Abstract
Group III metabotropic glutamate receptors (mGluRs), which are generally located presynaptically, modulate synaptic transmission by regulating neurotransmitter release. Previously we showed enhanced amygdala-dependent cued fear conditioning in mGluR4−/− mice 24 hr following training involving two tone-shock pairings. In this study, we assessed the effects of modulating mGluR4 signaling on acquisition and extinction of conditioned fear. mGluR4−/− and wild-type female and male mice received 10 tone-shock pairings during training. Compared to wild-type mice, mGluR4−/− mice showed enhanced acquisition and extinction of cued fear. Next, we assessed whether acute pharmacological stimulation of mGluR4 with the specific orthosteric mGluR4 agonist LSP1-2111 also affects acquisition and extinction of cued fear. Consistent with the enhanced acquisition of cued fear in mGluR4−/−, LSP1-2111, at 2.5 and 5mg/kg, inhibited acquisition of cued fear conditioning in wild-type male mice. The drug’s effect on extinction was less clear and only a subtle effect was seen at 5 mg/kg. Finally, analysis of microarray data of amygdala tissues from mGluR4−/− versus wild-type and from wild-type mice treated with a mGluR4 agonist versus saline revealed a significant overlap in pattern of gene expression. Together, these data support a role for mGluR4 signaling in acquisition of fear learning and memory.
Keywords: mGluR4, fear conditioning, mice, cued
1. Introduction
Ionotropic glutamate receptors mediate the fast actions of the excitatory neurotransmitter glutamate. In contrast, metabotropic glutamate receptors (mGluRs) modulate glutamatergic and GABAergic neurotransmission (Conn and Pin, 1997; Pin and Duvoisin, 1995). Group III receptors (mGluR4, mGluR6, mGluR7, and mGluR8) are generally located presynaptically and regulate neurotransmitter release (Cartmell and Schoepp, 2000), and they have been identified as attractive targets for treating anxiety disorders (Swanson et al., 2005). Alterations in fear learning mechanisms likely participate in the development and/or maintenance of anxiety disorders. Disorders such as phobias are primarily characterized by cue-specific fear and modeled by amygdala-dependent cued fear conditioning (Grillon and Davis, 1997; Mineka and Oehlberg, 2008).
In Pavlovian fear conditioning, mice learn to associate a conditioned stimulus (CS, e.g. a tone) with an unconditioned stimulus (US, e.g. foot shock). Contextual fear conditioning is assessed in the same environment but in the absence of the US, while cued fear conditioning is used in a new environment but in the presence of the CS. Recently, we showed enhanced cued fear conditioning in mGluR4−/− female and male mice 24 hours after receiving two CS-US pairings during training (Davis et al., 2012). In contrast, no changes were seen in hippocampus-dependent contextual fear conditioning. The fact that cued but not contextual freezing is enhanced in mGluR4−/− mice indicates specificity of the memory enhancing effects. This might involve anatomical specificity, as contextual, but not cued, fear conditioning is hippocampus-dependent while both require the amygdala (Ferbinteanu et al., 1999; Gerlai, 1998; Kim and Fanselow, 1992).
In the fear-potentiated startle paradigm, the selective mGluR7 allosteric agonist N,N'-dibenzyhydryl-ethane-1,2-diamine dihydrochloride (AMN082) impaired acquisition but enhanced extinction of conditioned fear, while mGluR7 knockdown using short interfering RNA attenuated extinction as assessed (Fendt et al., 2008). Together, these data support a role for mGluR7 in acquisition and extinction of conditioned fear. There have been successful efforts to develop mGluR4 selective agonists (Goudet et al., 2012), preferential mGluR4 agonist LSP1-2111 (Beurrier et al., 2009; Wieronska et al., 2010). As reported by Beurrier et al., 2009, and Wieronska et al., 2010, following systemic injection, LSP1-2111 does penetrate the brain (Doller et al., 2011) and causes anticataleptic effects in rats treated with haloperidol to a similar degree as that seen following central administration (Flor and Acher, 2012). In addition Doller et al. have measured the intracerebral concentration of LSP1-2111 after peripheral administration of the drug (Doller et al., 2011). In this study, mGluR4−/− and C57Bl6/J wild-type (WT) mice (Experiment 1) and LSP1-2111 or saline (Experiment 2) were used to assess the effects of modulating mGluR4 signaling on acquisition and extinction of conditioned fear. In addition, microarray data of amygdala tissues from untreated mGluR4−/− and WT mice and from wild-type mice treated with a mGluR4 agonist or saline (Experiment 3) were used to assess whether there is a potential overlap in pattern of gene expression.
2. Materials and methods
2.1. Animals
For Experiments 1 and 3, mGluR4−/− mice were obtained from Jackson Laboratory (stock #003619) and bred with wild-type C57BL/6 mice. Heterozygote mice were crossed to generate related mGluR4−/− and mGluR4+/+ (WT) mice. mGluR4−/− crosses and WT crosses were then made to generate the mice and the parents of the mice used for behavioral experiments. Experimentally naïve 3- and 6-month-old mGluR4−/− and WT female and male mice were used. For the pharmacological experiments in Experiments 2 and 3, wild-type C57BL/6 mice purchased from Jackson Laboratories were used. In Experiment 1, there were 75 mice, consisting of 39 WT mice (3-month old males, n = 10; 3-month-old females; n = 8; 6-month-old males, n = 10; and 6-month-old females, n = 11) and 36 mGluR4−/− mice (3-month-old males, n = 10; 3-month-old females, n = 8; 6-month-old males, n = 8; and 6-month old females, n = 10). In experiment 2, there were 39 WT male mice for the pre-training treatment experiment that were randomly assigned to the following treatment groups: vehicle (n = 10), 2.5mg/kg (n = 9), 5 mg/kg (n = 10), 7.5 mg/kg (n = 10). For the post-training treatment experiment, there were two groups: vehicle (n and 5 mg/kg (n = 10). In Experiment 3, there were 14 mGluR4−/− female mice divided among the two treatment groups, vehicle (n = 7) and 5mg/kg LSP1-2111 (n = 7). Males were not included as there was no significant effect of sex (see results, experiment 1). Animals were group housed in standard shoebox cages until three days prior to the start of behavioral testing. They were singly housed at this time through behavioral testing. The mice were maintained on a 12:12 light/dark schedule with standard laboratory chow (PicoLab Rodent diet 20, # 5,053; PMI Nutrition International, St. Louis, MO, USA) and water provided ad libitum. Experimenters were blinded to the genotype and drug treatment of the animals. All procedures were conducted in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University
2.2. Drugs
LSP1-2111 was synthesized in Francine Acher’s laboratory. The drug was prepared for injections on the day of the injection. The drug was dissolved in 0.9 % saline, and NaOH was added to bring the solution to a physiological pH. The drug was injected intraperitoneally 45 minutes prior to the start of the fear conditioning training trial unless mentioned otherwise.
