Abstract
While the role of collagen and elastin fibrous components in heart valve valvular biomechanics has been extensively investigated [see Sacks et al. 2009 J. Biomech. 42, 1804-24], the biomechanical role of the glycosaminoglycan (GAG) gelatinous-like material phase remains unclear. In the present study, we investigated the biomechanical role of GAGs in porcine aortic valve (AV) leaflets under tension utilizing enzymatic removal. Tissue specimens were removed from the belly region of porcine AVs and subsequently treated with either an enzyme solution for GAG removal, or a control (buffer with no enzyme) solution. A dual stress level test methodology was used to determine the effects at low and high (physiological) stress levels). In addition, planar biaxial tests were conducted both on-axis (i.e. aligned to the circumferential and radial axes) and at 45° off-axis to induce maximum shear, to explore the effects of augmented fiber rotations on the fiber-fiber interactions. Changes in hysteresis were used as the primary metric of GAG functional assessment. A simulation of the low force experimental setup was also conducted to clarify the internal stress system and provide viscoelastic model parameters fo this loading range. Results indicated that under planar tension the removal of GAGs had no measureable affect extensional mechanical properties (either on- or 45° off-axis) including peak stretch, hysteresis, or creep. Interestingly, in the low force range, hysteresis was markedly reduced from 35.96 ± 2.65% in control group to 25.00 ± 1.64% (p < 0.001) as a result of GAG removal. Collectively, these results suggest that GAGs do not play a direct role in modulating the time-dependent tensile properties of valvular tissues. Rather, they appear to be strongly connected with fiber-fiber and fiber-matrix interactions at low forces levels. Thus, we speculate that GAGs may be important in providing a damping mechanism to reduce leaflet flutter when the leaflet is not under high tensile stress.
Keywords: Glycosaminoglycan, Heart valve, Mechanical properties, Mechanical test
Introduction
Despite a phenomenally wide range of physiological function and variation in specific behaviors, soft connective (non-muscular) tissues typically share the same principle constituents: collagen, elastin, and glycosaminoglycans (GAGs). The unique properties of each tissue are a result of its distinctive structure determined by the constituents present and their physical arrangements. Type I and III collagen form undulated fibers that provide support under tension whereas elastin, an aggregate fiber from microfibrils, provides the ability to undergo large extensions and provides elastic properties. In contrast to these fibrous components, GAGs are composed of long unbranched polysaccharides usually attached to a protein core to form proteoglycans, whose high negative charge makes GAGs hydrophilic.
The native aortic valve (AV), a collagenous thin tissue structure, is a remarkable example of the highly developed structure and function [1]. When open, the AV allows for unobstructed flow of blood out of the ventricle during systole with flow deceleration it closes via Bernoulli effects, and the leaflet provides complete closure to prevent valve regurgitation. This is all achieved through the unique and complex structure of the AV tissue, which consists of three layers (the fibrosa, spongiosa, and ventricularis), constructed principally of collagen, elastin, and GAGs [2–7]. The ventricularis layer faces the left ventricle and is composed primarily of collagen and elastin [2, 4–9]. On the opposite face of the valve is the fibrosa layer. This layer faces the aorta and is composed predominantly of a dense network of Type I collagen fibers, which exhibit macroscopic crimp and are aligned primarily in the circumferential direction [2, 4]. The highly hydrophilic GAGs largely make up the central spongiosa, resulting in a highly hydrated layer [10–12]. Interestingly, all heart valves have similar micro-anatomical arrangements in that the GAG rich layer (spongiosa) is always in center of the leaflet [13, 14].
At the tissue level, all three layers within the AV tissue work in unison to produce the net leaflet mechanical behavior [13]. In diastole the AV leaflets are loaded as a result of diastolic pressure loading, and are virtually inextensible once fully straightened under full tensile load [2, 13, 15]. During systole the valve opens the collagen fibers recoil. The elastin fibers within the ventricularis, which are radially aligned, act to assist in the recovery of radial distention [4–6, 16] after full forward flow. In conjunction with aortic root distention [14], this allows for complete unobstructed blood flow. At the end of systole blood flow starts to decelerate and with very little reverse flow the valve closes due to the Bernoulli’s principle and the developing vortices in the sinuses behind the leaflets [13, 17]. Once again the collagen fibers are fully straightened rendering the leaflet inextensible to provide sufficient coaptation area (amount of leaflet overlap) to prevent valve prolapse [18].
Since GAGs are the primary component of the central spongiosa layer, it has been speculated that this layer serves to reduce the shearing resulting from the differential movement of the fibrosa and ventricularis during leaflet deformations [2, 8, 10, 19–21]. Moreover, the AV biaxial tensile biomechanical response results primarily from the collagenous fibrosa layer [22]. From these and other studies, we have hypothesized that under high tensile loading biomechanical behavior is dominated by collagen and elastin. This is all the more important as changes in behavior with various tissue pathologies that alter tissue composition (e.g. myxomatous valve disease) suggest substantial changes may occur in the tissue behavior. GAGs that occur in tendon, a dense collagenous tissue similar composition and structure to the fibrosa layer, have been shown to manifest themselves mechanically in the low stress regions, where the constituent collagen fibers are remain mostly in the undulated state [23–25]. Mechanistically, GAGs are hypothesized to contribute substantially to the time dependent behavior of the bulk tissue at lower physiological force levels through mechanical interactions with the partially undulated collagen fibers through direct bonding. This has been used to explain why the relative amount of stress relaxation is reduced at higher tissue stress levels, wherein the bulk tissue response is dominated by the fully loaded and straightened collagen fibers [26]. While some insight into collagen-GAG interactions has been performed in the mitral valve chordae [27], it remains unknown whether such mechanisms occur in valvular tissues.
