Abstract
Fibronectin (FN) is a plasma glycoprotein that circulates in the near micromolar concentration range and is deposited along with locally produced FN in the extracellular matrices of many tissues. Control of FN deposition is tightly controlled by cells. Agents that modulate FN assembly may be useful therapeutically in conditions characterized by excessive FN deposition, such as fibrosis, inflammatory diseases, and malignancies. To identify such agents by high throughput screening (HTS), we developed a microtiter assay of FN deposition by human fibroblasts. The assay provides a robust read-out of FN assembly. Alexa 488-FN (A488-FN) was added to cell monolayers, and the total fluorescence intensity of deposited A488-FN was quantified. The fluorescence intensity of deposited A488-FN correlated with the presence of FN fibrils visualized by fluorescence microscopy. The assay Z’ values were 0.67 or 0.54, respectively, when using background values of fluorescence either with no added A488-FN or with A488-FN added together with a known inhibitor of FN deposition. The assay was used to screen libraries comprising 4160 known bioactive compounds. Nine compounds were identified as non- or low-cytotoxic inhibitors of FN assembly. Four (ML-9, HA-100, tyrphostin and imatinib mesylate) are kinase inhibitors, a category of compounds known to inhibit FN assembly; two (piperlongumine and cantharidin) are promoters of cancer cell apoptosis; and three (maprotiline, CGS12066B, and aposcopolamine) are modulators of biogenic amine signaling. The latter six compounds have not been recognized heretofore as affecting FN assembly. The assay is straight-forward, adapts to 96- and 384-well formats, and should be useful for routine measurement of FN deposition and HTS. Screening of more diverse chemical libraries and identification of specific and efficient modulators of FN fibrillogenesis may result in therapeutics to control excessive connective tissue deposition.
Keywords: fibronectin, fibrillogenesis, extracellular matrix, HTS, kinase inhibitors, piperlongumine, cantharidin
1. Introduction
Fibronectin (FN) is a glycoprotein that circulates in soluble form at a concentration range of 200-600 μg/ml (0.4-1.2 μM) in blood and is deposited into detergent-insoluble fibrils in the extracellular matrices in many tissues (Magnusson and Mosher, 1998; Zerlauth and Wolf, 1984). Deposited FN is derived from plasma and also from the cells present in the tissues (Moretti et al., 2007; Oh et al., 1981). Fibrillar FN provides signals for cells to adhere, spread, migrate, proliferate, or differentiate, depending on the context of the microenvironment (Mao and Schwarzbauer, 2005; Pankov and Yamada, 2002). FN fibrillogenesis has been shown to be important for the deposition of other extracellular matrix molecules, including collagen (McDonald et al., 1982; Shi et al., 2010; Sottile and Hocking, 2002), fibrinogen (Pereira et al., 2002), fibrillin (Kinsey et al., 2008; Sabatier et al., 2009), TGF-β binding protein (Dallas et al., 2005), and fibulin (Sasaki et al., 1996), suggesting an orchestrator role for FN in the deposition of connective tissue (Sottile and Hocking, 2002). Thus, FN assembly is fundamental to processes that are restorative, such as wound healing; deleterious, such as malignant growth or fibrosis; or both, such as angiogenesis (To and Midwood, 2011). To enhance or suppress these effects of FN in vivo, one must identify specific modulators of FN fibrillogenesis that can be developed for systemic administration. To this end, there is a need for assays of FN assembly that can be used in high throughput screening (HTS) of small molecule libraries.
Assembly of plasma FN is catalyzed by adherent cells and is dependent on interactions of FN with cell-membrane molecules; these interactions enable conversion of FN from a compact soluble form to an extended one that forms the detergent-insoluble fibrils (Magnusson and Mosher, 1998; Singh et al., 2010; Tomasini-Johansson et al., 2006). Methods to quantify FN assembly have included measurement of cell monolayer-bound 125I-labeled FN (Allen-Hoffmann and Mosher, 1987; McKeown-Longo and Mosher, 1983; Tomasini-Johansson et al., 2001) and densitometry of extracted FN detected on Western blots (Cho and Mosher, 2006; Wierzbicka-Patynowski et al., 2004; Xu et al., 2009). These methods are cumbersome, time-consuming, and not scalable. FN assembly can also be assessed by fluorescence microscopy of fluorophore-tagged FN or immunofluorescent detection with anti-FN antibodies (Pankov and Momchilova, 2009). Microscopy offers rich information about fibril appearance, but suffers from field-to-field variation and ambiguity about which fields are most representative. Herein we present the development and validation of a robust, straight-forward FN fibrillogenesis assay that can be used in a 96-well plate format for experimental studies or in a 384-well format for HTS. Also presented are the results of a pilot screen of small libraries of compounds with known bioactivity from which a set of compounds has been identified as reproducible, dose-dependent inhibitors of FN assembly.
2. Results and Discussion
The assay was designed to allow sequential addition of components to microtiter plate wells with wash steps only at the end. The first step is a 1-h incubation of human skin fibroblasts in 2% fetal bovine serum (FBS) to allow cell adhesion and spreading, which is required for binding and assembly of FN (Zhang et al., 1997). FBS at 2% contains adequate vitronectin to mediate cell adhesion (Hayman et al., 1985). Cell adhesion and spreading in wells was monitored by phase microscopy and observed to be complete 1 h after plating (not shown). The second step is addition of fluorescently labeled FN, 10-40 nM (4.5-18 μg/ml), in the presence or absence of test compounds. The concentration of FN in FBS is 20-30 μg/ml (Hayman and Ruoslahti, 1979), so the concentration in 2% FBS is < 1 μg/ml (< 2 nM), considerably less than the concentration of labeled FN. At the end of an incubation period, non-assembled FN is removed by washing, and fluorescence is read on a microtiter plate reader. The number of viable cells remaining in the well is estimated by a luminescent measurement of ATP content using the commercial kit, Cell Titer Glo, thus allowing the amount of assembled FN to be normalized for the number of adherent cells that catalyze assembly and providing a HTS counter-assay for compounds that are cytotoxic or disturb cell adhesion.
The assay was optimized in a 96-well plate format with flat transparent bottom and black wall wells. We utilized a locally-derived strain of foreskin fibroblasts (AH1F) that synthesize FN, co-assemble endogenous and exogenously added FN, and have been studied previously to identify antibodies that inhibit FN assembly (Chernousov et al., 1991; Peters et al., 1990). We focused on assembly of fluorescently labeled exogenous FN rather than tagging assembled total FN at the end of the assay to avoid the additional incubation and wash steps that would be required for the latter. The assay has been tested with embryonic dermal fibroblasts (C1-1-F) and IMR-90 lung fibroblasts obtained from the American Type Culture Collection with similar results (not shown) and should be transferable to almost any fibroblast that adheres to and spreads on microplates in serum-containing medium.