2.3. Fear Conditioning
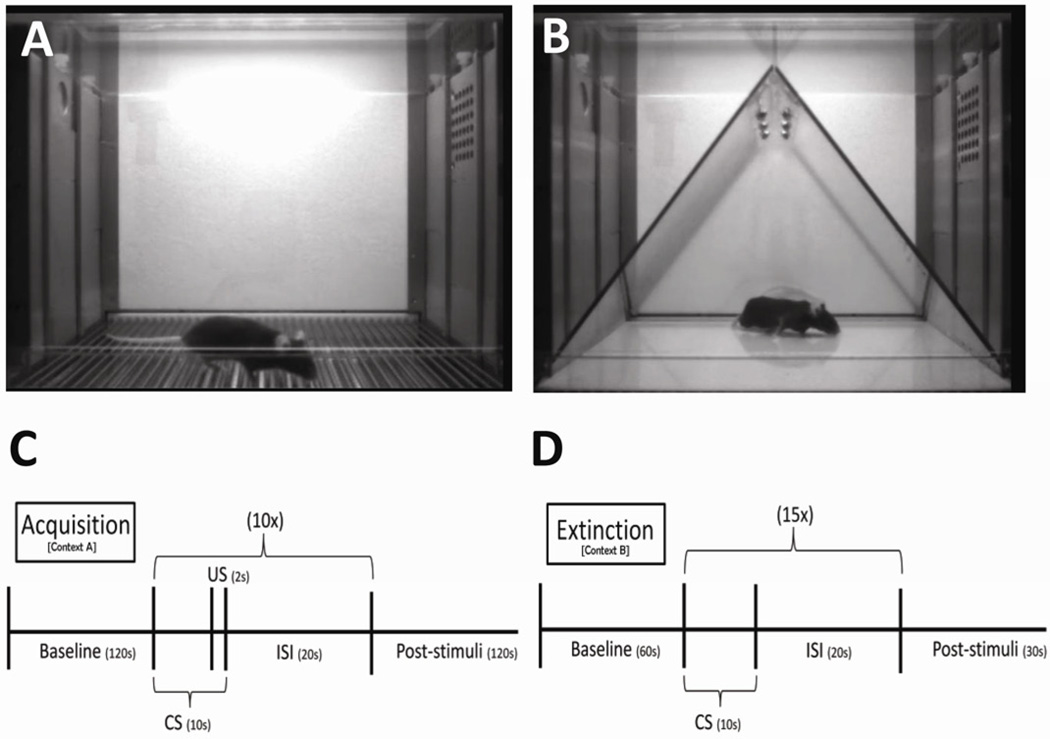
The mice were tested for fear conditioning using Med Associates NIR Video and automated analysis (Med Associates, St. Albans, VT, USA) utilizing Med Associates Video Freeze automated scoring system. This system is described in detail and validated against traditional hand scoring methods (Anagnostaras et al., 2010). Pavlovian fear conditioning is a versatile and well-understood method of assessing associative learning and memory (Maren, 2001). In this task, mice learn to associate a conditioned stimulus (CS, e.g. a tone) with an unconditioned stimulus (US, e.g. foot shock). CS–US pairings are preceded by a short habituation period, from which a baseline measure of locomotor activity and other behavior can be scored. Freezing is defined as the absence of all movement except for respiration. The freezing response is a widely used indictor of a conditioned fear response (Fanselow, 1994). On day 1, training, the mice were placed inside a white LED lit (100 lux) fear conditioning chamber (Context A). Context A consisted of a metal grid floor with gray and white walls (Fig. 1A). There was a 120 second baseline followed by ten CS-US pairings. During acquisition, the 10-second tones (80dB, 2.8Hz) co-terminated with 2-second footshocks (0.35 mA) (US). The intertone interval (ISI) was 20 sec. Motion during shock (arbitrary units) was measured to explore potential genotype and treatment-induced differences in response to the aversive stimulus. Percent time freezing during each minute of the day 1 was measured to assess acquisition of the fear response. On days 2–6, mice were exposed to Context B and extinction was analyzed. Context B consisted of a smooth white plastic floor, with a “tented” black plastic ceiling and scented with a 10% isoproponol solution (Fig. 1B). There was a 60 second baseline and 15 10-sec tones with an ISI of 20 sec. As we wanted to assess between trial extinction (Meyers and Davis, 2007), and not within trial extinction, we analyzed the freezing during the first 5 tones on each day, as described by the group of Anagnostaras (Carmack et al., 2010). Experiment 1 and 2 had the same acquisition protocol, but experiment 1 had 6 days of extinction, while experiment 2 had 4 days of extinction. For the last part of experiment 2, LSP1-2111 (5 mg/kg) was given immediately after training.
Fig. 1.
The fear conditioning environments and training and testing protocols. (A) Context ‘A’ was used during the training trial for experiments 1 and 2. The chamber consisted of grey and white and was scented with 0.5% acetic acid. (B) Context ‘B’ was used during the extinction trials for experiments 1 and 2. It consisted of a smooth white floor and a black “tented” roof. The chamber was scented with 10% isopropanol. (C) Scheme of stimuli presentation during the training trials. (D) Scheme of the stimuli presentation during the extinction trials.
For experiment 3, we used a different conditioning and extinction protocol to induce higher freezing responses to the conditioned stimulus on the first day of extinction. Mice were placed in Context A and exposed to a 60-second baseline period, followed by a 60-second tone (CS, 2.8kHz, 80db) which co-terminated with a 2-second shock (US, 0.35mA). These CS-US presentations were separated by a 60-second interval, and the pattern was repeated for a total of 5 presentations, giving a trial duration of 10 minutes. Next, the mice received 7 consecutive days of extinction trials. Each extinction trial consisted of 60-second of no-tone and 60-second of tone, repeated 5 times, giving a total trial length of 10 minutes.
2.4. Pattern of gene expression
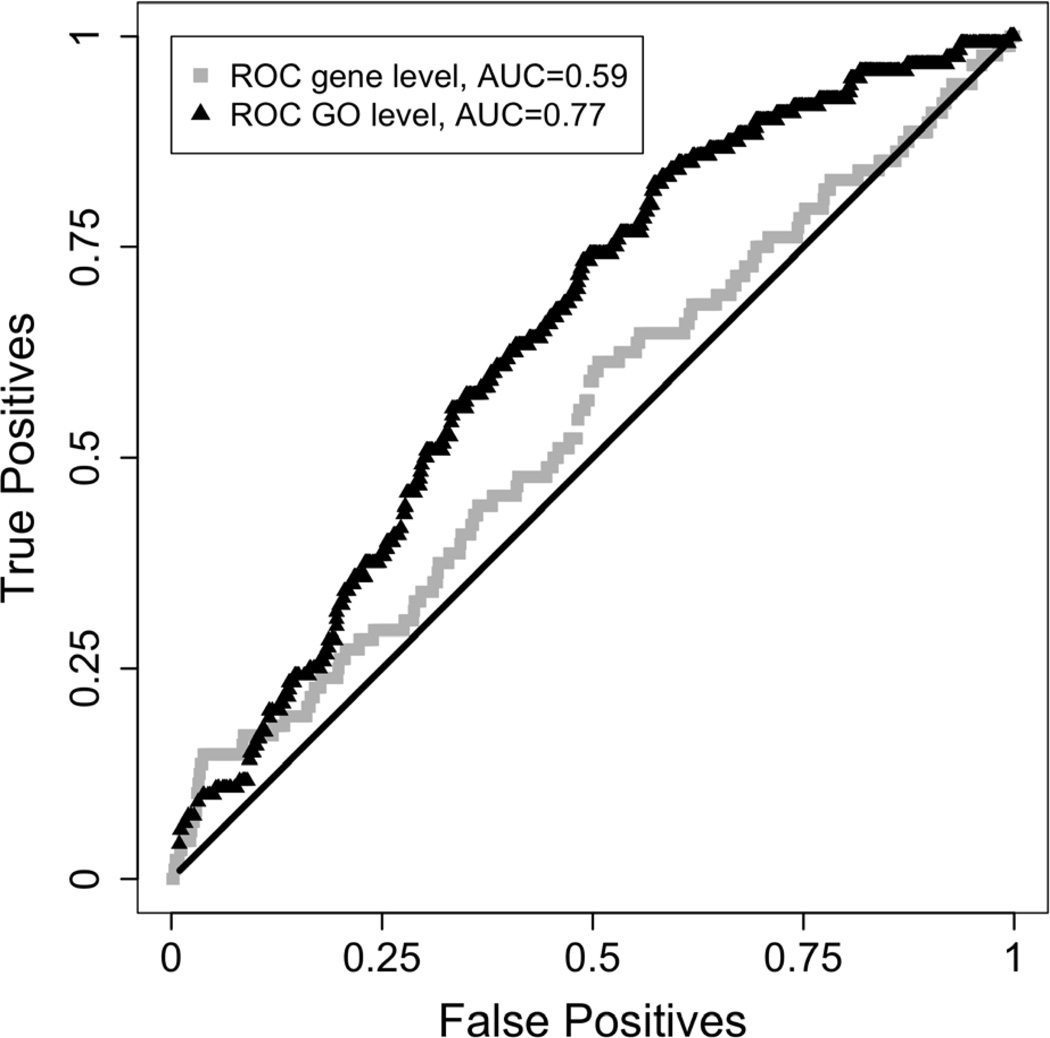