However, how the GAGs contribute to heart valve tissue behavior, especially as they relate to the observed valvular tissue quasi-elastic at high tensile loading [28–30], remains unclear. Such insights are important not only for our understanding of native tissue function, but also to lay the basis of novel approaches to chemically modify biologically derived tissues for heart valve biomaterials. The present study was thus undertaken to clarify and quantify the mechanical contribution of GAGs to AV leaflet tensile behavior. We developed a dual stress-level experimental design to elucidate both a low stress (strip-biaxial configuration) and high-stress (planar biaxial) tensile modes under both quasi-static and creep configurations. This novel two-tier measurement method was utilized due to the substantial differences in force levels required to encompass the physiologic ranges of extensional deformation known to occur in the native valve in-vivo [13]. Finally, we simulated of the low stress experimental configuration to gain insight into the internal stress distribution in this deformation mode to better interpret the experimental results. To elucidate the biomechanical role of GAGS, we preformed these tests on pre- and post GAG removal specimens using an enzymatic technique to remove the GAGs from the leaflet tissue.
Methods
Tissue harvest
Fresh porcine aortic valves were acquired at the local abattoir from adult animals of both sexes (Thoma’s Meat Market, Saxonburg, PA). Valves were excised intact along with the aortic root on site by cutting the aorta distal to the aortic sinuses and then cutting the left ventricle outflow. Valves were then transported back to lab in ice chilled phosphate buffered saline (PBS). Valves were rinsed again with ice cold PBS and then the individual leaflets were cut away from the aortic root with no differentiation between the non-, right, and left coronary leaflets. Valve leaflets were then divided into three groups; native, enzyme treated, and control.
Leaflets in the native group were stored covered in ice cold PBS and underwent mechanical testing within 24 hours of harvesting. The leaflets from each valve in the enzyme treated group were processed using a standard protocol [31], modified to utilize higher concentrations of enzymes for shorter periods of time in order to minimize total processing time. The specimens were placed in a conical tube with 2.4 ml of enzyme degradation solution per leaflet, containing 30 Units/ml Hyaluronidase and 0.6 Units/ml Chondroitinase (Sigma Aldrich #H3631 and #C3667) in 100 mM Ammonium Acetate Buffer solution (AABS) with an adjusted pH of 7.0. The tubes were continuously shaken for 2 hours at 37 °C. The enzyme degradation solution was then removed and the leaflets were washed with ice cold PBS three times, for five minutes each time. The enzyme treated specimens were then stored covered in ice cold PBS and underwent mechanical testing within 48 hours of harvesting. The leaflets from the each valve in the control non-treated group were placed in a conical tube with 2.4 ml per leaflet of AABS with an adjusted pH of 7.0. The tubes were continuously shaken for 2 hours at 37 °C. The buffer solution was then removed and the leaflets were washed with ice cold PBS three times, for five minutes each time. The control specimens were then stored covered in ice cold PBS and underwent mechanical testing within 48 hours of harvesting.
GAG content quantification
Quantification of GAG content was performed by hexosamine analysis using previously published methods [31, 32]. In brief, leaflets were lyophilized, weighed, and then hydrolyzed in 2 ml of 2 M HCL at 95 °C for 20 hours. The solution was then evaporated under nitrogen gas and the hydrolysates dissolved in 2 ml of 1 M NaCl. This was followed by the addition of 2 ml of 3% acetylacetone in 1.25 M sodium carbonate. Next, 4 ml of absolute ethanol and 2 ml of Ehrlich’s reagent (0.18 M p-diemethyl-aminobenzaldahyde in 50% ethanol) were added. Incubation at room temperature for 45 minutes yielded a color product indicative of hexosamine quantities, which was read for absorbance at 540 nm with a set of D (+) glucosamine (0–200 µg) controls. All values were normalized to their respective dry tissue weights and corrected for the fact that hexosamine assay accounts for ~90 µg / 10 mg of non-GAG related hexosamines as previously done [31, 33].
Planar biaxial mechanical behavior
Specimens (native: group 1, control: group 2, enzyme treated: group 3; n=8 for each group) were cut from the central belly region of the leaflet into an approximately 10 mm × 10 mm square (Figure 1-a), with the thickness measurement. A fourth group of native tissue only specimens (group 5, n=5) were similarly prepared but with their edges aligned at a 45 degree angle to the circumferential axis as in [34] induce a state of high in-plane shear [35]. This was done to investigate the role of large fiber rotations that occur in the high in-plane shear state on the viscoelastic responses under high stress. Our assumption here is that larger rotations will induce greater fiber-matrix interactions, and if true increase viscous drag and thus the energy loss per test cycle.
Figure 1.
(a) The biaxial testing specimens were cut approximately 10 mm × 10 mm from the central belly region of the leaflet. (b) A photo and schematic of the low force experimental configuration.
A detailed description of the planar biaxial testing methods has been previously presented [28, 29]. Briefly, four markers were glued using cyanoacrylate adhesive marking an approximately 4 mm × 4 mm square. Specimens were then mounted in the biaxial testing device with their edges aligned with the device axes, with the experiment performed in PBS at room temperature. The applied axial forces were measured while marker positions were continuously recorded using the digital imaging system incorporated into the biaxial testing device. The resulting membrane stress T and deformation gradient F tensors were determined, and from the later the shear angle α (quantifying the degree of in-plane shear) and rigid body rotation ⊝ during deformation computed.
To characterize the high force planar biaxial behavior, specimens from each orientation group were loaded up to 90 N/m to approximate physiologic loading along both axes as in [1] (i.e. T=diag[T11,T22,0], where T11=T22=90 N/m) over a 15 second period, then unloaded at the same rate. Nine contiguous preconditioning cycles were applied to the specimen, and then the tenth cycle was recorded as the representative cycle. Hysteresis, which represents the energy loss per cycle, was calculated as the difference between the respective loading and unloading curve areas, expressed as percent loading curve area. Note that in the present study, hysteresis was computed both over the full curve (to 90 N/m) and to 10 N/m, the later by truncating the data. Specimens were frozen at −80°C after mechanical testing for later GAG analysis.