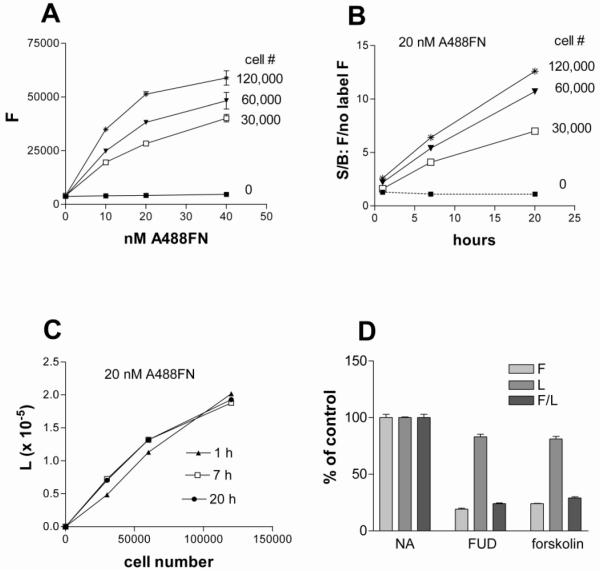
Shown in Figure 1A is fluorescence after addition of 0, 10, 20 or 40 nM A488-FN to 0, 30,000, 60,000, or 120,000 cells per well for 20 h. The signal in wells with no cells or with cells and no A488-FN was approximately 10% of the value with 60,000 cells incubated with 20 nM A488-FN and did not vary when more A488-FN was added in the absence of cells or more cells were added in the absence of A488-FN. These results indicate that the background is not due to intrinsic fluorescence of cells or non-specific adsorption of A488-FN to wells.
Figure 1. Dependence of FN fibrillogenesis on cell number, concentration of A488-FN, time, and presence of known inhibitors.
A) The indicated numbers of AH1F cells were plated in 96-well plates in DMEM containing 2% FBS, and following a 1-h incubation A488-FN was added to wells for varying concentrations and incubated for 20-h. Plates were washed and fluorescence was determined. B) The indicated numbers of cells were plated, given 20 nM (9 μg/ml) A488-FN, and then incubated for 1-, 7-, or 20-h at 37°C. Fluorescence (signal, S) was divided by fluorescence from wells without added labeled FN (background, B) to generate signal to background ratios (S/B). C) Luminescence values corresponding to cell number of wells described in panel B) following addition of Cell Titer Glo reagent. D) Cells (60,000 per well) were incubated 20-h with 20 nM Alexa 488-FN in the presence or absence of 500 nM FUD or 100 μM forskolin. Following PBS washes, fluorescence (F) and luminescence (L) were measured and F, L, and F/L of wells containing FUD or forskolin were expressed as percent of values in wells containing no inhibitor (NA, no additions). F values were obtained by subtracting fluorescence from cell monolayers without added label. Error bars = SEM of triplicate wells.
Incorporation of A488-FN measured after 20 h incubation reached plateaus at 40 nM for each cell number, whereas 20 nM was at the cusps of the curves (Figure 1A); A488-FN at 20 nM (9 μg/ml) was thus chosen as the optimal concentration for the fibrillogenesis assay. We tested whether shorter incubation periods of 1- or 7-h provide a suitable end point, which is reflected by the signal (monolayers with 20 nM A488-FN) to background (monolayers without added label) ratio, S/B. Figure 1B shows that S/B ratios of approximately 5 can be obtained after a 7-h incubation with 60,000 or 120,000 cells per well, but a higher ratio of about 10 was obtained after a 20-h incubation. Figure 1C shows that luminescence corresponding to cellular ATP content increases as expected with cell number per well, with 120,000 cells per well starting to fall off the linear portion of the curve. We therefore chose to use 60,000 cells per well, which made efficient use of cells while preserving signal to background ratio.
The luminescence component of the assay provides a measure of the mass of viable cells after quantitation of fluorescence. As shown in Figure 1D, 500 nM FUD (Functional Upstream Domain) and 100 μM forskolin, known inhibitors of FN fibrillogenesis (Allen-Hoffmann and Mosher, 1987; Tomasini-Johansson et al., 2001), inhibited normalized (F/L) and raw fluorescence (F) to a similar extent. There was a 15-20% decrease in luminescence promoted by the inhibitors in this context. Importantly, the known inhibitors work by different mechanisms. FUD is a 56-residue polypeptide derived from the F1 adhesin of Streptococcus pyogenes that inhibits FN assembly by binding by β-strand addition to multiple N-terminal FN type 1 (F1) modules (2F1-9F1), thus preventing interaction of FN with cell surface molecules involved in deposition (Maurer et al., 2010; Tomasini-Johansson et al., 2001). Because of its high affinity (nM range) and specificity (Ensenberger et al., 2001; Hanski et al., 1996; Maurer et al., 2010), we used FUD as the preferred prototype inhibitor in further development of the FN assembly assay. Forskolin is a small molecule that acts intracellularly to activate adenylyl cyclase and generate cAMP, causing activation of protein kinase A (Chen et al., 1998).
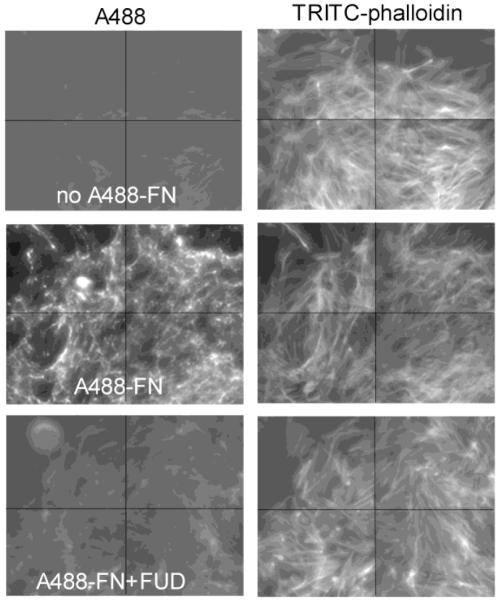
The 96-well format assay was transferred to a 384-well format with a 4-fold reduction in number of added cells and final volume per well. Addition of cells, library compounds, A488-FN, and washes were performed by robotic systems available at Small Molecule Screening Facility (SMSF) of the University of Wisconsin Carbone Cancer Center (UWCCC). To corroborate that the HTS assay measuring fluorescence represents fibrillar FN deposition, an inverted fluorescent microscope (BD Pathway) was utilized to image multiple fields in wells of a 384-well plate set up in tandem with fluorescence readings at SMSF. Following washes after 20 h of A488-FN incubation, cell monolayers were fixed with 3.7% paraformaldehyde and permeabilized with 0.2% Tween in PBS followed by incubation with rhodamine-phalloidin for 1 h to stain actin cytoskeleton. As shown in Figure 2, A488-FN was assembled by AH1F cells into a fibrillar matrix with the expected apical meshwork pattern over cells, which was absent in wells treated with FUD. Each panel is presented as a montage of 4 fields imaged from a given well. Actin stress fibers in the wells treated with FUD appeared similar to those in the A488-FN untreated control. This is consistent with previous results showing FUD does not visibly affect cell morphology (Chiang et al., 2009; Tomasini-Johansson et al., 2001). Thus, the quantitative microtiter fluorescence assay reflects FN fibrillogenesis.