For Experiment 4, total RNA was prepared from the amygdala of 3 WT and 3 mGluR4−/−, mice, as well as from the amygdala of WT mice treated with the mGluR4-selective allosteric agonist VU 0155041 (Niswender et al., 2008), purchased from (Tocris, Ellisville, Missouri), or vehicle, followed by cDNA synthesis and linear amplification. After verifying the quality of the RNA, samples were applied to Illumina whole genome 6 (WG6) mouse arrays by the OHSU Gene Microarray Shared Resource. Gene expression data were imported into the R application environment (www.r-project.org) using the lumi package (Du et al., 2008). Subsequently, we performed quantile normalization using the default lumiExpresso settings. Differential gene expression was evaluated using the eBayes modified t-statistic (Smyth, 2004), which is available in Bioconductor (www.bioconductor.org); raw p values were further adjusted using False Discovery Rate (FDR) (Benjamini and Hochberg, 1995). Gene set enrichment analysis was performed using the GSEA package (Subramanian et al., 2007) and gene sets corresponding to all Gene Ontology (GO) categories. We used our own ranked list of p values obtained using the eBayes procedure. Overlap in differential expression between the mGluR4 agonist and genotype conditions was evaluated based on receiver operator characteristic (ROC) curves. First, we selected the differentially expressed genes in the mGluR4−/− versus WT mice, at raw p values of 0.005 (slightly different values resulted in nearly identical results). Next, we varied the p value threshold for the mGluR4 agonist versus saline comparison between 0 and 1; for each p value, true positive (TP) and false positive (FP) values were computed based on the overlap between differentially expressed genes in the two categories. The collection of TP and FP points traced the ROC curve inside the unit square. A ROC curve bending above the diagonal signifies overlap above chance level; this also corresponds to area under the curve (AUC) values above 0.5 (chance level) up to a maximum of 1. The ROC analysis was repeated for the GSEA analysis results. Each GO category corresponded to a set of p values; GSEA analysis assigned a single collective p value to each GO category.
2.5. Statistical analyses cognitive data
Data are reported as means ± the standard error of the mean (SEM) and were analyzed using SPSS 16.0 software (IBM, Armonk, NY, USA). Results were considered significant at an α level of 0.05. For experiment 1, a three-way ANOVA (univariate, MANOVA, or repeated measures), with sex (male vs. female), age (3-month-old versus 6-month-old), and genotype (wild-type versus mGluR4−/−) as factors, was used to explore the potential group differences in the dependent variable. If sex or age did not interact with the primary independent variable of interest, genotype, then sex and/or age were dropped from the model. When a significant effect was observed, multiple-comparisons (e.g. t-tests) with Bonferroni corrections were applied to the model to determine the pattern of group differences. For Experiment 2, a 1-way ANOVA was used, with drug dose as factor (0, 2.5 mg/kg, 5 mg/kg, and 7.5mg/kg).
3. Results
3.1. Experiment 1
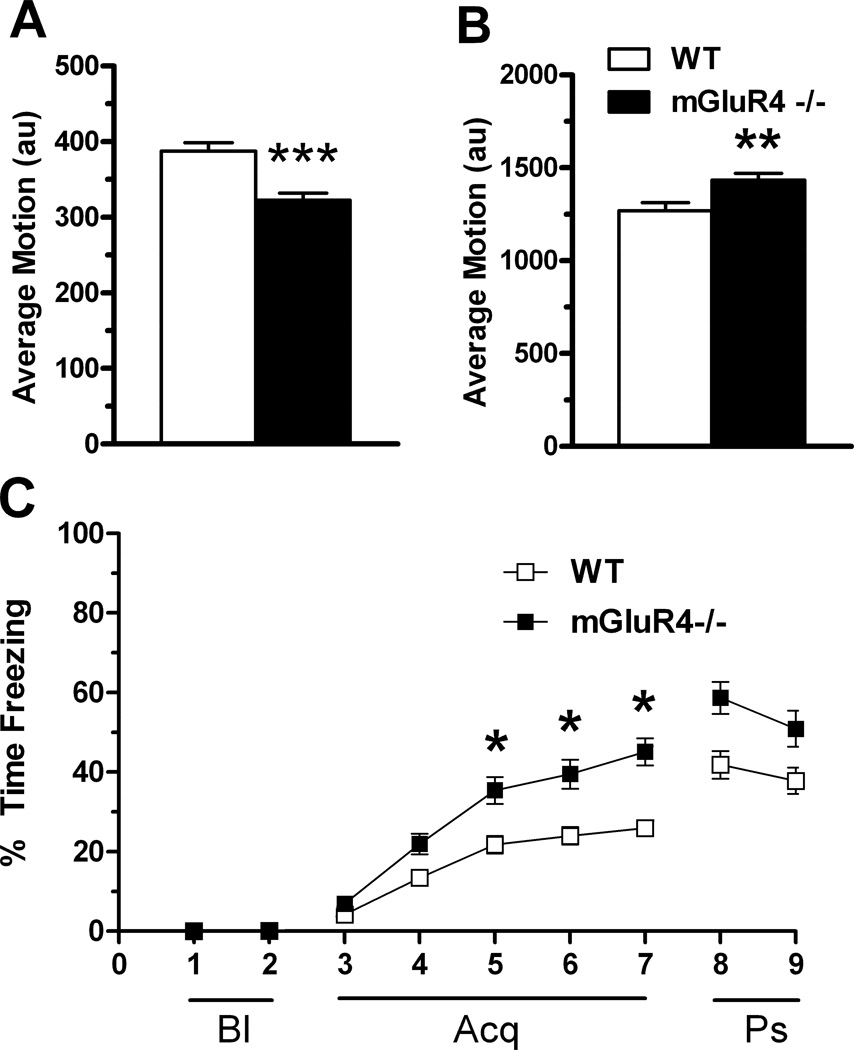
In Experiment 1, acquisition and extinction of cued fear was assessed mGluR4−/− and WT mice. A three-way univariate ANOVA revealed a significant effect of main effect of age (p < 0.001) and genotype (p < 0.001) on average baseline movement, but there were no interactions. Therefore, age and sex were dropped from the model. A one-way ANOVA demonstrated that there was a significant effect of genotype on average baseline movement (F(1, 73) = 20.552, p < 0.001) (Fig. 2A). WT mice (388 ± 11 arbitrary units (au)) were more active than mGluR4−/− mice (323 ± 9 au). Next, we analyzed the effect of the independent variables on the average motion in response to the shocks (all ten shocks were averaged). A three-way univariate ANOVA revealed a significant main effect of genotype (F(1, 67) = 11.369, p < 0.001), age (F(1, 67) = 6.420, p = 0.014), and sex (F(1, 67) = 19.051, p < 0.001). As there were no interactions between genotype and sex or genotype and age, sex and age were dropped from the model. A 1-way ANOVA revealed a main effect of genotype (F(1, 73) = 8.153, p = 0.006). In general, WT mice (1269 ± 43 au) moved less during the shock than mGluR4−/− mice (1434 ± 37 au).