To characterize the effects of larger fiber rotations during creep in native tissues, a third and fourth group of native tissue specimens were prepared aligned to the circumferential and radial axes and at a 45 deg angle, as for groups 1 and 2, respectively, as described above. As before, each specimen underwent nine precondition cycles to 90 N/m equi-biaxial stress, on the tenth cycle the specimen was loaded to 90 N/m in 100 ms and held at that tension level for 30 minutes as in [28, 36]. Both T and F were continuously measured over the entire test period.
Low stress mechanical behavior
A novel aspect of this work is the use of a low tensile stress deformation experimental design to elucidate viscoelastic behaviors in low force region for valvular tissues. The experimental design was based on previously presented flexure testing techniques [37–39] with modifications described in the following. We adopted this particular experimental configuration rather than the standard strip biaxial as done in [22] since the present configuration allows specimen contact at three locations rather than two. This is important when dealing with extremely compliant valvular tissue specimens at low forces.
For these studies, a final group of rectangular specimens (n=8) were cut from the leaflet approximately 20 mm × 3 mm oriented in the circumferential direction, 3–4 mm in from the coaptation edge. Six red glass microbeads (color chosen to enhance optical tracking) were affixed evenly spaced to the transmural face of the specimen using cyanoacrylate adhesive (Fig. 1-b). A transverse linkage was fabricated from 20-gauge hypodermic tubing and attached to the ventricularis side of the specimen using cyanoacrylate adhesive. Specimens were then mounted in the holder and held in place by clamp assemblies on each end, with the ventricularis side facing down. Two red glass microbeads were attached on the face of the holder, outside of the clamp assemblies. The holder was then loaded into a PBS-filled optical acrylic tank on the testing device, and a bending bar constructed of 316V stainless steel was slid through the transverse linkage attachment. A final red glass microbead was attached to the bending bar approximately three mm above the specimen to track its motion. A camera placed perpendicularly to the holder tracked the markers, while custom software controlled the motor and determined the position of each marker per iteration. Testing began by preconditioning for ten cycles with a vertical displacement sufficient to induce an extensional strain of < ~7% strain, an amount that is below the estimated straightened strain of the AV collagen fibers [13]. Then a final testing protocol was completed in the same manner and the final cycle recorded for analysis. The applied force was calculated by measuring the displacement of the bending bar and the predetermined force-displacement relationship for the given bar.
Finite element simulation of the low stress experiment
As the low force experimental design induces primarily extensional but also a small degree of a flexural deformation state, we developed the following finite element model to clarify the actual specimen internal stress distribution. We utilized the commercial finite element software package ABAQUS (Dassault Systemes Simulia Corporation, Providence, RI). Four-node bilinear hybrid plane strain elements with reduced integration CPE4RH in ABAQUS/Standard were used to model the AV leaflet tissues. The four posts and the middle bending bar were modeled as rigid bodies, with the middle bending bar (glued to the tissue) modeled as a tie constraint. Loading and unloading displacements were applied on the bending bar directly in the y direction, while the four posts remained fixed. The contact interactions between the tissue and the posts were modeled as perfectly clamped, with the nodes that contact with the posts thus fixed. We have previously shown that in the low-stress range in bending the valvular tissue can be simulated using a Neo-Hookean isotropic hyperelastic material model [13]. Extending this approach, the following isotropic hyperelastic model was utilized based on a reduced Yeoh model (or reduced polynomial model) form
| (1) |
where C10 and C20 are the material parameters, and I1 the first invariant of left Cauchy-Green strain tensor. The leaflet tissues were assumed to be incompressible, with viscous effects modeled using the following relaxation modulus GR(t) defined by a Prony series
| (2) |
where are constants and G0 = 2C10 and represents the instantaneous shear modulus. In preliminary investigations we found that only the first term of Prony series was required, so that the shear relaxation modulus GR(t) was simplified to
| (3) |
.
Model parameters were determined using a semi-automated iterative approach so that the load-deflection curve from simulation matched that from experiments, with a criterion of r2>0.95.
Statistical analysis
All computed values were represented as mean ± standard error of the mean (SEM), a one way ANOVA test was used for statistical analysis between the three groups. When significant, Bonferroni pairwise multiple comparisons were performed to determine significance between groups. A t-test was used to compare biomechanical properties between the non-treated control and enzyme treated groups. Statistical significance was assumed for all tests when p < 0.05.
Results
GAG content
The modified enzymatic GAG removal protocol was verified utilizing quantitative GAG measurements. Incubation in 30 U/ml hyaluronidase and 0.6 U/ml chondroitinase resulted in a significant difference in GAG content between the normal, control non-treated, and enzyme treated groups, p < 0.001 (Fig. 2). Bonferroni pairwise multiple comparisons showed a significant change in leaflet GAG content between the control non-treated group and the enzyme treated group (318.36 ± 16.66 µg GAG/10 mg tissue vs. 167.59 ± 9.10 µg GAG/10 mg tissue, p < 0.001). There was also a significant decrease in GAG content between the native group and the enzyme treated group (369.06 ± 6.37 µg GAG/10 mg tissue vs. 167.59 ± 9.10 µg GAG/10 mg tissue, p < 0.001). Bonferroni pairwise comparison did not show a significant change in GAG content between the native and control non-treated groups (369.06 ± 6.37 µg GAG / 10 mg tissue vs. 318.36 ± 16.66 µg GAG / 10 mg tissue, p = 0.066).
Figure 2.
GAG quantification by hexosamine assay. The enzyme treated group showed a significant decrease from the native (p < 0.001) and control (p < 0.001) groups. The GAG content was not significantly different between the native and control groups (p = 0.066).