Figure 2. Imaging of FN fibrils and stress fibers.
AH1F cells (15,000 per well) were dispensed in transparent 384-well plates with 20 nM Alexa 488-FN in the presence or absence of 500 nM FUD, and monolayers were incubated for 20-h. Microplates were washed twice with PBS containing Ca2+ and Mg2+, fixed, permeabilized and stained with TRITC-phalloidin to identify actin stress fibers. Wells were imaged on an inverted fluorescent microscope using Attovision software. Shown are montages from 4 areas captured per well of fluorescence for Alexa 488 showing FN fibrils inhibited by FUD (compare the middle left and lower left panels), and the corresponding panels to the right for TRITC channels showing stress fibers were unaffected by FUD treatment.
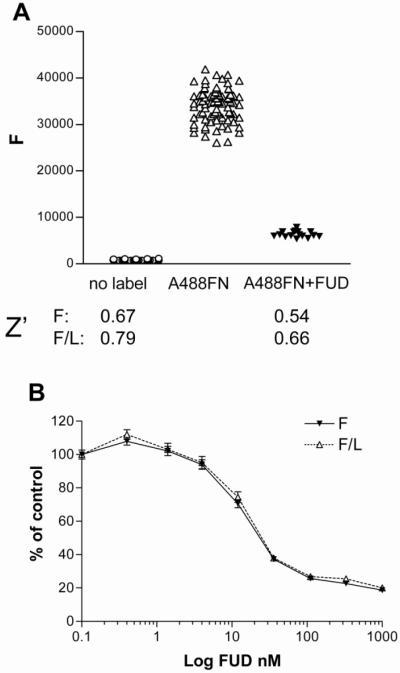
Shown in figure 3A are the averages for positive and negative controls (non-label and FUD) from a 384-well HTS control plate, indicating an S/B ratio of >10. Robustness of a HTS assay is estimated by the Z’ value (Zhang et al., 1999), which is calculated using the formula: Z' = 1- [(3sdc+ + 3sdc-)/(mc+ - mc-)]
Figure 3. Validation of the assay in a HTS format.
AH1F cells in 384-well plates were incubated with nothing, or 20 nM Alexa 488-FN without or with 1 μM FUD followed by 20-h incubation, washing, and quantitation of fluorescence (F) and luminescence (L). (A) Scatter plot of values from wells without or with A488-FN (n=80) or with A488-FN and FUD (n=16). F and F/L data were used for determination of the Z’ values shown under scatter plot. (B) Effect of increasing amounts of FUD on F and F/L as a percentage of positive control (A488-FN without FUD). Error bars = SEM of 16 wells per dose.
where sd = standard deviation; m = mean; c+ = positive control (fluorescent label and no inhibitor); c− = negative control (no fluorescent label or fluorescent label in the presence of a known inhibitor). A Z’ value of 0.4 is considered minimal robustness for an assay to perform well in HTS (Zhang et al., 1999). We obtained fluorescence Z’ values of 0.67 (n=80) and of 0.54 (n= 16) for the no label and label along with1 μM FUD, respectively (Figure 3A). The coefficient of variation was 0.1 (n=80) for both fluorescence and luminescence assays. When fluorescence was normalized for luminescence, Z’ values were higher, 0.79 and 0.66 for no label and for label along with FUD, respectively. In figure 3B are shown dose-response curves of F and F/L values obtained with varying concentrations of FUD expressed as a percent of values in wells without FUD. F/L and F were almost identical, indicating little if any effect on cell viability by FUD at any concentration tested. The concentration of FUD that inhibited 50% (IC50) was ~25 nM, which is consistent with values obtained previously in assays of assembly of 125I-labeled FN (Tomasini-Johansson et al., 2001).
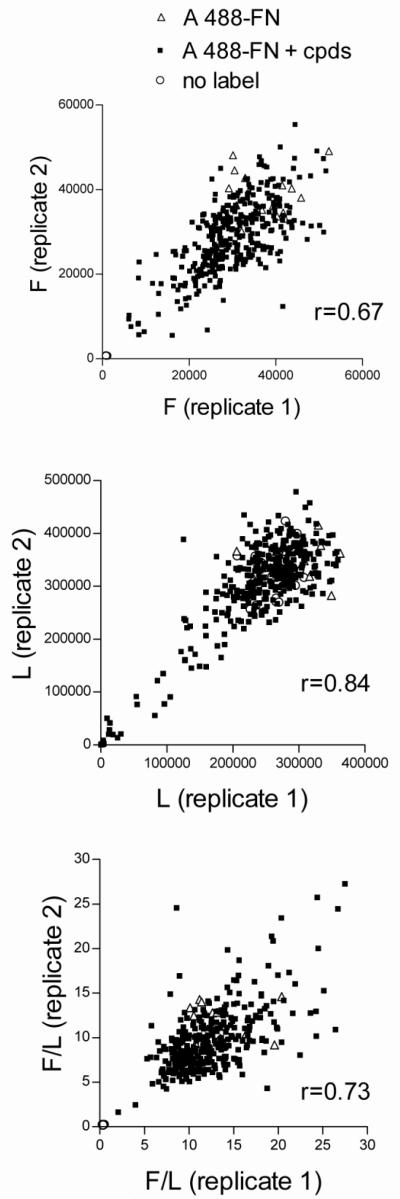
The FN HTS assay was used to screen 4160 compounds selected by the SMSF from libraries of known bioactive compounds (Prestwick, Sigma Lopac, Spectrum, and NIH Clinical Collection). Compounds were tested in 15 384-well plates. Each compound was tested once by addition of 0.1-0.2 μl of 10 mM compound to yield a final concentration of 20-40 μM. Each plate contained 48 positive control wells with A488-FN and no added compounds and 16 negative control wells with no added A488-FN. Compounds that inhibited or increased fluorescence by at least 50% of the positive control and did not inhibit luminescence by more than 30% were selected for further testing, i.e., as “cherry picks”.. In this manner, 337 compounds were chosen, 127 inhibitors and 210 activators. Figure 4 shows the correlation between values for the same cherry-picked compounds assayed on two separate plates. Pearson correlation (r) values for raw fluorescence (Figure 4A), luminescence (Figure 4B), and F/L (Figure 4C), were 0.67, 0.84, and 0.73, respectively. The moderate correlation value for the fluorescence component is consistent with the coefficients of variation found in the positive control wells on these plates (0.14 and 0.17, n=16).