Fig. 2.
Day 1 (training) fear conditioning of WT and mGluR4−/− mice in ‘Context A’ (see methods). (A) Average baseline motion (arbitrary units) was recorded to assess general locomotor activity. Wild-type mice tended to be more active in the baseline period than mGluR4−/− mice. (B) Average motion (arbitrary units) during the shocks (the 10 shocks were averaged). (C) Percent time freezing during the entire training trial, which was analyzed using the following time periods: baseline (Bl), acquisition (Acq), and post-stimuli (Ps). Baseline consisted of a 2-minute period with no stimuli in which mice generally had zero freezing. Therefore freezing was not analyzed during this period. During the acquisition phase, mice received a total of ten tone-shock pairings that were administered at equal intervals. mGluR4−/− mice tended to freeze more than wild-type mice during minutes 5,6, and 7. The post-stimuli period represents the immediate freezing to the context after all tone-shocks ceased. *p < 0.05, **p < 0.01, ***p < 0.001. Error bars represent SEM values.
No statistical analysis was performed for percent freezing during the baseline period because most mice did not freeze during this period (Fig. 2C, Bl). Next, percent time freezing during acquisition was analyzed using a MANOVA (Fig. 2C, Acq). Minute was the within-subjects variable and sex, age, and genotype the between-subjects variables. There was no main effect of sex and sex did not interact with any other variables. Therefore, another MANOVA was performed with sex removed from the model. There was significant effect of minute (λ = 0.182, F(4, 68) = 76.165, p < 0.001) and a minute x genotype interaction (λ = 0.761, F(4, 68) = 5.332, p = 0.001). Additionally, there was a main effect of age (F(1, 71) = 10.253, p = 0.002) and a main effect of genotype (F(1, 71) = 24.294, p < 0.001). As age did not interact with any other variable, it was removed from the model. A repeated-measures ANOVA revealed a significant day x genotype interaction (F(5,365) = 4.894, p < 0.001). Multiple comparisons with a Bonferroni correction confirmed that mGluR4−/− mice showed more freezing than controls in minutes 5, 6, and 7 (all p values < 0.05).
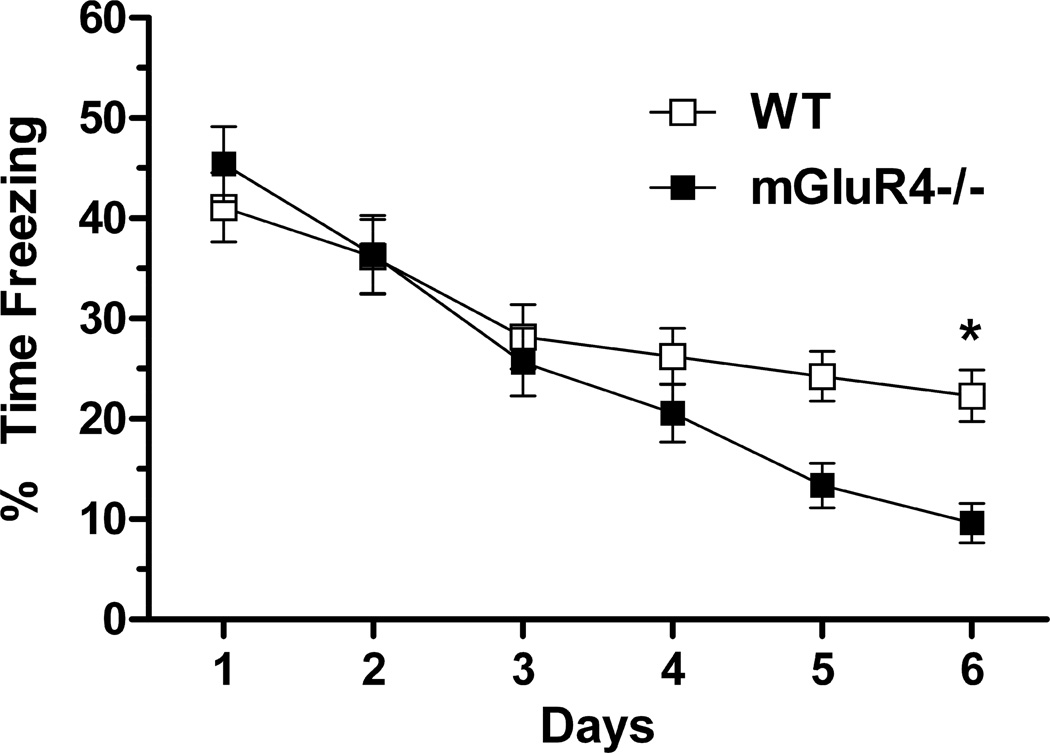
Extinction was analyzed by averaging the percent time freezing during the first five tones for each day (Fig. 3). We did not analyze the remaining tones, as we were more interested in between-trial extinction, rather than within-trial extinction. Between-trial extinction is more relevant to animal models of psychiatric disorders (e.g. post-traumatic stress disorder (PTSD)) (Carmack et al., 2010). Percent time freezing over 6 days of extinction was analyzed using a MANOVA. Day was the within-subjects variable and sex, age, and genotypes were the between-subjects variables. There were significant effects of day (λ = 0.312, F(5,63) = 27.769, p < 0.001), a day x genotype interaction (λ = 0.784, F(5,63) = 3.467, p = 0.008), and a day x age interaction (λ = 0.672, F(5,63) = 6.138, p < 0.001). There were also significant effects of sex (F(1,67) = 11.483, p < 0.001) and age (F(1,167) = 20.277, p < 0.0001). There was no interaction between genotype and age or genotype and sex. Therefore, age and sex were removed from the model, and a new MANOVA revealed a main effect of day (λ = 0.312, F(5,69) = 27.769, p < 0.001), and a day x genotype interaction (λ = 0.8327, F(5,69) = 2.692, p = 0.028). Multiple comparisons using a Bonferroni correction showed that mGluR4−/− mice had lower freezing levels than WT mice on day 6.
Fig. 3.
Days 2–6 of the fear extinction trials in WT and mGluR4−/− mice. Fifteen ten-second tones were presented with twenty second interval, but only the first five tones were averaged to assess daily freezing levels. mGLuR4−/− mice tended to extinguish faster, but the difference in daily freezing levels did not reach significance until day 6. *p < 0.05. Error bars represent SEM values.