Planar tensile mechanical behavior
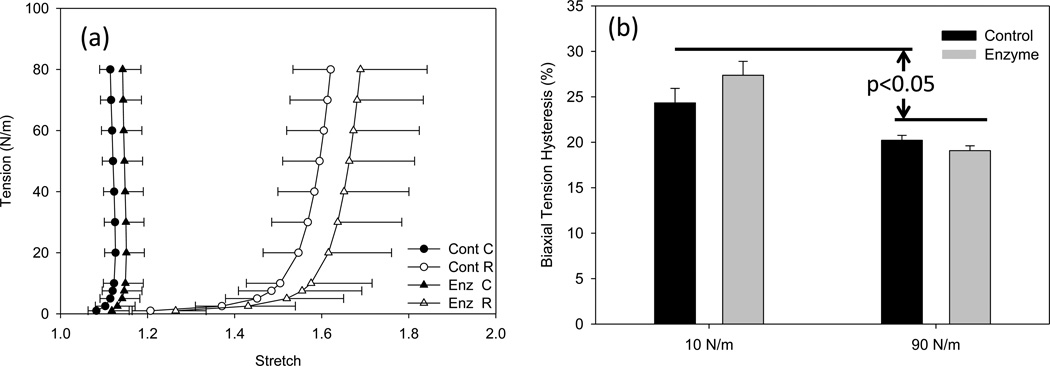
All groups demonstrated a smooth, non-linear anisotropic response under planar equi-biaxial tension (Fig. 3-a). There were no measurable differences with the removal of GAGs for the circumferential stretch (p=0.570) and radial stretch (p = 0.701) (Fig. 3-a). Similarly, the hysteresis values under planar biaxial physiologic tension (90 N/m) for the control and enzyme treated, 20.21 ± 0.54 % and 19.07 ± 0.54 % respectively, were not significantly different (p = 0.885) (Fig. 3-b). Interestingly, at low tension (10 N/m) calculated hysteresis for control and enzyme treated, 24.33 ± 1.60% and 27.36 ± 1.53%, were not significantly different from each other (p = 0.400), but were significantly higher than the full tension hysteresis values (p<0.05).
Figure 3.
(a) High stress planar biaxial testing results of the control and enzyme treated groups, where C and R correspond to the circumferential and radial directions, respectively. Note here the enzyme treated group exhibited an insignificant increase in compliance while maintaining similar anisotropy. (b) Areal tensile hysteresis of the same data which showed no significant change between the control group and enzyme treated at low tension (10 N/m) (p = 0.400) and physiologic tension (90 N/m) (p = 0.855). However, the hysteresis at the low tension was significantly larger than the high stress group.
As expected, the tension-stretch relationship for shear specimens demonstrated a more isotropic response with the peak stretches laying in-between the on-axis stretches (Fig 4-a), and exhibited an average α of 9.74 ± 1.62°. In spite of the presence of larger fiber rotations, no difference in the hysteresis level was observed between the on- and off-axes specimens. These results suggest that the majority of the viscous loss occurred in the low-tension region, and were unaffected by larger fiber rotations in the high stress range. This is consistent with proportionally larger effects of collagen fiber uncrimping, as well as potentially fiber rotations, that occur at the lower stress level [26].
Figure 4.
(a) Representative biaxial loading and unloading curves for on-axis (radial and circumferential) specimen and 45° shear (axis 1 and axis 2), showing a decreased anisotropy for the shear test as the result of averaging the two directions. (b) Representative creep response at 90 N/m equi-biaxial tension, for on-axis (radial and circumferential) specimen and 45° shear (axis 1 and axis 2).
Planar tensile behavior under creep loading
Under planar biaxial creep testing there were no measurable increases in creep stretch throughout the loading duration, consistent with previous work which showed no creep in native valvular tissues [36]. Consistent with this is the off-axis creep test also demonstrated no measurable changes over the duration of the 30-minute test (figure 4-b). Thus, within our ability to measure specimen orientation, and associated larger fiber rotations, had no affect on creep behavior.
Low force mechanical behavior
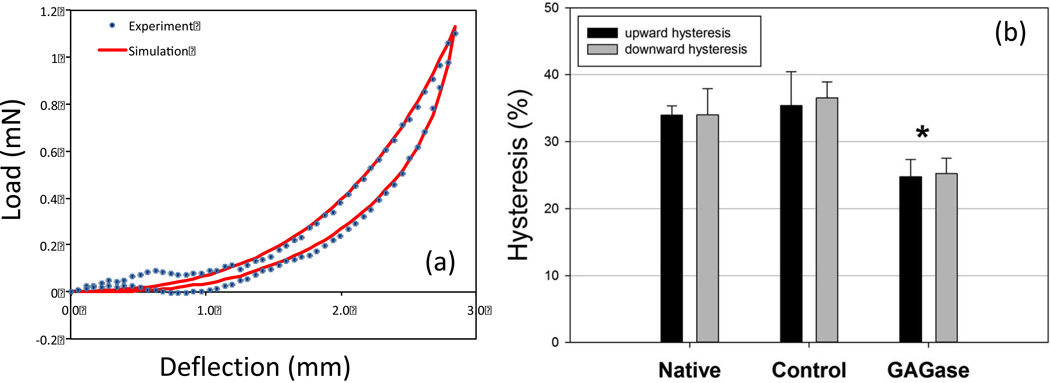
The low-force response produced a smooth, exponential response, with notable hysteresis (Fig. 5-a). Interestingly, in this deformation range GAG removal resulted in a substantial decrease in hysteresis. This decrease was also uniform in both flexure directions and changed from ~35% in the native and control groups to ~25% in enzyme treated group (p<0.001). This reduction was comparable to that previously found by our lab for porcine bioprosthetic heart valve tissues in flexure [40].
Figure 5.
(a) Representative load-deflection curve from the enzyme treated group. (b) Resulting flexural hysteresis values for each group in both flexural directions. While no group exhibited measureable differences in flexural direction, the enzyme group exhibited a significant (*=p<0.05) decrease in hysteresis compared to the control or native tissue.
For the simulations, the native tissue the load-deflection curve simulations agreed very well with the experimental results for the normal group. As an example, the load-deflection curve between the simulation and the experimental data for a representative specimen in the normal group had an r2=0.9931 (Fig. 5-b), using material parameters listed in Table 1. Simulated hysteresis was 32.9% compared to 30.9% measured. It should be noted that while localized bending occurred at the force application and grips, by far the predominant deformation mode was clearly extensional strain, with an average extensional strain of 7.5% (Fig. 6).