Figure 4. Replicate assays of cherry-picked compounds.
AH1F cells were dispensed into duplicate 384-well plates in DMEM and incubated for 1 h before addition of 337 compounds chosen from among 4160 compounds as causing enhancement or inhibition of FN assembly. A488-FN, 20 nM, was added followed by 20-h incubation, washing, and quantitation of (A) fluorescence (F), (B) luminescence (L), and (C) normalized fluorescence (F/L). Values from each well in replicate plates are plotted against each other to demonstrate degree of inter-plate variability. Pearson correlation coefficients (r) were, respectively, 0.67, 0.84, and 0.73 for fluorescence, luminescence, and F/L.
Of the cherry-picked activator set, 17 compounds acted consistently as activators (not shown). Several fell under categories previously described to increase FN assembly. For instance, the screen identified vinblastine sulfate and podofilox, which are inhibitors of microtubular formation and work similarly to nocodazole and vinblastine, which are known enhancers of assembly by fibroblasts (Zhang et al., 1997). Also identified was methylprednisolone which is similar to dexamethasone, a known enhancer of FN assembly by osteosarcoma cells (McKeown-Longo and Etzler, 1987). To develop the identification of activators, we would need to minimize exposure of cells to lysophosphatidic acid, a known assembly enhancer in the 2% serum (Zhang et al., 1994). Therefore, most of our attention was directed to the 127 inhibitors.
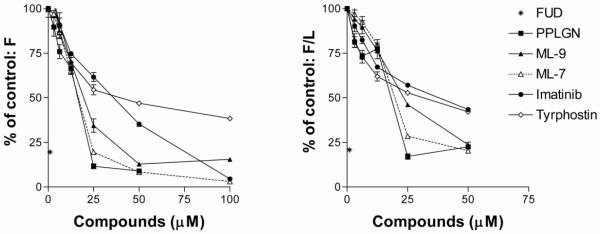
Table 1 lists the nine cherry-picked compounds that by HTS format consistently inhibited FN assembly by at least 40% and did not promote cell loss by more than 30%, as tested on a total of 5 replicate plates on 2 separate occasions. We obtained and tested piperlongumine, ML-9, Imatinib mesylate, and tyrphostin from commercial sources and performed dose-response experiments in a 96-well format alongside ML-7 and FUD as known inhibitors (Lin et al., 2002; Somers and Mosher, 1993; Tomasini-Johansson et al., 2001). Figure 5 shows that the four compounds inhibited A488-FN incorporation into AH1F monolayers, with different dose responses. Comparison of F versus F/L (luminescence-normalized fluorescence, Figure 5 A and B), expressed as percentages of positive control, reveals the concentration at which compounds are cytotoxic, i.e., as luminescence decreases, F/L increases. Such was the case for all compounds at 100 μM and for piperlongumine at 50 μM. The relative FN assembly inhibition potency of the compounds tested was: FUD>piperlongumine=ML-7>ML-9 >imatinib mesylate>tyrphostin. With IC50s in the 20-50 μM range, none of the tested inhibitors approximate the 25 nM IC50 obtained for FUD inhibition of FN assembly.
Table 1. Identities of cherry picked compounds that inhibited deposition of A488-FN by ≥ 40% and did not decrease cell mass by ≥30%.
| Name | CAS # | Inhibition % n=5 | Cell loss % n=5 | Mode of Action | ||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | (Ki) | ||
| ML-9 | 105637-50-1 | 54 | 4 | 3 | 11 | MLCK inhibitor (5-50 μM, Saitoh et al 1987) |
| HA-100 | 84468-24-6 | 50 | 9 | 20 | 6 | PKC and MLCK inhibitor (20 μM, Deshpande et al, 1997) |
| Tyrphostin AG538 |
133550-18-2 | 54 | 5 | 14 | 5 | tyrosine kinase inhibitor (5 μM, Blum et al, 2003) |
| Imatinib mesylate |
220127-57-1 | 42 | 7 | -9 | 2 | abl tyrosine kinase inhibitor (0.1-5 μM, Krystal et al, 2000) |
| Piperlongumine | 20069-09-4 | 74 | 3 | 4 | 22 | glutathione S-transferase inhibitor (10 μM, Raj et al, 2011) |
| Cantharidin | 56-25-7 | 63 | 6 | 21 | 3 | protein phosphatase 2 inhibitor (10 μM, Li et al, 2010) |
| Maprotiline HCl | 10347-81-6 | 52 | 5 | 25 | 0.4 | norepinephrine re-uptake inhibitor (10nM, Tatsumi et al 1997) |
| CGS12066B | 109028-09-3 | 49 | 10 | 17 | 0.4 | 5 HT1B serotonin agonist (50 nM, Pauwels and Palmier, 1994) |
| Aposcopolamine | 535-26-2 | 73 | 2 | 7 | 20 | Muscarinic receptor (M1) antagonist (270 pM, Anisuzzaman, et al, 2011) |
Note: Inhibition and cell loss values are the mean and SD from 1 well in 5 replicate HTS format plates. Values are percentages of positive control (wells with A488-FN and cells).
Figure 5. Dose-dependence of FN assembly inhibition by four compounds identified from cherry pick testing.
AH1F cells were plated into 96-well plates and incubated with increasing doses of piperlongumine (PPLGN), ML-9, imatinib mesylate or tyrphostin. ML-7 and 1 μM FUD were included as known inhibitors of FN assembly. Wells were analyzed for fluorescence (F) and luminescence (L). Points are the averages of 3 assays, with fluorescence (F) and fluorescence/luminescence (F/L) expressed as percentages of values obtained from A488-FN incubated on cells without compounds. Error bars = SD of 3 experiments, each assay point in triplicate.