3.2. Experiment 2
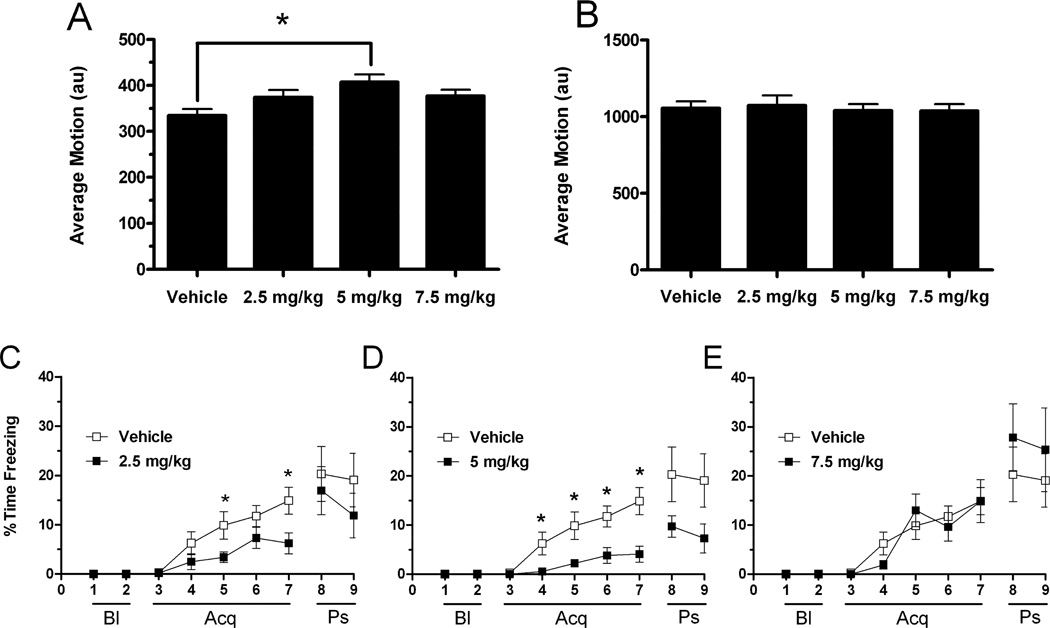
In Experiment 2, acquisition and extinction of cued fear was assessed in WT mice treated with the mGluR4 agonist LSP1-2111 or saline. A one-way ANOVA revealed a main effect of treatment (F(3, 35) = 3.879, p = 0.017) on baseline average motion (Fig. 4A). Post-hoc comparisons using the Tukey HSD test indicated that baseline average motion was significantly higher in the 5 mg/kg treatment group (407 ± 17 au) than the vehicle group (334 ± 16 au). When average motion during the average of all the shocks was analyzed using a one-way ANOVA, there was no effect of treatment (F(3, 35) = 0.107, p = 0.956) (Fig. 4B). Administration of LSP1-2111 did not affect this measure of reactivity to shock.
Fig. 4.
Day 1 (training) fear conditioning of mice administered vehicle, 2.5 mg/kg, 5 mg/kg, or 7.5 mg/kg of the mGlurR4 orthosteric agonist LSP1-2111 45 minutes before being placed in ‘Context A’ (see methods). (A) Average baseline motion (arbitrary units) was recorded to assess general locomotor activity. Mice that received 5 mg/kg of drug tended to move more than vehicle-treated mice. (B) Average motion (arbitrary units) during the shocks (the 10 shocks were averaged). All groups exhibited similar average motion in response to shock. (C) Percent time freezing during the entire training trial, which was analyzed using the following time periods: baseline (Bl), acquisition (Acq), and post-stimuli (Ps). Baseline consisted of a 2-minute period with no stimuli in which mice generally had zero freezing. Therefore, freezing was not assessed during this period. During the acquisition phase, mice received a total of ten tone-shock pairings that were administered at equal intervals. The 2.5 mk/kg treated group exhibited lower freezing, compared to controls, in minutes 5 and 7; the 5 mg/kg group froze less in minutes 4–7; the 7.5 mg/kg performed the same as controls. The post-stimuli period represents the immediate freezing to the context after all tone-shocks ceased. *p < 0.05, Error bars represent SEM values.
Next, percent time freezing during acquisition was analyzed using a repeated-measures ANOVA. Minute was the within-subjects variable and dose was the between-subjects variable. There was a main effect of minute (λ = 0.210, F(4,33) = 31.013, p < 0.001), a minute x dose interaction (λ = 0.441, F(12,87.6) = 12.034, p < 0.0001), and a main effect of treatment (F(3, 36) = 3.110, p = 0.038). Next, the three LSP1-2111 treated groups were separately compared to the vehicle group, using a repeated measures ANOVA. In the 2.5 mg/kg versus vehicle analysis, there was a minute x dose interaction (F(4,68) = 3.373, p = 0.014). Post hoc comparisons showed that LSP1-2111-treated animals showed lower freezing levels at minutes 5 and 7. A similar analysis with the mice treated with LSP1-2111 at a dose of 5 mg/kg group also revealed a minute x dose interaction (F(4,72) = 3.708, p = 0.008). Post hoc comparisons confirmed that LSP1-2111-treated mice showed less freezing than vehicle-treated mice in minutes 4–7 (all p values <0.05). In contrast to the 2.5 and 5 mg/kg dose, there was no minute x dose interaction (F(4,72) = 1.184, p = 0.325) in the analysis with the 7.5 mg/kg group.
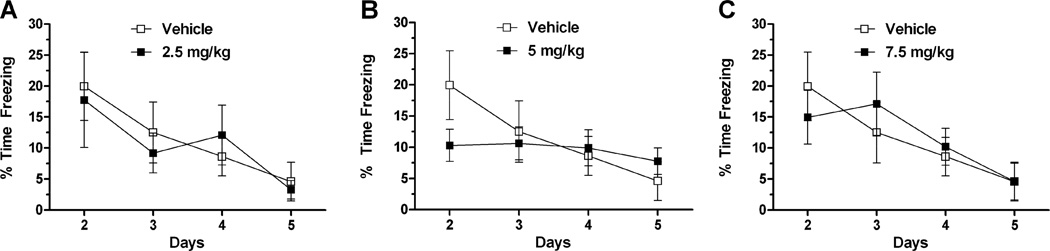
To analyze the potential effect of LSP1-2111 on extinction, percent time freezing during each extinction day and for each dose was compared to that of the vehicle in 3 separate repeated-measures ANOVAs (Fig. 5). For the 5 mg/kg versus vehicle analysis, there was an effect of day (F(3, 54) = 5.097, p = 0.004) and day x dose interaction (F(3, 36) = 2.862, p = 0.045). While the vehicle-treated mice showed between day extinction, the 5 mg/kg dose group did not. Post hoc analysis comparing the treatment groups on individual days did not reveal a significant drug effect on any day. In contrast to the 5 mg/kg dose, there was no day x treatment interaction for either the 2.5 mg/kg (F(3,51) = 0.340, p = 0.796) or the 7.5 mg/kg (F(3,54) = 1.072, p = 0.369). When LSP1-2111 at 5 mg/kg was given after acquisition, no treatment effect on extinction was observed (data not shown). Thus, the freezing levels in the 5 mg/kg group over the days might be due more to alterations in acquisition than post-acquisition memory consolidation.
Fig. 5.
Days 2–6 of the fear extinction trials of mice administered vehicle versus 2.5 mg/kg (A), 5 mg/kg (B), or 7.5 mg/kg (C) of LSP1-2111 before the training trial (Day 1). Fifteen ten-second tones were presented with a 20-second interval, but only the first five tones were averaged to assess daily freezing levels. The mice administered 5 m/kg of LSP1-2111 showed genotype x dose interaction on the level of freezing over days, indicating an effect on extinction. Error bars represent SEM values.
The effects of the 5 mg/kg dose on extinction did not seem due to a potential bottom effect, as the 5 mg/kg dose group did show within day extinction on day 4 ((F(2,18) = 4.491, p = 0.026)). However, the reduced acquisition and freezing levels in the 5 mg/kg group on day 1 might have contributed to the level of freezing of this group on subsequent days. When the paradigm described below in Experiment 3 was used, there was no drug effect on extinction (data not shown).