Table 1.
Hyperelastic-viscoelastic material parameters for eqn. 3 for FE simulation for the native heart valve tissue in the normal group.
| C10 | C20 |
|
|
||
|---|---|---|---|---|---|
| 3.9 | 39.0 | 0.55 | 0.05 |
Figure 6.
Fully loaded configuration from FE simulation at full deflection with the contours showing local strain along the specimen long axis (E11), superimposed on the experimental configuration. This simulation clearly demonstrated the predominance of extensional strains within the specimen.
Discussion
Functionally elastic tensile valve biomechanical behavior
GAGs are known to play an important role in a wide range of soft tissues, especially in providing articular cartilage with its time dependent compressive properties [41–43]. In native valve tissues, it has been shown that despite the presence of GAGs hysteresis under tensile loading is small and creep negligible [36]. This is a distinct difference from other soft tissues such as cartilage, tendons, and ligaments where more pronounced viscoelastic behavior has been observed [44–46]. Although heart valves do not exhibit formal viscoelastic behaviors, the importance of GAGs in time dependent behavior is controlled by the specific arrangement of GAGs within the leaflet. It has been shown that valvular tissues have an increased content of water binding GAGs in regions that experience compression [47] as well as valvular interstitial cells demonstrating in vitro GAG production that is strain magnitude dependent [48]. The contributions of GAGs to valvular tissue are further highlighted through the recent research in bioprosthetic heart valves (BHVs), in which GAG loss has been implicated with early BHV failure [31, 49, 50].
Interestingly, in the present study we have observed that GAGs had no measurable contribution to the tensile viscoelastic properties of AV leaflet tissue under maximum physiological loading (on-axis) and supra-physiological (45 degree orientation to induce larger fiber rotations) conditions. This further serves as evidence that the fiber-matrix interactions, as measured at the bulk level under tension, are not measurably influenced by the presence of GAGs. Collectively, our results indicate that the high level tensile properties of the AV leaflet are entirely dominated by the functionally elastic behavior of the collagen and elastin fiber networks.
Low stress behavior
The only measurable biomechanical effect of GAG removal was at low strain levels (Figs. 3 and 5). This result is entirely consistent with previous studies on tendon that have demonstrated a pronounced effect at low strains wherein the collagen is still highly crimped [25, 44]. Our ability to detect a mechanical effect of GAG loss in this deformation range is most likely a result of collagen fiber-matrix interactions that are detectable only at this portion of the stress-strain response, which while present is negligible at high stresses. Thus, GAGs do not appear to directly modulate the mechanical behavior of valvular collagen network in the physiological stress range. Thus, once the collagen fiber network is straightened and loaded, its response is dictated by the relatively elastic collagen network. However, given that hysteresis is a result of energy dissipation, this suggests that the gel like GAGs act to provide at least some level of damping, potentially to reducing flutter during maximum forward systolic flow.
FE modeling results demonstrated that use of a simple Prony series (Eq. 6) simulated the low stress behavior of the aortic valve leaflet (Figs. 5). Simulation results also confirmed that the test configuration induced a tensile stress field within the specimen. As mentioned above we adopted this test configuration to simply maintaining a regular specimen geometry given the extreme pliancy of valvular tissues at low strains. While the effects of the measured viscous dissipation are not known, it is likely they will strongly influence the precise opening/closing leaflet shape and potentially the presence of flutter. Thus, the present approach is important for both correct interpretation of the experimental data, as well as accurate simulations of the dynamic behavior native and potentially engineered tissue valve leaflets in the full dynamic sense.
GAGs in prosthetic valve design
Currently, a wide range of research is underway to improve heart valve therapies, including improved bioprosthetic heart valves (BHVs) as well as tissue engineered heart valve approaches. In both cases, the goal is to replicate native valve function via preserving or imitating native valve structure [31, 51, 52]. Clearly, the structure-function relationship of the native valve needs to be fully elucidated. Mechanical failure of BHVs occurs by structural dysfunction that is either calcific or noncalcific [2, 19, 53, 54]. In noncalcific failure, the stress during heart valve function makes BHVs extremely vulnerable to tearing at highly stressed regions, such as the cuspal commissures and points of maximal flexion [2, 55]. Implanted valve tissue undergoes a 50% reduction in mechanical strength after only four years of implantation [2, 56]. One of the shortcomings of glutaraldehyde fixation used in the fabrication of BHVs is its inability to stabilize multiple important components of the extracellular matrix (ECM) [2, 8]. GAG loss has been documented during fixation and storage via hexosamine analysis as well as using transmission electron microscopy [10]. This reduction in the amount of GAGs is also thought to be involved in this failure process, possibly resulting in tissue buckling and the depletion of the valve’s ability to sustain high compressive loads during valve operation [2, 8, 57].
The results of this work are consistent with research concerning the role of GAGs in BHVs. Removal of GAGs from BHVs have not shown any significant changes in peak stretch under equi-biaxial tension or calculated hysteresis [58]. The flexural results provided similar trends with significant changes in hysteresis but no significant changes in flexural rigidity as a result of GAG removal [58]. The application of the results of this study can be applied in the improved development of BHVs. While biomechanical properties as well as fiber structure of the valve are profoundly altered by the chemical cross-linking process utilized in BHV manufacture, the necessity of GAGs to provide critical damping likely remains. As a result, the mechanical evaluation of GAG retention techniques in BHVs should largely focus on the flexural mechanical properties as assessment of flutter in assembled valve function.