Four protein kinase inhibitors were identified. HA-100 inhibits PKC (Deshpande et al., 1997); and ML-9, like the previously reported FN assembly inhibitor ML-7 (Somers and Mosher, 1993), is an inhibitor of myosin light chain kinase, MLCK (Saitoh et al., 1987), an enzyme involved in the RhoA signaling pathway leading to cell contractility, which is important for FN assembly (Zhang et al., 1997; Zhong et al., 1998). Tyrphostin and imatinib mesylate are inhibitors of tyrosine kinases (Blum et al., 2003; Krystal et al., 2000) and to our knowledge had not been reported to inhibit FN matrix assembly. However, another tyrosine kinase inhibitor, genistein, has been reported to inhibit FN assembly (Lin et al., 2002). Imatinib mesylate is well-known as Gleevec and used therapeutically to inhibit the oncogenic bcr-abl tyrosine kinase that causes chronic myelogenous leukemia. Imatinib mesylate also binds normal abl kinase, c-kit, and PDGFR; it has been used successfully in the treatment of other malignancies (Stegmeier et al., 2010), and has also been shown to be effective in experimental lung fibrosis (Daniels et al., 2004), and a systemic sclerosis model (Distler et al., 2007). In the systemic sclerosis model, imatinib mesylate caused collagen and FN mRNAs to be decreased. In this regard, we cannot rule out that compounds affecting incorporation of exogenously added A488FN also affect synthesis of endogenous fibronectin. However, experiments using FN-null fibroblastic cells (Saoncella et al., 1999) incorporated similar levels of A488FN as AH1F cells (not shown), indicating that, at least during the 20 h assay period, endogenously synthesized FN does not significantly affect incorporation of the labeled FN. As listed in Table 1, reported active concentrations of the kinase inhibitors vary according to cell type and assay read-out but are mostly in the μM range, consistent with the dose responses in Figure 5.
A second group of inhibitors are antagonists or inhibitors of biogenic amine receptors. This group is more difficult to assess because, to our knowledge, the receptors involved have not been characterized in fibroblastic cells. Catecholamine and serotonin receptors are G protein-coupled and activate second messengers. The receptors for maprotiline (Tatsumi et al., 1997), CGS-12066B (Pauwels and Palmier, 1994), and aposcopolamine (Md Anisuzzaman et al., 2011) may interact with a variety of G proteins, depending on cell type. As indicated in Table 1, the three compounds interact with their receptors at nM concentrations, that is, 1000-fold lower concentration than was needed to inhibit fibronectin assembly. This discrepancy suggests alternative mode(s) of action, perhaps even direct inhibition of the extracellular interaction between FN and its binding molecules at the cell surface.
Piperlongumine is a terpenoid extracted from Piper longum that has been investigated as an inhibitor of platelet aggregation (Park et al., 2008);(Iwashita et al., 2007). Recently, piperlongumine was also identified as the primary hit in a HTS for compounds promoting apoptosis of cancer cells (Raj et al., 2011). Piperlongumine was found to act by increasing the already high levels of reactive oxygen species in cancer but not normal cells, promoting apoptosis specifically in cells with high levels of glutathione S transferase. Cantharidin is derived from beetles and other insects and known to inhibit protein phosphatase 2A (Li et al., 2010; Zhang et al., 2010). Like piperlongumine, cantharidin promotes apoptosis of cancer cells, although in an oxidative stress-independent manner. Many cell types undergo a special type of apoptosis called anoikis when deprived of a suitable adhesive substratum (Frisch and Ruoslahti, 1997). It is possible that inhibition of FN assembly promotes detachment of cancer cells and synergizes with other pro-apoptotic effects of piperlongumine, or cantharidin, thus increasing the anti-cancer activities of these compounds.
Considering fluorescence and luminescence criteria, the hit rates from the original library content were 8% cherry-picked modulators (activators and inhibitors), 3% cherry-picked inhibitors,and 0.22% confirmed inhibitors. Factors possibly contributing to the initial hit rate were the relatively high final concentration of compounds tested (20-40 μM), and use of libraries of compounds that are known to affect cell function, some of which would be expected to affect cell adhesion and matrix interactions. Considering luminescence alone, 7% of the known bioactive compounds caused greater than 30% cell loss compared to controls without compound addition.
Future screening of unbiased libraries should result in lower hit rates. Indeed, a pilot screen of 2000 diverse chemical compounds tested at 10 μM resulted in a hit rate for inhibitor compounds of 0.4% (not shown).This type of screening may yield compounds that act similarly to FUD, i.e., with an extracellular mode of action, and the expectation of avoiding the pleiotropic consequences of targeting intracellular signaling pathways. FUD has provided proof-of-concept for such a target in a carotid artery disease mouse model where it inhibited FN and collagen deposition, immune cell infiltration, and decreased carotid lining thickness (Chiang et al., 2009). FUD inhibition of FN assembly also demonstrated the importance of this process in an in vitro model of angiogenesis (Zhou et al., 2008) and in an osteoblast mineralization model (Brunner et al., 2011). Active compounds may provide insight into mechanisms underlying the control of FN assembly in addition to becoming leads that can be developed for testing in disease models in which FN plays a role in excessive pathological deposition of extracellular matrix.
3. Experimental procedures
3.1 Reagents
Alexa 488 was purchased from Invitrogen (Carlsbad, CA). Cell Titer Glo was purchased from Promega (Madison, WI). Cells AH1F human foreskin fibroblasts passage number 4 were a gift from Lynn Allen-Hoffman, Dept. of Pathology, UW-Madison, and used after expansion at passages 6 to 18; FBS, DMEM, penicillin and streptomycin were purchased from Mediatech (Manassas, VA), trypsin/EDTA solution was from Clonetics (Walkersville, MD); and PBS containing Mg2+, Ca2+ (proprietary concentration) was from Gibco (Invitrogen, Carlsbad, CA). Microtiter tissue culture plates in 96- and 384-well configurations with transparent flat-bottoms and black-walled sides were from Costar (Corning, NY) and Nunc (Rochester, NY). Piperlongumine was purchased from Indofine (Hillsborough, NJ). Forskolin, ML-9, ML-7, tyrphostin AG 538, and sterile-filtered dimethyl sulfoxide (DMSO; Hybrimax) were purchased from Sigma (St. Louis, MO). Imatinib mesylate (now brand name Gleevec) was a gift to Dr. Fran Fogerty of our laboratory from Novartis, Basel, Switzerland). Piperlongumine and ML-9 were dissolved in methanol; and forskolin, imatinib mesyate tyrphostin and ML-7 were dissolved in DMSO; FUD was dissolved in TBS. FUD was expressed and purified as described previously (Maurer et al., 2010) as a 56-amino acid polypeptide containing the original 49 amino acid FUD sequence (Ensenberger et al., 2001) and a total of 7 residues at the ends due to the expression vector pET-ELMER. The His-tag used for purification by affinity chromatography on nickel-nitrilotriacetic acid-agarose was removed (Maurer et al., 2010). The libraries used in high-throughput screening were composed of 4160 unique compounds of known bioactivity from the NIH Clinical Collection, Prestwick Chemical Library, Spectrum and Sigma Lopac libraries.