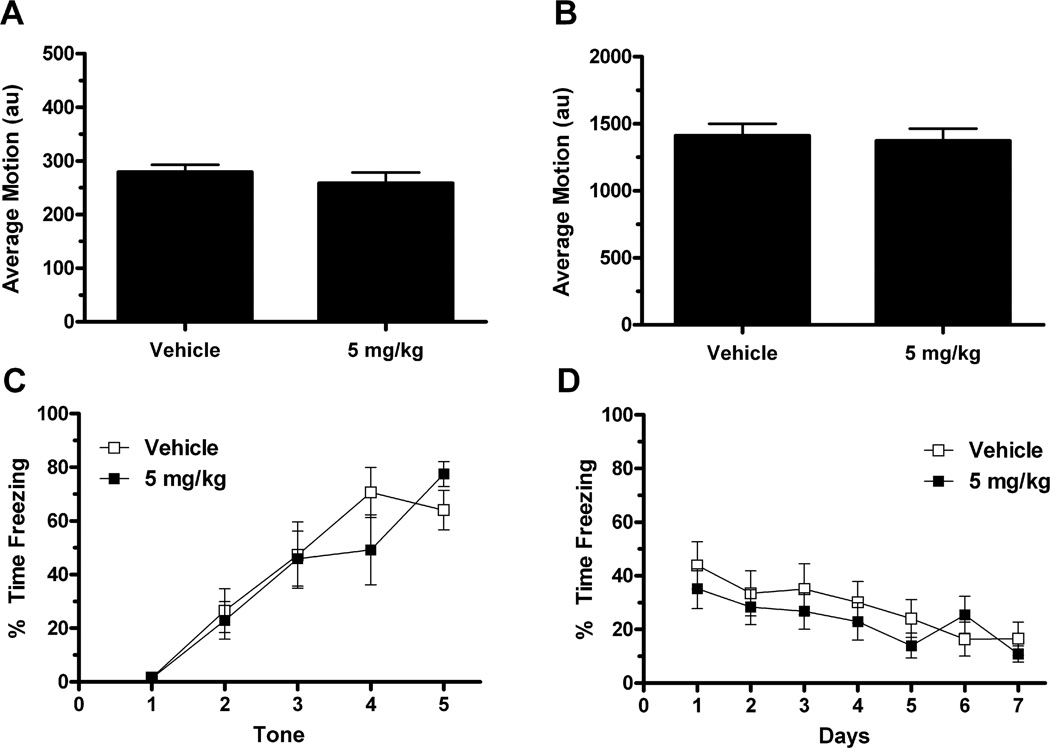
3.3. Experiment 3
Baseline average motion was measured in the 60-second period prior to the first tone on the first day (Fig. 6A). An independent samples t-test revealed no effect of treatment on baseline average motion (t(12) = 0.857, p = 0.408; vehicle = 279.2 + 13.53; LSP1-2111 = 258.56 + 19.9). Five shocks were presented as the unconditioned stimuli in the acquisition trial (Fig. 6B). Average motion during the five shocks was averaged, this value was used in an independent samples t-test, which revealed no effect of treatment (t(12) = 0.295, p = 0.773; vehicle = 1410.7 + 89.3; LSP1-2111 = 1373.0 + 91.6). Percent time freezing during the five 60 second tones were measured as a means of assessing acquisition of conditioned fear (Fig. 6C). These data were analyzed with a repeated-measures ANOVA, with day as the within-subjects variable and treatment as the between-subjects variable. There was a main effect of day (F(4,48) = 26.282, p < 0.001), with but no day x treatment interaction (p = 0.279). Also, there was no overall effect of treatment (p = 0.714). Taken together, this analysis indicates that both groups, LSP1-2111 and vehicle treated mGluR4−/− mice, were able to acquire conditioned fear. However, the treatment did not alter acquisition in contrast to the results in wild-type mice (experiment 2).
Fig. 6.
mGluR4−/− mice were given either the mGlur4 agonist LSP1-2111 (n = 7) or vehicle (n = 7) 45 minutes prior to a fear conditioning training session. Treatment with LSP1-2111 did not affect general locomotor activity (A), reactivity to the shock (B), or acquisition of conditioned fear (C). Furthermore, there was no effect of treatment on the subsequent extinction trials (D).
Percent time freezing over the 7 days of extinction was analyzed using repeated-measures ANOVA (Fig. 7D). Only freezing during the first 60-second tone was analyzed. Days was the within-subjects variable and treatment was the between-subjects variable. There was a main effect of day (F(6,72) = 10.994, p < 0.001), with but no day x treatment interaction (p = 0.949). Additionally, there was no overall effect of treatment (p = 0.450). This analysis indicates that both groups demonstrated extinguished conditioned fear to the tone; however, treatment with LSP1-2111, compared to vehicle, during training did not affect performance on subsequent extinction trials. Thus, treatment with 5 mg/kg of LSP1-2111 or vehicle in mGluR4−/− mice did not produce significant differences in gross locomotion, reactivity to shock, acquisition or extinction of conditioned fear. These results demonstrate that the effects of LSP1-2111 were mGluR4 specific.
Fig. 7.
Receiver operator characteristic curves for comparison of transcriptional effects. Curves above the diagonal indicate significant overlap in differential expression. Gene Ontologoy based comparison (black curve) reveals more concordance than individual transcript comparison (gray curve).
3.4. Experiment 4
We evaluated differential gene expression between mGluR4−/− and WT mice, as well as between the mGluR4 agonist- and vehicle-treated WT mice. The goal of the microarray experiments was to determine whether genetic manipulation and agonist exposure resulted in similar transcriptional effects. We evaluated the transcriptional effects both at the level of individual transcripts and at the level of groups of transcripts, as defined by the Gene Ontology (GO) annotations. If genetic and agonist effects on the transcriptome are similar, then this should be detectable by inspection of the p values resulted from the two experiments. Because relatively few transcripts had large changes in expression levels, but many more transcripts had small changes, we employed a comparison procedure that was designed to evaluate the cumulative effects of small but widespread changes in expression levels. Our approach was based on a Receiver Operator Characteristic (ROC) (Li et al., 2008) analysis (see Methods).
A number of transcriptional effects were found: at a raw p value of 0.01, we detected 180 and 616 differentially expressed transcripts for the genotype and agonist effects, respectively, with a number of 8 transcripts (Ehmt2, Fads2, Fbxo18, Gripap1, LOC383330, Raf1, Swap70, Ypel3) being affected in both groups. This level of overlap is unlikely to arise by pure chance (p<0.003, Fisher Exact Test). However, after adjustment at a false discovery rate (FDR) =0.1, none of the transcriptional changes remained significant; this implies that transcriptional changes are subtle and dispersed among a relatively large number of transcripts, potentially affecting a number of distinct transcriptional processes.
In order to investigate in more depth the transcriptional effects, we employed a gene set enrichment analysis (GSEA) of the expression changes, using the GSEA package (Subramanian et al., 2007). This strategy evaluates the relative ranking of p values of predefined groups of genes. In our case, we selected groups of genes sharing Gene Ontology (GO) annotations. Out of 972 separate GO categories evaluated, 166 appeared affected in the genotypic comparison and 51 appeared affected in the agonist group; of these, 15 were affected in both cases (see supplemental tables 1 and 2).
We evaluated to what extent genotypic manipulation and agonist administration resulted in similar transcriptional effects. This analysis revealed that at the transcript level, there was modest but significant overlap in differential expression (area under ROC curve (AUC) 0.59 on a scale from 0.5 to 1, Fig. 7). However, when transcriptional effects were evaluated at the level of GO categories, a more significant overlap was detected at an AUC = 0.77.
4. Discussion
The data presented here show enhanced acquisition and extinction of fear learning and memory in mGluR4−/− mice compared to WT mice as well as reduced acquisition of cued fear in WT mice following pharmacological stimulation with the specific orthosteric mGluR4 agonist LSP1-2111 at a dose of 5 mg/kg compared to vehicle treatment. The optimal pharmacological effect of a dose of 5 mg/kg is in agreement with other behavioral measures (Wieronska et al., 2010, 2012). Consistent with the opposite effects seen in mGluR4−/− versus WT and LSP1-2111-treated versus vehicle-treated WT mice, analysis of microarray data of amygdala tissues from mGluR4−/− versus WT and from WT mice treated with a mGluR4 agonist versus vehicle revealed a significant overlap in pattern of gene expression. Together, these data support a role of mGluR4 signaling in acquisition of fear learning and memory. Type I mGluRs have also been shown to modulate fear conditioning. For example, the mGluR5 antagonist 2-methyl-6-(phenylethynyl) pyridine hydrochloride (MPEP) impairs acquisition of conditioned fear (for a review, see Rodrigues et al., 2004)).