Limitations
The removal of GAGs utilizing a combination of hyaluronidase and chondroitinase has previously been utilized in valve tissue studies [31, 58]. Our GAG quantification analysis showed that the modified protocol was effective in the removal of GAGs on the same level as these previous studies. The control group further suggested the instability of GAGs in porcine valve tissue as seen by the decrease in GAGs from processing alone. However, our enzymatic GAG removal techniques only removed half of the total GAG mass present in the native valve. While significant, it could be argued that more complete removal should be obtained. However, the remaining GAGs after enzymatic degradation are closely associated with collagen fibers [58], so that attempts at their removal would require significant disruption of the collagen structure. Given that half of the GAGs present, the majority of which are un-bound, were removed should be sufficient to elucidate the role of the GAGs in the functioning heart valve. Additionally, due to current experimental limitations we were not able to perform studies at physiological loading rates. We have previously shown that over a 100 fold change in strain rate there are no observable changes in planar biaxial tensile behavior for the aortic and mitral valve leaflets [36]. However, while we anticipate hysteresis would be greater at physiological rates, the presented results likely represent a faithful estimate of the hysteretic behavior of the valvular tissue.
Summary and conclusions
The results of this study support our hypothesis that GAGs do not play a measurable, direct role in tensile biomechanics of aortic valve tissues. Under physiological extensional deformations collagen fibers rotate and straighten; GAGs interacting with the collagen fibers to contribute to the biomechanics under this loading state would induce viscous effects via fiber – matrix interactions, yet none were observed. In-plane shear results imply that GAGs to not affect fiber rotations under high stress. We know that heart valve collagen fibers to undergo larger rotations during loading, yet the fiber-matrix interactions are evidently not modulated by the GAGs. This result maybe partly due to the parallel need for heart valve tissues to be “leaky” in that all nutrients must come from the surrounding blood. Thus, HV tissues are designed to both permit large fiber rotations (fibers are thus not tightly bound) and high permeability. However, in low the low strain range the biomechanical behavior is likely dominated by the crimped collagen fibers (not straightened) interacting with the GAGs. This result suggests a damping role of GAGs in the aortic valve leaflet, reducing flutter during full systolic flow. This information can be utilized in the design and evaluation of novel engineered heart valves, minimizing valve flutter via enforcing hysteretic properties in flexure could assist in the improvement of long term durability of these valve replacement options.
Acknowledgements
This work was supported by the NIH grant R01- HL070969.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sacks MS, Yoganathan AP. Heart valve function: a biomechanical perspective. Philos Trans R Soc Lond B Biol Sci. 2007;362:1369–1391. doi: 10.1098/rstb.2007.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoen F, Levy R. Tissue heart valves: Current challenges and future research perspectives. Journal of Biomedical Materials Research. 1999;47:439–465. doi: 10.1002/(sici)1097-4636(19991215)47:4<439::aid-jbm1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Vesely I, Boughner D. Analysis of the bending behaviour of porcine xenograft leaflets and of natural aortic valve material: bending stiffness, neutral axis and shear measurements. Journal of Biomechanics. 1989;22:655–671. doi: 10.1016/0021-9290(89)90016-x. [DOI] [PubMed] [Google Scholar]
- 4.Vesely I, Noseworthy R. Micromechanics of the fibrosa and the ventricularis in aortic valve leaflets. Journal of Biomechanics. 1992;25:101–113. doi: 10.1016/0021-9290(92)90249-z. [DOI] [PubMed] [Google Scholar]
- 5.Scott M, Vesely I. Aortic valve cusp microstructure: The role of elastin. Annals of Thoracic Surgery. 1995;60:S391–S394. doi: 10.1016/0003-4975(95)00263-k. [DOI] [PubMed] [Google Scholar]
- 6.Scott MJ, Vesely I. Morphology of porcine aortic valve cusp elastin. J Heart Valve Dis. 1996;5:464–471. [PubMed] [Google Scholar]
- 7.Christie GW. Anatomy of aortic heart valve leaflets: the influence of glutaraldehyde fixation on function. European Journal of Cardio-Thoracic Surgery. 1992;6:S25–S33. [PubMed] [Google Scholar]
- 8.Vyavahare N, Ogle M, Schoen FJ, Zand R, GLoeckner DC, Sacks MS, et al. Mechanisms of bioprosthetic heart valve failure: Fatigue causes collagen denaturation and glycosaminoglycan loss. Journal of Biomedical Materials Research. 1999;46:44–50. doi: 10.1002/(sici)1097-4636(199907)46:1<44::aid-jbm5>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 9.Vesely I, Boughner D. Analysis of the bending behaviour of porcine xenograft leaflets and of neutral aortic valve material: bending stiffness, neutral axis and shear measurements. J Biomech. 1989;22:655–671. doi: 10.1016/0021-9290(89)90016-x. [DOI] [PubMed] [Google Scholar]
- 10.Simionescu DT, Lovekamp JJ, Vyavahare NR. Degeneration of bioprosthetic heart valve cusp and wall tissues is initiated during tissue preparation: an ultrastructural study. J Heart Valve Dis. 2003;12:226–234. [PubMed] [Google Scholar]