3.2 Purification of FN
FN was purified from a globulin fraction of human plasma precipitated with 9% polyethylene glycol 8000 also containing 80 mM BaCl2. Precipitated proteins (10 g) were resolubilized in 100 ml 10 mM Tris, 150 mM NaCl, pH 7.4, 0.1 mM EDTA (TBS/EDTA) and subjected to affinity chromatography on gelatin-Sepharose with elution by 1M NaBr in 50 mM sodium acetate, pH 5.0 (Mosher and Schad, 1979; Mosher et al., 1980). Eluted protein was dialyzed against TBS/EDTA, precipitated with 35% (NH4)2SO4, and dissolved in TBS at a concentration of 5-10 mg/ml. FN was determined to be intact by polyacrylamide gel electrophoresis in sodium dodecyl sulfate (SDS-PAGE) without and with reduction, and concentration was measured by UV spectroscopy at 280 nm (ε=1.28; dimer MW=450 kDa). Extent of aggregation was measured by light scattering at 320 nm. Acceptable preparations were intact by SDS-PAGE, and had a ratio of A320nm/A280 nm of <0.2. FN was aliquoted, snap frozen and stored at −80°C. All molar amounts of FN were calculated based on its dimeric MW.
3.3 Labeling of FN with Alexa 488
FN at 2 mg/ml previously dialized against PBS pH 8.8, was reacted with a 30-fold molar excess of Alexa 488 dye over FN dimer for 1 h at room temperature with gentle mixing. The reaction mixture, 2 ml, was dialyzed against 3 changes, 2 L each of PBS, pH 8. FN labeled with Alexa 488 (A488-FN) was assessed by spectroscopy for absorbance at λ= 280nm, 320 nm, and 494 nm for protein concentration, aggregation and incorporation of dye, respectively. Alexa 488-labeled FN was aliquoted, snap-frozen, and stored at −80°C. Using ε=71,000 for Alexa 488 (according to manufacturer instruction, Invitrogen, Carlsbad, CA), incorporation of approximately 15 mol fluorochrome per mol of FN dimer was typical.
3.4 FN fibrillogenesis assay: 96-well and HTS formats
AH1F cells were grown in DMEM containing 10% FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Confluent cells were trypsinized and resuspended in DMEM containing 2% FBS at densities that allowed delivery of 60,000 cells in 50 μl or 15,000 cells in 40 μl to wells of 96-well or 384-well plates, respectively. Cells were incubated for 1 h at 37°C with 8% CO2 and checked for spreading and confluence. For the 96-well format, compounds were added in 25 μl at 4-fold the desired final concentration whereas for HTS format, the library of compounds was dispensed by automation with 0.1-0.2 μl of 10 mM stocks added per well of cell monolayers to give a final concentration of 20-40 μM compound. Following compound addition, A488-FN was added to monolayers to a final concentration of 20 nM (9 μg/ml) delivering 25 μl for a total volume of 100 μl in the 96-well plate format; or 10 μl for a total volume of 50 μl in the 384-well plates. To minimize non-specific loss of A488-FN on surfaces during addition to wells, the ligand was dispensed in DMEM containing 2% FBS.
Microplates were incubated overnight in a humidified 37°C, 8% CO2 incubator. Wells containing cells and ligand and no potential modifier were checked microscopically for the presence of confluent monolayers. Microplates were washed thrice with PBS containing Ca2+, Mg2+ by automation for the 384-well plates (60 μl/well) or using a multichannel pipet for the 96-well plates (100 μl/well). Fluorescence in 96-well or 384-well plates was read using, respectively, a Genios Pro or Saphire II (Tecan) microplate reader equipped with bottom-readout capabilities at 485 nm excitation and 525 nm emission with 20 nm bandwidths. To estimate number of cells remaining in the wells after the washes and measurement of fluorescence, an equal volume of luminescence reagent (Cell Titer Glo) was added followed by a 10 min incubation and then quantification of luminescence (Genios Pro or Biotek instruments for 96-well or 384-well plates, respectively). Fluorescence of wells to which A488-FN had not been added, i.e. intrinsic fluorescence of wells with only cells was subtracted from fluorescence values of wells containing cells and ligand, and corrected fluorescence was normalized for cell mass per well with luminescence values.
3.5 Fluorescence Microscopy
Visual assessment of FN fibrillogenesis was carried out by fluorescence microscopy of cells on glass coverslips using fluorescent microscopes to view monolayers on slides (Olympus BM60, not shown) or in 384-well plates (BD Pathway at Flow Facility, UWCCC, UW-Madison). AH1F monolayers were prepared in 384-well plates as described above for the FN fibrillogenesis assay. Instead of adding luminescence reagent after the PBS washing steps, monolayers were fixed with 3.7% paraformaldehyde in PBS for 10 min, followed by permeabilization with 0.2% Tween in PBS for 5 min, blocking with 2% bovine serum albumin in PBS for 1 h at room temperature, incubation with 1 μg/ml TRITC-phalloidin for 1 h, and further washes in PBS. These steps were performed at room temperature. Microtiter plates containing the fixed and actin-stained monolayers were then visualized by fluorescence microscopy using the appropriate filters for Alexa 488 and TRITC. Images are composed of a montage from 4 regions per well.
Highlights of “Quantitative microtiter fibronectin fibrillogenesis assay: use in high throughput screening for identification of inhibitor compounds”.
-
-
Development and validation of a HTS fibronectin assembly assay is described.
-
-
The results of a pilot screen of 4160 small molecules from known bioactive libraries are presented.
-
-
New compounds that are reproducible inhibitors of fibronectin assembly were identified.