The LSP1-2111 pharmacological data indicate a bell-shape dose-response curve. This pattern is similar to that seen for the mGluR4 agonists (1S,3R,4S)-1-aminocyclopentane-1,3,4-tricarboxylic acid (ACPT-I) (Lopez et al., 2007), (1S,2R)-1-amino-2-phosphonomethylcyclopropanecarboxylic acid (APCPr) (Sibille et al., 2007), LSP1-2111 (Beurrier et al., 2009) and LSP4-2022 (Goudet et al., 2012) in animal models of Parkinson disease. These compounds display an increasing selectivity for mGluR4 versus mGluR8 (no selectivity for ACPT-I and (1S,2R)-APCPr, about 40 fold for LSP1-2111, and 300 fold for LSP4-2022). For all these compounds, mGluR7 may be activated at higher concentrations. Interestingly, in the same type of Parkinson’s disease models, AMN082, the selective mGlu7 allosteric agonist also showed benefits and similarly to orthosteric agonists a loss of effect when higher concentrations were administered (Greco et al., 2010). These results indicate that activation of mGluR4 and mGluR7 only works at low drug concentrations. Like LSP1-2111, the mGluR7 agonist AMN082 impairs acquisition of conditioned fear (Fendt et al., 2008), supporting a role for both mGluR7 and mGluR4 in acquisition of conditioned fear. The amygdala plays an important role in the acquisition and expression of conditioned fear (Johansen et al., 2010; Phelps et al., 2004). This role is modulated by input from the ventromedial prefrontal cortex (vmPFC) (Morgan and LeDoux, 1999), which is thought to inhibit the expression of conditioned fear following extinction training (Quirk et al., 2006). However, in contrast to LSP1-2111, AMN082 enhances extinction of conditioned fear (Fendt et al., 2008). Interestingly, opposing effects of mGluR4 and mGluR7 are also seen in animal models of positive symptoms of schizophrenia (Wieronska et al., 2012).
In contrast to enhanced cued fear conditioning, mGluR4−/− mice did not show altered hippocampus-dependent contextual fear conditioning (Davis et al., 2012). Interestingly, deficiency of the 65 kDa form of glutamic acid decarboxylase (GAD65) also impairs cued but not contextual fear conditioning (Sangha et al., 2009). Because the effects of LSP1-2111 on measures of anxiety involve GABA-ergic systems (Wieronska et al., 2010), we propose that the effects of LSP1-2111 on conditioned fear might also involve GABA-ergic signaling, consistent with the proposed mechanism of mGluR4 inhibiting GABA-ergic signaling in Parkinson’s disease (Beurrier et al., 2009). Thus, mGluR4 deficiency would be expected to enhance GABA-ergic signaling and to show the opposite phenotype of GAD65 deficiency.
While mGluR4−/− mice showed enhanced acquisition of cued fear conditioning, they showed reduced acquisition of hippocampus-dependent hidden platform training in the water maze; they swam farther away from the hidden platform than age-matched wild-type mice (Davis et al., 2012). These opposite effects on acquisition of cued fear conditioning and hidden water maze learning in mGluR4−/− mice indicate that there might be anatomical specificity to the direction of the effect on acquisition in mGluR4−/− mice. A potential concern with therapeutic modulation of fear-related learning and memory is that other forms of learning and memory might be similarly affected. mGluR4 seems an attractive drug target in this regard because while mGluR4 agonists reduce acquisition of cued fear, they are expected to enhance hippocampus-dependent learning.
Increased measures of anxiety could contribute to enhanced fear conditioning. However, while acquisition of fear conditioning was enhanced in both female and male mGluR4−/− mice, measures of anxiety were affected in a sex-dependent fashion. Compared to sex-matched wild-type mice, mGluR4−/− male mice showed enhanced, but mGluR4−/− female mice reduced, measures of anxiety in the open field and elevated zero maze. In addition, mGluR4−/− male mice did not show increased significantly increased measures of anxiety at 6 months of age but only at 12 months of age. As 3- and 6-month-old mGluR4−/− mice were used in the current study, these data indicate that there is no simple relationship between measures of anxiety and cued fear conditioning. In mGluR4−/− mice, enhanced acquisition was associated with reduced baseline motion and enhanced motion in response to shock. However, in LSP1-2111-treated mice, enhanced acquisition was associated with enhanced baseline motion and no difference in motion in response to shock. Therefore, potential differences in baseline motion or response to shock do not seem required for enhanced acquisition supporting a mGluR4-mediated learning effect. However, we cannot exclude that potential genotype differences in pain sensitivity might have contributed to the genotype differences in motion in response to shock and it would be important to assess this possibility in the future. Nevertheless, as the drug affected acquisition but not motion during the shock, altered response during the shock does not seem required for the observed acquisition effect.
Compared to other studies of fear conditioning, in some experiments, especially in Experiment 2, the freezing levels were relative low. Potential differences in the age, strain, and sex of the mice, handling of the mice and other environmental conditions might have contributed to this.
Analysis of the transcriptional effects suggests several conclusions. First, changes in expression do not appear to affect strongly a small number of genes, but rather a relatively large number of genes displaying moderate changes. This is evident by the fact that, particularly for the mGluR4−/− effects, FDR adjustment does not reveal any particularly strong effects on individual transcripts but nevertheless hundreds of GO categories are significantly affected. Second, it appears that mGluR4−/− effects, at the same p value, affect a smaller number of transcripts but a larger number of distinct GO categories. This implies that mGluR4 deficiency results in a more coherent compensatory response as compared to mGluR4 agonist administration. Third, we are able to detect significant overlap between the transcriptional response to agonist injection and the transcriptional response to mGluR4 deficiency. Importantly, this overlap is more detectable at the level of GO categories and is less detectable or pronounced at the individual transcript level. This observation suggests that in some cases transcriptional effects are dispersed between a large number of genes and evaluation is most appropriate at the level of gene groups.
In summary, compared to WT mice, mGluR4−/− mice show enhanced acquisition and extinction of fear learning and memory. There were no obvious alterations in the maternal care of mGluR4−/− mice. However, while litter sizes were similar to those seen in wild-type mice and upon casual observation there were no alterations in the maternal care of mGluR4−/− mice, maternal care was not recorded and analyzed continuously. Therefore, we cannot conclude that potential differences in maternal care might have contributed to the observed cognitive effects. Developmental compensations do not seem to be required for the effects on acquisition, as pharmacological stimulation with LSP1-2111 at a dose of 5 mg/kg reduced acquisition of cued fear in WT mice. Consistent with this hypothesis, there was a significant overlap between the transcriptional response to mGluR4 administration and the transcriptional response to mGluR4 deficiency. Especially because hippocampus-dependent acquisition and memory retention were differentially affected than cued fear conditioning, these data strongly support mGluR4 as target to modulate acquisition of fear learning and memory. Future studies are warranted to determine the molecular mechanisms and brain area(s) involved in these effects.
Supplementary Material
Highlights.