- 11.Talman E, Boughner DR. Internal shear properties of fresh porcine aortic valve cusps: implications for normal valve function. Journal of Heart Valve Disease. 1996;5:152–159. [PubMed] [Google Scholar]
- 12.Talman EA, Boughner DR. Glutaraldehyde fixation alters the internal shear properties of porcine aortic heart valve tissue. Annals of Thoracic Surgery. 1995;60:S369–S73. doi: 10.1016/0003-4975(95)00250-o. [DOI] [PubMed] [Google Scholar]
- 13.Sacks MS, David Merryman W, Schmidt DE. On the biomechanics of heart valve function. J Biomech. 2009;42:1804–1824. doi: 10.1016/j.jbiomech.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thubrikar M, Bosher LP, Nolan SP. The mechanism of opening of the aortic valve. J Thorac Cardiovasc Surg. 1979;77:863–870. [PubMed] [Google Scholar]
- 15.Joyce EM, Liao J, Schoen FJ, Mayer JE, Jr, Sacks MS. Functional collagen fiber architecture of the pulmonary heart valve cusp. Ann Thorac Surg. 2009;87:1240–1249. doi: 10.1016/j.athoracsur.2008.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vesely I. The role of elastin in aortic valve mechanics. Journal of Biomechanics. 1998;31:115–123. doi: 10.1016/s0021-9290(97)00122-x. [DOI] [PubMed] [Google Scholar]
- 17.Reul H, Talukder N. The Heart. 7th ed. McGraw Hill; 1989. Heart valve mechanics. [Google Scholar]
- 18.Vesely I, Lozon A, Talman E. Is zero-pressure fixation of bioprosthetic valves truly stress free? J Thorac Cardiovasc Surg. 1993;106:288–298. [PubMed] [Google Scholar]
- 19.Schoen FJ. Future directions in tissue heart valves: impact of recent insights from biology and pathology. J Heart Valve Dis. 1999;8:350–358. [PubMed] [Google Scholar]
- 20.Thubrikar M, Eppink RT. A method for analysis of bending and shearing deformations in biological tissue. J Biomech. 1982;15:529–535. doi: 10.1016/0021-9290(82)90006-9. [DOI] [PubMed] [Google Scholar]
- 21.Thubrikar MJ, Skinner JR, Eppink RT, Nolan SP. Stress analysis of porcine bioprosthetic heart valves in vivo. J Biomed Mater Res. 1982;16:811–826. doi: 10.1002/jbm.820160607. [DOI] [PubMed] [Google Scholar]
- 22.Stella JA, Sacks MS. On the biaxial mechanical properties of the layers of the aortic valve leaflet. J Biomech Eng. 2007;129:757–766. doi: 10.1115/1.2768111. [DOI] [PubMed] [Google Scholar]
- 23.Ali AF, Taha MM, Thornton GM, Shrive NG, Frank CB. Biomechanical study using fuzzy systems to quantify collagen fiber recruitment and predict creep of the rabbit medial collateral ligament. J Biomech Eng. 2005;127:484–493. doi: 10.1115/1.1894372. [DOI] [PubMed] [Google Scholar]
- 24.Boorman RS, Thornton GM, Shrive NG, Frank CB. Ligament grafts become more susceptible to creep within days after surgery: evidence for early enzymatic degradation of a ligament graft in a rabbit model. Acta Orthop Scand. 2002;73:568–574. doi: 10.1080/000164702321022866. [DOI] [PubMed] [Google Scholar]
- 25.Thornton GM, Shrive NG, Frank CB. Ligament creep recruits fibres at low stresses and can lead to modulusreducing fibre damage at higher creep stresses: a study in rabbit medial collateral ligament model. J Orthop Res. 2002;20:967–974. doi: 10.1016/S0736-0266(02)00028-1. [DOI] [PubMed] [Google Scholar]
- 26.Thornton GM, Frank CB, Shrive NG. Ligament creep behavior can be predicted from stress relaxation by incorporating fiber recruitment. Journal of Rheology. 2001;45:493–507. [Google Scholar]
- 27.Liao J, Vesely I. Relationship between collagen fibrils, glycosaminoglycans, and stress relaxation in mitral valve chordae tendineae. Ann Biomed Eng. 2004;32:977–983. doi: 10.1023/b:abme.0000032460.97278.e9. [DOI] [PubMed] [Google Scholar]
- 28.Grashow JS, Sacks MS, Liao J, Yoganathan AP. Planar biaxial creep and stress relaxation of the mitral valve anterior leaflet. Ann Biomed Eng. 2006a;34:1509–1518. doi: 10.1007/s10439-006-9183-8. [DOI] [PubMed] [Google Scholar]
- 29.Grashow JS, Yoganathan AP, Sacks MS. Biaxial stress-stretch behavior of the mitral valve anterior leaflet at physiologic strain rates. Ann Biomed Eng. 2006b;34:315–325. doi: 10.1007/s10439-005-9027-y. [DOI] [PubMed] [Google Scholar]
- 30.Liao J, Yang L, Grashow J, Sacks MS. The relation between collagen fibril kinematics and mechanical properties in the mitral valve anterior leaflet. J Biomech Eng. 2007;129:78–87. doi: 10.1115/1.2401186. [DOI] [PubMed] [Google Scholar]
- 31.Lovekamp JJ, Simionescu DT, Mercuri JJ, Zubiate B, Sacks MS, Vyavahare NR. Stability and function of glycosaminoglycans in porcine bioprosthetic heart valves. Biomaterials. 2006;27:1507–1518. doi: 10.1016/j.biomaterials.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raghavan D, Starcher BC, Vyavahare NR. Neomycin binding preserves extracellular matrix in bioprosthetic heart valves during in vitro cyclic fatigue and storage. Acta Biomater. 2009;5:983–992. doi: 10.1016/j.actbio.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mercuri JJ, Lovekamp JJ, Simionescu DT, Vyavahare NR. Glycosaminoglycan-targeted fixation for improved bioprosthetic heart valve stabilization. Biomaterials. 2007;28:496–503. doi: 10.1016/j.biomaterials.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Billiar KL, Sacks MS. Biaxial mechanical properties of the natural and glutaraldehyde treated aortic valve cusp- -Part I: Experimental results. Journal of Biomechanical Engineering. 2000a;122:23–30. doi: 10.1115/1.429624. [DOI] [PubMed] [Google Scholar]
- 35.Sun W, Sacks MS, Sellaro TL, Slaughter WS, Scott MJ. Biaxial mechanical response of bioprosthetic heart valve biomaterials to high in-plane shear. Journal Biomechanical Engineering. 2003;125:372–380. doi: 10.1115/1.1572518. [DOI] [PubMed] [Google Scholar]
