients with Niemann-Pick disease type C can be anesthetized without airway instrumentation; however, a decision to intubate the trachea should take into consideration preexisting history of aspiration. As such, in our series we decided to electively intubate the trachea of patients with history of recurrent aspiration.
We did observe some morbidity during the anesthesia of patients with Niemann-Pick disease type C, which is in concert with findings previously reported with the use of similar anesthesia technique in patients with American Society of Anesthesia physical status III.22 We observed that some of our younger children (11 children during 15 general anesthetics) presented profuse sweating while in the 3-T MRI scanner without significant changes in temperature. Although we did not observe hyperthermia, the findings of profuse sweating are in concert with reports indicating that substantial patient warming can occur in a 3-T magnet.3 Interestingly, we also had 4 older patients with long-term, severe neurological impairment who had hypothermia during MRI. Therefore, careful monitoring of temperature of children and adolescents with Niemann-Pick disease type C undergoing MRI is certainly warranted.
In this series, we observed episodes of decreases in oxygen saturation in 4 patients. Those patients had a history of dysphagia, increased oral secretion, drooling, chronic persistent cough, or history of aspiration; 2 patients had to be reintubated to enable suction of tracheal secretions and improve oxygenation. Although there were no episodes of witnessed aspiration, it is conceivable that these episodes of hypoxia resulted from a combination of atelectasis associated with increased abdominal pressure and possible aspiration of oral secretions. Those patients who had episodes of decreases in oxygen saturation during anesthetics were transported to the intensive care unit for overnight observation and later discharged without sequelae. Therefore, patients with Niemann-Pick disease type C are at risk for episodes of decreases in oxygen saturation that can possibly be associated with aspiration of oral secretions or preexisting lung involvement.
Summary
We prospectively examined the administration of anesthesia in 32 patients with Niemann-Pick disease type C with confirmed NPC1 mutation who underwent a total of 64 inductions of anesthesia for diagnostic procedures. For those treating and preparing children with Niemann-Pick disease type C disease to undergo anesthesia, a clear understanding of the extent of visceral and neurological involvement is of utmost importance. As our series illustrates, children with Niemann-Pick disease type C are at risk of perianesthetic morbidity; however, once all possible comorbidities in patients with Niemann-Pick disease type C disease are defined, perianesthesia morbidity can be anticipated and addressed, and the anesthetic courses are likely to be, for the most part, uneventful.
Acknowledgments
The work was conducted at the National Institutes of Health Clinical Center. We are grateful for the care that the staff from the Department of Perioperative Medicine at the National Institutes of Health provided to our patients.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institutes of Health Clinical Center, National Institutes of Health. The work was also supported by a Bench to Bedside award from the Office of Rare Diseases and National Institutes of Health Clinical Center and grants from the Ara Parseghian Medical Research Foundation and Dana Angels Research Trust (Nicole Yanjanin).
Footnotes
Author Contributions
NM designed the study, collected the data, and prepared and reviewed the manuscript. XL designed the study, collected the data, and prepared the manuscript. NPO collected the data and prepared and reviewed the manuscript. NY collected the data and prepared and reviewed the manuscript. FDP designed the study, collected the data, and reviewed the manuscript. ZMNQ designed the study, collected and interpreted the data, and reviewed the manuscript.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
The study was conducted after approval from the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
References
- 1.Imrie J, Dasgupta S, Besley GT, et al. The natural history of Niemann-Pick disease type C in the UK. J Inherit Metab Dis. 2007;30(1):51–59. doi: 10.1007/s10545-006-0384-7. [DOI] [PubMed] [Google Scholar]