-
-
mGluR4−/− mice showed enhances acquisition and extinction of cued fear
-
-
LSP1-2111, at 2.5 and 5mg/kg, inhibits acquisition of fear learning
-
-
modulating mGluR4 signaling attractive target for treating anxiety disorders
Acknowledgements
This work was supported by NIMH R01 MH77647 and the Era-Net Neuron program (ANR-08-NEUR-006-02). We thank Tammie Haley for her help with the breeding and genotyping and Iwona Strycharska-Orczyk for her help in processing amygdala tissues for RNA, Reid Olsen for his assistance in behavioral testing and drug administration, and Delphine Rigault (UMR8601) for the synthesis of LSP1-2111.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anagnostaras S, Wood S, Shuman T, Cai D, Leduc A, Zurn K, Zurn J, Sage J, Herrera G. Automated assessment of pavlovian conditioned freezing and shock reactivity in mice using the video freeze system. Frontiers Beahv Neurosci. 2010;4:1–11. doi: 10.3389/fnbeh.2010.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B (Methodological) 1995;57:289–300. [Google Scholar]
- Beurrier C, Lopez S, Revy D, Selvam C, Goudet C, Lherondel M, Gubellini P, Kerkeria-LeGoff L, Acher F, Pin J-P, Amalric M. Electrophysiological and behavioral evidence that modulation of metabotropic glutamate receptor 4 with a new agonist reverses experimental parkinsonism. FASEB J. 2009;23:3619–3628. doi: 10.1096/fj.09-131789. [DOI] [PubMed] [Google Scholar]
- Carmack S, Wood S, Anagnostaras S. Amphetamine and extinction of cued fear. Neurosci Lett. 2010;468:18–22. doi: 10.1016/j.neulet.2009.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Davis M, Haley T, Duvoisin R, Raber J. Measures of anxiety, sensorimotor function, and memory in male and female mGluR4−/− mice. Beh Brain Res. 2012 doi: 10.1016/j.bbr.2011.12.037. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doller D, Hong S-P, Liu KG, Bacolod MD, Uberti MA, Cajina M, Acher F, Gubellini P. Disposition characteristics and functional activity of LSP 1-2111, an orthosteric Group III metabotropic glutamate receptor agonist. American Chemical Society 242nd National meeting: MEDI285. 2011 [Google Scholar]
- Du P, Kibbe W, Lin S. lumi: a pipeine for processing Illumina microarray. Bioinformatics. 2008;24:1547–1548. doi: 10.1093/bioinformatics/btn224. [DOI] [PubMed] [Google Scholar]
- Fanselow M. Neural organization of the defensive behavior system responsible for fear. Psychonom Bull Rev. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Fendt M, Schmid S, Thakker D, Jacobson L, Yamamoto R, Mitsukawa K, Maier R, Natt F, Husken D, Kelly P, McAllister K, Hoyer D, van der Putten H, Cryan J, Flor P. mGluR7 facilitates extinction of aversive memories and controls amygdala plasticity. Mol Psychiatr. 2008:1–10. doi: 10.1038/sj.mp.4002073. [DOI] [PubMed] [Google Scholar]
- Ferbinteanu J, Holsinger RMD, McDonald RJ. Lesions of the medial or lateral perforant path have different effects on hippocampal contributions to place learning and on fear conditioning to context. Behav.Brain Res. 1999;101:65–84. doi: 10.1016/s0166-4328(98)00144-2. [DOI] [PubMed] [Google Scholar]
- Flor PJ, Acher FC. Orthosteric versus allosteric GPCR activation: The great challenge of group-III mGluRs. Biochem. Pharmacol. 2012 doi: 10.1016/j.bcp.2012.04.013. In press, http://dx.doi.org/10.1016/j.bcp.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Contextual learning and cue association in fear conditioning in mice: a strain comparison and a lesion study. Behav.Brain Res. 1998;95:191–203. doi: 10.1016/s0166-4328(97)00144-7. [DOI] [PubMed] [Google Scholar]
- Greco B, Lopez S, van der Putten H, Flor PJ, Almaric M. Metabotropic glutamate7 receptor subtype modulates motor symptoms in rodent models of Parkinson’s disease. J Pharmacol Exp Ther. 2012;32:1064–1071. doi: 10.1124/jpet.109.162115. [DOI] [PubMed] [Google Scholar]
- Goudet C, B V, T C, Deltheil T, Bessiron T, Brabet I, N O, Rigault D, Bertrand H-O, McLean H, Daniel H, M A, F A, Pin J-P. A novel selective metabotropic glutamate receptor 4 agonist reveals new possibilities for developing subtype selective ligands with therapeutic potential. FASEB J published on line January 5. 2012 doi: 10.1096/fj.11-195941. [DOI] [PubMed] [Google Scholar]
- Grillon C, Davis M. Fear-potentiated startle conditioning in humans: Explicit and contextual cue conditioning following paired versus unpaired training. Psychophysiology. 1997;34:451–458. doi: 10.1111/j.1469-8986.1997.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT, LeDoux JE. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12692–12697. doi: 10.1073/pnas.1002418107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Li J, Pine JP. ROC analysis with multiple classes and multiple tests: methodology and its application in microarray studies. Am J Clin Nutrition. 2008;91:184–190. doi: 10.1093/biostatistics/kxm050. [DOI] [PubMed] [Google Scholar]
- Lopez S, Turle-Lorenzo N, F A, De Leonibus E, Mele A, M A. Targeting Group III Metabotropic Glutamate Receptors Produces Complex Behavioral Effects in Rodent Models of Parkinson’s Disease. J Neurosci. 2007;27:6701–6711. doi: 10.1523/JNEUROSCI.0299-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Ann Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Meyers K, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychology. 2008;127:567–580. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Morgan M, LeDoux J. Contribution of ventrolateral prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Neurobiol Learn Mem. 1999;72:244–251. doi: 10.1006/nlme.1999.3907. [DOI] [PubMed] [Google Scholar]
- Niswender C, Johnson K, Weaver C, Jones C, Xiang Z, Luo Q, Rodriguez A, Marlo J, de Paulis T, Thompson A, Days E, Nalywajko T, Austin C, Williams M, Ayala J, Williams R, Lindsley C, Conn P. Discovery, characterization, and antiparkinsonian effect of novel positive allosteric modulators of metabotropic glutamate receptor 4. Mol Pharmacol. 2008;74:1345–1358. doi: 10.1124/mol.108.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E, Delgrado M, Nearing K, LeDoux J. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garci R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Rodrigues S, Schafe G, LeDoux J. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Sangha S, Narayanan R, Bergado-Acosta J, O S, Seidenbecher T, Pape H-C. Deficiency of the 65 kDa Isoform of Glutamic Acid Decarboxylase Impairs Extinction of Cued But Not Contextual Fear Memory. J Neurosci. 2009;29:15713–15720. doi: 10.1523/JNEUROSCI.2620-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille P, Lopez S, Brabet B, O V, N O, Gaven F, Goudet C, Bertrand H-O, Neyton J, Marino M, Amalric M, JP P, Acher F. Synthesis and Biological Evaluation of 1-Amino-2-Phosphonomethylcyclopropanecarboxylic Acids, New Group III Metabotropic Glutamate Receptor Agonists. J Med Chem. 2007;50:3585–3595. doi: 10.1021/jm070262c. [DOI] [PubMed] [Google Scholar]
- Smyth G. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Bio. 2004;3 doi: 10.2202/1544-6115.1027. Article 3. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Kuehn H, Gould J, Tamayo P, Mesirov JP. GSEA-P: a desktop application for Gene Set Enrichment Analysis. Bioinformatics. 2007;23:3251–3253. doi: 10.1093/bioinformatics/btm369. [DOI] [PubMed] [Google Scholar]
- Swanson C, Bures M, Johnson M, Linden A-M, Monn J, Schoepp D. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nature Rev Drug Disc. 2005;4:131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- Wieronska JM, Stachowicz K, Palucha-Poniewiera A, Acher F, Branski P, T, Pilc A. Metabotropic glutamate receptor 4 novel agonist LSP1-2111 with anxiolytic, but not antidepressant-like activity, mediated by serotonergic and GABAergic systems. Neurophamacology. 2010;59:627–634. doi: 10.1016/j.neuropharm.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Wieronska JM, Stachowicz K, Acher F, Lech T, Pilc A. Opposing efficacy of group III mGlu receptor activators, LSP1-2111 and AMN082, in animal models of positive symptoms of schizophrenia. Psychoparmacology. 2012;220:481–494. doi: 10.1007/s00213-011-2502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.