- 36.Stella JA, Liao J, Sacks MS. Time-dependent biaxial mechanical behavior of the aortic heart valve leaflet. J Biomech. 2007;40:3169–3177. doi: 10.1016/j.jbiomech.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirnajafi A, Raymer J, Scott MJ, Sacks MS. The effects of collagen fiber orientation on the flexural properties of pericardial heterograft biomaterials. Biomaterials. 2005;26:795–804. doi: 10.1016/j.biomaterials.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Gloeckner DC, Billiar KL, Sacks MS. Effects of mechanical fatigue on the bending properties of the porcine bioprosthetic heart valve. Asaio J. 1999;45:59–63. doi: 10.1097/00002480-199901000-00014. [DOI] [PubMed] [Google Scholar]
- 39.Engelmayr GC, Jr, Hildebrand DK, Sutherland FW, Mayer JE, Jr, Sacks MS. A novel bioreactor for the dynamic flexural stimulation of tissue engineered heart valve biomaterials. Biomaterials. 2003;24:2523–2532. doi: 10.1016/s0142-9612(03)00051-6. [DOI] [PubMed] [Google Scholar]
- 40.Sacks MS, Mirnajafi A, Sun W, Schmidt P. Bioprosthetic heart valve heterograft biomaterials: structure, mechanical behavior and computational simulation. Expert Rev Med Devices. 2006;3:817–834. doi: 10.1586/17434440.3.6.817. [DOI] [PubMed] [Google Scholar]
- 41.Gray ML, Burstein D, Xia Y. Biochemical (and functional) imaging of articular cartilage. Semin Musculoskelet Radiol. 2001;5:329–343. doi: 10.1055/s-2001-19043. [DOI] [PubMed] [Google Scholar]
- 42.Yoon JH, Halper J. Tendon proteoglycans: biochemistry and function. J Musculoskelet Neuronal Interact. 2005;5:22–34. [PubMed] [Google Scholar]
- 43.Almond A. Hyaluronan. Cell Mol Life Sci. 2007;64:1591–1596. doi: 10.1007/s00018-007-7032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thornton GM, Oliynyk A, Frank CB, Shrive NG. Ligament creep cannot be predicted from stress relaxation at low stress: a biomechanical study of the rabbit medial collateral ligament. J Orthop Res. 1997;15:652–656. doi: 10.1002/jor.1100150504. [DOI] [PubMed] [Google Scholar]
- 45.Provenzano P, Lakes R, Keenan T, Vanderby R., Jr Nonlinear ligament viscoelasticity. Ann Biomed Eng. 2001;29:908–914. doi: 10.1114/1.1408926. [DOI] [PubMed] [Google Scholar]
- 46.Setton LA, Mow VC, Howell DS. Mechanical behavior of articular cartilage in shear is altered by transection of the anterior cruciate ligament. J Orthop Res. 1995;13:473–482. doi: 10.1002/jor.1100130402. [DOI] [PubMed] [Google Scholar]
- 47.Grande-Allen KJ, Calabro A, Gupta V, Wight TN, Hascall VC, Vesely I. Glycosaminoglycans and proteoglycans in normal mitral valve leaflets and chordae: association with regions of tensile and compressive loading. Glycobiology. 2004;14:621–633. doi: 10.1093/glycob/cwh076. [DOI] [PubMed] [Google Scholar]
- 48.Gupta V, Tseng H, Lawrence BD, Grande-Allen KJ. Effect of cyclic mechanical strain on glycosaminoglycan and proteoglycan synthesis by heart valve cells. Acta Biomater. 2009;5:531–540. doi: 10.1016/j.actbio.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grande-Allen KJ, Mako WJ, Calabro A, Shi Y, Ratliff NB, Vesely I. Loss of chondroitin 6-sulfate and hyaluronan from failed porcine bioprosthetic valves. J Biomed Mater Res A. 2003;65:251–259. doi: 10.1002/jbm.a.10475. [DOI] [PubMed] [Google Scholar]
- 50.Simionescu DT, Lovekamp JJ, Vyavahare NR. Glycosaminoglycan-degrading enzymes in porcine aortic heart valves: implications for bioprosthetic heart valve degeneration. J Heart Valve Dis. 2003;12:217–225. [PubMed] [Google Scholar]
- 51.Stephens EH, Chu CK, Grande-Allen KJ. Valve proteoglycan content and glycosaminoglycan fine structure are unique to microstructure, mechanical loads, and age: relevance to an age-specific tissue engineered heart valve. Acta Biomaterial. 2008 doi: 10.1016/j.actbio.2008.03.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi Y, Ramamurthi A, Vesely I. Towards tissue engineering of a composite aortic valve. Biomed Sci Instrum. 2002;38:35–40. [PubMed] [Google Scholar]
- 53.Schoen FJ, Levy RJ. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg. 2005;79:1072–1080. doi: 10.1016/j.athoracsur.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 54.Sidhu P, O'Kane H, Ali N, Gladstone DJ, Sarsam MAI, Campalani G, et al. Mechanical or Bioprosthetic Valves in the Elderly: A 20-Year Comparison. Annals of Thoracic Surgery. 2001;71:S257–S260. doi: 10.1016/s0003-4975(01)02522-x. [DOI] [PubMed] [Google Scholar]
- 55.Mirnajafi A, Raymer JM, McClure LR, Sacks MS. The flexural rigidity of the aortic valve leaflet in the commissural region. J Biomech. 2006;39:2966–2973. doi: 10.1016/j.jbiomech.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 56.Purinya B, Kasyanov V, Volkolakov J, Latsis R, Tetere G. Biomechanical and structural properties of the explanted bioprosthetic valve leaflets. J Biomech. 1994;27:1–11. doi: 10.1016/0021-9290(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 57.Mako W, Calabro A, Ratliff N, Vesely I. Loss of glycosaminoglycans from implanted bioprosthetic heart valves. Orlando, FL: American Heart Association 70th Scientific Session; 1997. [Google Scholar]
- 58.Friebe VM, Mikulis B, Kole S, Ruffing CS, Sacks MS, Vyavahare NR. Neomycin enhances extracellular matrix stability of glutaraldehyde crosslinked bioprosthetic heart valves. Journal of biomedical materials research Part B Applied biomaterials. 2011 doi: 10.1002/jbm.b.31889. [DOI] [PubMed] [Google Scholar]