- 2.Wraith JE, Baumgartner MR, Bembi B, et al. Recommendations on the diagnosis and management of Niemann-Pick disease type C. Mol Genet Metab. 2009;98(1–2):152–165. doi: 10.1016/j.ymgme.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Wraith JE, Guffon N, Rohrbach M, et al. Natural history of Niemann-Pick disease type C in a multicentre observational retrospective cohort study. Mol Genet Metab. 2009;98(3):250–254. doi: 10.1016/j.ymgme.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Vanier MT. Niemann-Pick disease type C. Orphanet J Rare Dis. 2010;5:16. doi: 10.1186/1750-1172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wraith JE, Vecchio D, Jacklin E, et al. Miglustat in adult and juvenile patients with Niemann-Pick disease type C: long-term data from a clinical trial. Mol Genet Metab. 2010;99(4):351–357. doi: 10.1016/j.ymgme.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Yanjanin NM, Velez JI, Gropman A, et al. Linear clinical progression, independent of age of onset, in Niemann-Pick disease, type C. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(1):132–140. doi: 10.1002/ajmg.b.30969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patterson MC, Vecchio D, Jacklin E, et al. Long-term miglustat therapy in children with Niemann-Pick disease type C. J Child Neurol. 2010;25(3):300–305. doi: 10.1177/0883073809344222. [DOI] [PubMed] [Google Scholar]
- 8.Patterson MC. A riddle wrapped in a mystery: understanding Niemann-Pick disease, type C. Neurologist. 2003;9(6):301–310. doi: 10.1097/01.nrl.0000094627.78754.5b. [DOI] [PubMed] [Google Scholar]
- 9.Ory DS. The Niemann-Pick disease genes: regulators of cellular cholesterol homeostasis. Trends Cardiovasc Med. 2004;14(2):66–72. doi: 10.1016/j.tcm.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Nixon RA. Niemann-Pick Type C disease and Alzheimer’s disease: the APP-endosome connection fattens up. Am J Pathol. 2004;164(3):757–761. doi: 10.1016/S0002-9440(10)63163-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garver WS, Francis GA, Jelinek D, et al. The National Niemann-Pick C1 disease database: report of clinical features and health problems. Am J Med Genet A. 2007;143A(11):1204–1211. doi: 10.1002/ajmg.a.31735. [DOI] [PubMed] [Google Scholar]
- 12.Patterson MC, Vecchio D, Prady H, et al. Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. Lancet Neurol. 2007;6(9):765–772. doi: 10.1016/S1474-4422(07)70194-1. [DOI] [PubMed] [Google Scholar]
- 13.Schilling T, Kozian A, Pfau G, et al. Anesthetic management of a patient with Niemann-Pick type B disease undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2007;21(3):428–431. doi: 10.1053/j.jvca.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Bujok LS, Bujok G, Knapik P. Niemann-Pick disease: a rare problem in anaesthesiological practice. Paediatr Anaesth. 2002;12(9):806–808. doi: 10.1046/j.1460-9592.2002.00922.x. [DOI] [PubMed] [Google Scholar]
- 15.Fu R, Yanjanin NM, Bianconi S, et al. Oxidative stress in Niemann-Pick disease, type C. Mol Genet Metab. 2010;101(2–3):214–218. doi: 10.1016/j.ymgme.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattsson N, Zetterberg H, Bianconi S, et al. Gamma-secretase-dependent amyloid-beta is increased in Niemann-Pick type C: a cross-sectional study. Neurology. 2011;76(4):366–372. doi: 10.1212/WNL.0b013e318208f4ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grewal RP, Yu KT, Barton NW, et al. Liver biopsies in patients with lysosomal storage disease: experience with effective sedation. Indian J Pediatr. 1997;64(6):887–891. doi: 10.1007/BF02725518. [DOI] [PubMed] [Google Scholar]
- 18.Vakkuri A, Jantti V, Sarkela M, et al. Epileptiform EEG during sevoflurane mask induction: effect of delaying the onset of hyperventilation. Acta Anaesthesiol Scand. 2000;44(6):713–719. doi: 10.1034/j.1399-6576.2000.440609.x. [DOI] [PubMed] [Google Scholar]
- 19.Mohanram A, Kumar V, Iqbal Z, et al. Repetitive generalized seizure-like activity during emergence from sevoflurane anesthesia. Can J Anaesth. 2007;54(8):657–661. doi: 10.1007/BF03022961. [DOI] [PubMed] [Google Scholar]
- 20.Constant I, Seeman R, Murat I. Sevoflurane and epileptiform EEG changes. Paediatr Anaesth. 2005;15(4):266–274. doi: 10.1111/j.1460-9592.2004.01538.x. [DOI] [PubMed] [Google Scholar]
- 21.Griese M, Brasch F, Aldana VR, et al. Respiratory disease in Niemann-Pick type C2 is caused by pulmonary alveolar proteinosis. Clin Genet. 77(2):119–130. doi: 10.1111/j.1399-0004.2009.01325.x. [DOI] [PubMed] [Google Scholar]
- 22.Kiringoda R, Thurm AE, Hirschtritt ME, et al. Risks of propofol sedation/anesthesia for imaging studies in pediatric research: eight years of experience in a clinical research center. Arch Pediatr Adolesc Med. 2010;164(6):554–560. doi: 10.1001/archpediatrics.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melloni C. Morbidity and mortality related to anesthesia outside the operating room. Minerva Anestesiol. 2005;71(6):325–334. [PubMed] [Google Scholar]