Acknowledgements
We thank the staff of the Small Molecule Screening Facility at UWCCC, Song Guo, and Noel Peters, for implementation of the bioactive library screen; and Mary Gillis for tissue culture maintenance, purification of fibronectin, and production of recombinant FUD. This work was supported by NIH/NINDS R21 NS07647 (BTJ), NIH/NHLBI RO1 HL21644 (DFM), and NIH/NHLBI P01 HL088584 (DFM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen-Hoffmann BL, Mosher DF. Matrix assembly sites for exogenous fibronectin are decreased on human fibroblasts after treatment with agents which increase intracellular cAMP. J Biol Chem. 1987;262:14361–14365. [PubMed] [Google Scholar]
- Blum G, Gazit A, Levitzki A. Development of new insulin-like growth factor-1 receptor kinase inhibitors using catechol mimics. J Biol Chem. 2003;278:40442–40454. doi: 10.1074/jbc.M305490200. [DOI] [PubMed] [Google Scholar]
- Brunner M, Millon-Fremillon A, Chevalier G, Nakchbandi IA, Mosher D, Block MR, Albiges-Rizo C, Bouvard D. Osteoblast mineralization requires beta1 integrin/ICAP-1-dependent fibronectin deposition. J Cell Biol. 2011;194:307–322. doi: 10.1083/jcb.201007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TC, Hinton DR, Zidovetzki R, Hofman FM. Up-regulation of the cAMP/PKA pathway inhibits proliferation, induces differentiation, and leads to apoptosis in malignant gliomas. Lab Invest. 1998;78:165–174. [PubMed] [Google Scholar]
- Chernousov MA, Fogerty FJ, Koteliansky VE, Mosher DF. Role of the I-9 and III-1 modules of fibronectin in formation of an extracellular fibronectin matrix. J Biol Chem. 1991;266:10851–10858. [PubMed] [Google Scholar]
- Chiang HY, Korshunov VA, Serour A, Shi F, Sottile J. Fibronectin is an important regulator of flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol. 2009;29:1074–1079. doi: 10.1161/ATVBAHA.108.181081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Mosher DF. Characterization of fibronectin assembly by platelets adherent to adsorbed laminin-111. J Thromb Haemost. 2006;4:943–951. doi: 10.1111/j.1538-7836.2006.01862.x. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Sivakumar P, Jones CJ, Chen Q, Peters DM, Mosher DF, Humphries MJ, Kielty CM. Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J Biol Chem. 2005;280:18871–18880. doi: 10.1074/jbc.M410762200. [DOI] [PubMed] [Google Scholar]
- Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, Leof EB. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande RV, Peterson RH, Moore MA. Granulocyte colony-stimulating factor-induced activation of protein kinase-C in myeloid cells. J Cell Biochem. 1997;66:286–296. doi: 10.1002/(sici)1097-4644(19970901)66:3<286::aid-jcb2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Distler JH, Jungel A, Huber LC, Schulze-Horsel U, Zwerina J, Gay RE, Michel BA, Hauser T, Schett G, Gay S, Distler O. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arth and Rheum. 2007;56:311–322. doi: 10.1002/art.22314. [DOI] [PubMed] [Google Scholar]
- Ensenberger MG, Tomasini-Johansson BR, Sottile J, Ozeri V, Hanski E, Mosher DF. Specific interactions between F1 adhesin of Streptococcus pyogenes and N-terminal modules of fibronectin. J Biol Chem. 2001;276:35606–35613. doi: 10.1074/jbc.M105417200. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- Hanski E, Jaffe J, Ozeri V. Proteins F1 and F2 of Streptococcus pyogenes. Properties of fibronectin binding. Adv Exp Med Biol. 1996;408:141–150. doi: 10.1007/978-1-4613-0415-9_16. [DOI] [PubMed] [Google Scholar]
- Hayman EG, Pierschbacher MD, Ruoslahti E. Detachment of cells from culture substrate by soluble fibronectin peptides. J Cell Biol. 1985;100:1948–1954. doi: 10.1083/jcb.100.6.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman EG, Ruoslahti E. Distribution of fetal bovine serum fibronectin and endogenous rat cell fibronectin in extracellular matrix. J Cell Biol. 1979;83:255–259. doi: 10.1083/jcb.83.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita M, Oka N, Ohkubo S, Saito M, Nakahata N. Piperlongumine, a constituent of Piper longum L., inhibits rabbit platelet aggregation as a thromboxane A(2) receptor antagonist. Eur J Pharmacol. 2007;570:38–42. doi: 10.1016/j.ejphar.2007.05.073. [DOI] [PubMed] [Google Scholar]
- Kinsey R, Williamson MR, Chaudhry S, Mellody KT, McGovern A, Takahashi S, Shuttleworth CA, Kielty CM. Fibrillin-1 microfibril deposition is dependent on fibronectin assembly. J Cell Sci. 2008;121:2696–2704. doi: 10.1242/jcs.029819. [DOI] [PubMed] [Google Scholar]
- Krystal GW, Honsawek S, Litz J, Buchdunger E. The selective tyrosine kinase inhibitor STI571 inhibits small cell lung cancer growth. Clin Can Res. 2000;6:3319–3326. [PubMed] [Google Scholar]
- Li W, Xie L, Chen Z, Zhu Y, Sun Y, Miao Y, Xu Z, Han X. Cantharidin, a potent and selective PP2A inhibitor, induces an oxidative stress-independent growth inhibition of pancreatic cancer cells through G2/M cell-cycle arrest and apoptosis. Can Sci. 2010;101:1226–1233. doi: 10.1111/j.1349-7006.2010.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Wang SM, Huang TF, Fu WM. Differential regulation of fibronectin fibrillogenesis by protein kinases A and C. Conn Tiss Res. 2002;43:22–31. [PubMed] [Google Scholar]
- Magnusson MK, Mosher DF. Fibronectin: Structure, assembly, and cardiovascular implications. Arterioscler.Thromb.Vasc.Biol. 1998;18:1363–1370. doi: 10.1161/01.atv.18.9.1363. [DOI] [PubMed] [Google Scholar]
- Mao Y, Schwarzbauer JE. Stimulatory effects of a three-dimensional microenvironment on cell-mediated fibronectin fibrillogenesis. J Cell Sci. 2005;118:4427–4436. doi: 10.1242/jcs.02566. [DOI] [PubMed] [Google Scholar]
- Maurer LM, Tomasini-Johansson BR, Ma W, Annis DS, Eickstaedt NL, Ensenberger MG, Satyshur KA, Mosher DF. Extended binding site on fibronectin for the functional upstream domain of protein F1 of Streptococcus pyogenes. J Biol Chem. 2010;285:41087–41099. doi: 10.1074/jbc.M110.153692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JA, Kelley DG, Broekelmann TJ. Role of fibronectin in collagen deposition: Fab’ to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. J Cell Biol. 1982;92:485–492. doi: 10.1083/jcb.92.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo PJ, Etzler CA. Induction of fibronectin matrix assembly in human fibrosarcoma cells by dexamethasone. J.Cell Biol. 1987;104:601–610. doi: 10.1083/jcb.104.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo PJ, Mosher DF. Binding of plasma fibronectin to cell layers of human skin fibroblasts. J Cell Biol. 1983;97:466–472. doi: 10.1083/jcb.97.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Md Anisuzzaman AS, Nishimune A, Yoshiki H, Uwada J, Muramatsu I. Influence of tissue integrity on pharmacological phenotypes of muscarinic acetylcholine receptors in the rat cerebral cortex. J Pharm Exp Pharma. 2011;339:186–193. doi: 10.1124/jpet.111.182857. [DOI] [PubMed] [Google Scholar]
- Moretti FA, Chauhan AK, Iaconcig A, Porro F, Baralle FE, Muro AF. A major fraction of fibronectin present in the extracellular matrix of tissues is plasma-derived. J Biol Chem. 2007;282:28057–28062. doi: 10.1074/jbc.M611315200. [DOI] [PubMed] [Google Scholar]
- Mosher DF, Schad PE. Cross-linking of fibronectin to collagen by blood coagulation Factor XIIIa. J Clin Invest. 1979;64:781–787. doi: 10.1172/JCI109524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher DF, Schad PE, Vann JM. Cross-linking of collagen and fibronectin by factor XIIIa. Localization of participating glutaminyl residues to a tryptic fragment of fibronectin. J Biol Chem. 1980;255:1181–1188. [PubMed] [Google Scholar]
- Oh E, Pierschbacher M, Ruoslahti E. Deposition of plasma fibronectin in tissues. Proc Natl Acad Sci U S A. 1981;78:3218–3221. doi: 10.1073/pnas.78.5.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankov R, Momchilova A. Fluorescent labeling techniques for investigation of fibronectin fibrillogenesis (labeling fibronectin fibrillogenesis) Methods Mol Biol. 2009;522:261–274. doi: 10.1007/978-1-59745-413-1_18. [DOI] [PubMed] [Google Scholar]
- Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- Park BS, Son DJ, Choi WS, Takeoka GR, Han SO, Kim TW, Lee SE. Antiplatelet activities of newly synthesized derivatives of piperlongumine. Phytother Res. 2008;22:1195–1199. doi: 10.1002/ptr.2432. [DOI] [PubMed] [Google Scholar]
- Pauwels PJ, Palmier C. Differential functional activity of 5-hydroxytryptamine receptor ligands and beta adrenergic receptor antagonists at 5-hydroxytryptamine1B receptor sites in Chinese hamster lung fibroblasts and opossum renal epithelial cells. J Pharm Exp Ther. 1994;270:938–945. [PubMed] [Google Scholar]
- Pereira M, Rybarczyk BJ, Odrljin TM, Hocking DC, Sottile J, Simpson-Haidaris PJ. The incorporation of fibrinogen into extracellular matrix is dependent on active assembly of a fibronectin matrix. J Cell Sci. 2002;115:609–617. doi: 10.1242/jcs.115.3.609. [DOI] [PubMed] [Google Scholar]
- Peters DM, Portz LM, Fullenwider J, Mosher DF. Co-assembly of plasma and cellular fibronectins into fibrils in human fibroblast cultures. J Cell Biol. 1990;111:249–256. doi: 10.1083/jcb.111.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, Stern AM, Mandinova A, Schreiber SL, Lee SW. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sabatier L, Chen D, Fagotto-Kaufmann C, Hubmacher D, McKee MD, Annis DS, Mosher DF, Reinhardt DP. Fibrillin assembly requires fibronectin. Mol Biol Cell. 2009;20:846–858. doi: 10.1091/mbc.E08-08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M, Ishikawa T, Matsushima S, Naka M, Hidaka H. Selective inhibition of catalytic activity of smooth muscle myosin light chain kinase. J Biol Chem. 1987;262:7796–7801. [PubMed] [Google Scholar]
- Saoncella S, Echtermeyer F, Denhez F, Nowlen JK, Mosher DF, Robinson SD, Hynes RO, Goetinck PF. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc Natl Acad Sci U S A. 1999;96:2805–2810. doi: 10.1073/pnas.96.6.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Wiedemann H, Matzner M, Chu ML, Timpl R. Expression of fibulin-2 by fibroblasts and deposition with fibronectin into a fibrillar matrix. J Cell Sci. 1996;109(Pt 12):2895–2904. doi: 10.1242/jcs.109.12.2895. [DOI] [PubMed] [Google Scholar]
- Shi F, Harman J, Fujiwara K, Sottile J. Collagen I matrix turnover is regulated by fibronectin polymerization. Am J Cell Physiol. 2010;298:C1265–1275. doi: 10.1152/ajpcell.00341.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Ann Rev Cell Dev Biol. 2010;26:397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers CE, Mosher DF. Protein kinase C modulation of fibronectin matrix assembly. J Biol Chem. 1993;268:22277–22280. [PubMed] [Google Scholar]
- Sottile J, Hocking DC. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol Biol Cell. 2002;13:3546–3559. doi: 10.1091/mbc.E02-01-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmeier F, Warmuth M, Sellers WR, Dorsch M. Targeted cancer therapies in the twenty-first century: lessons from imatinib. Clin Pharm Therap. 2010;87:543–552. doi: 10.1038/clpt.2009.297. [DOI] [PubMed] [Google Scholar]
- Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340:249–258. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- To WS, Midwood KS. Plasma and cellular fibronectin: distinct and independent functions during tissue repair. Fibro Tiss Rep. 2011;4:21. doi: 10.1186/1755-1536-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasini-Johansson BR, Annis DS, Mosher DF. The N-terminal 70-kDa fragment of fibronectin binds to cell surface fibronectin assembly sites in the absence of intact fibronectin. Matrix Biol. 2006;25:282–293. doi: 10.1016/j.matbio.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasini-Johansson BR, Kaufman NR, Ensenberger MG, Ozeri V, Hanski E, Mosher DF. A 49-residue peptide from adhesin F1 of Streptococcus pyogenes inhibits fibronectin. J.Biol.Chem. 2001;276:23430–23439. doi: 10.1074/jbc.M103467200. [DOI] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski I, Mao Y, Schwarzbauer JE. Analysis of fibronectin matrix assembly. Curr Prot Cell Biol. 2004 doi: 10.1002/0471143030.cb1012s25. Ed. Juan S. Bonifacino … [et al.] Chapter 10. [DOI] [PubMed] [Google Scholar]
- Xu J, Bae E, Zhang Q, Annis DS, Erickson HP, Mosher DF. Display of cell surface sites for fibronectin assembly is modulated by cell adherence to (1)F3 and C-terminal modules of fibronectin. PloS one. 2009;4:e4113. doi: 10.1371/journal.pone.0004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerlauth G, Wolf G. Plasma fibronectin as a marker for cancer and other diseases. The Am J Med. 1984;77:685–689. doi: 10.1016/0002-9343(84)90366-8. [DOI] [PubMed] [Google Scholar]
- Zhang C, Peng Y, Wang F, Tan X, Liu N, Fan S, Wang D, Zhang L, Liu D, Wang T, Wang S, Zhou Y, Su Y, Cheng T, Zhuang Z, Shi C. A synthetic cantharidin analog for the enhancement of doxorubicin suppression of stem cell-derived aggressive sarcoma. Biomaterials. 2010;31:9535–9543. doi: 10.1016/j.biomaterials.2010.08.059. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Checovich WJ, Peters DM, Albrecht RM, Mosher DF. Modulation of cell surface fibronectin assembly sites by lysophosphatidic acid. J Cell Biol. 1994;127:1447–1459. doi: 10.1083/jcb.127.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Magnusson MK, Mosher DF. Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol Biol Cell. 1997;8:1415–1425. doi: 10.1091/mbc.8.8.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Rowe RG, Hiraoka N, George JP, Wirtz D, Mosher DF, Virtanen I, Chernousov MA, Weiss SJ. Fibronectin fibrillogenesis regulates three-dimensional neovessel formation. Genes Dev. 2008;22:1231–1243. doi: 10.1101/gad.1643308. [DOI] [PMC free article] [PubMed] [Google Scholar]