Abstract
Objective
Myeloid dendritic cell (mDC) dysfunction during HIV infection may hinder the formation of both innate and adaptive immune responses and contribute to pathogenesis. Our objective was to determine whether circulating factors during chronic HIV infection impair mDC function with respect to secretion of IL-12, a pro-Th1 cytokine, and T cell stimulatory capacity. Particular focus was placed on the effect of combination anti-retroviral therapy (cART) and the role of HIV itself on mDC function.
Methods
Monocyte-derived DC (moDC) from uninfected donors were exposed to plasma from HIV-infected individuals prior to Toll-like receptor (TLR) stimulation. Cytokine secretion was measured via cytokine bead arrays, and T cell proliferation and IFNγ secretion was evaluated following co-culture with naive CD4+ T cells. Expression of genes central to TLR-mediated signal transduction was analyzed via qRT-PCR arrays and western blot.
Results
Exposure of moDC to plasma from untreated HIV-infected donors suppressed secretion of IL-12, and impaired Th1-skewing of CD4+ T cells. The suppressive effect was less by plasma donors receiving cART. Removal of virus from plasma did not relieve suppression, nor was IL-12 secretion decreased upon addition of HIV to control plasma. On a transcriptional level, decreased expression of IKKβ, a key regulator in the TLR/NF-kappaB signaling pathway, corresponded to suppressed cytokine secretion.
Conclusions
Plasma factors during chronic HIV infection impair mDC function in a manner that likely impacts the formation of immune responses to HIV, opportunistic pathogens, and vaccines. Despite partial alleviation by cART, this suppression was not directly mediated by HIV.
Keywords: HIV-1, Myeloid Dendritic Cell, Innate Immunity, Toll-like receptor, IL-12, I-kappa B kinase
Introduction
Myeloid dendritic cells (mDC) are potent antigen presenting cells (APC) that bridge the innate and adaptive immune systems in order to orchestrate responses to pathogens. mDC are powerful stimulators of both CD4+ and CD8+ T cells, and are required to prime naïve T cells in the formation of antigen-specific responses1. In order to become optimal APC, mDC undergo maturation upon encountering a pathogen; a process that involves upregulation of surface costimulatory molecules and secretion of cytokines in order to stimulate and modulate adaptive responses. However, during HIV-1 infection, accumulating evidence suggests that mDC function is dysregulated and may contribute to pathogenesis2–15. Several studies have demonstrated impaired cytokine secretion and T cell stimulatory capacity by mDC during HIV infection in response to toll-like receptor (TLR) ligands and other stimuli5, 7, 11, 14, 15, though results have been conflicting with certain studies describing normal reactivity and even hyperreactivity of mDC possibly contributing to generalized immune activation6, 10, 12, 16. These observed inconsistencies may be attributable to several factors including variability in the stage of disease and mDC subset studied, stimuli used, and functional parameters assayed. Furthermore, the etiology of mDC dysfunction during HIV infection remains largely unclear. Several reports have demonstrated viral-mediated mechanisms, including modulation of mDC function via HIV/C-type lectin interactions, and gp120 and vpr dependent mechanisms9, 11, 13, 14, 17.
Though multiple important questions remain regarding mDC dysfunction during HIV infection, of particular interest is how HIV impacts the ability of mDC to secrete the pro-Th1 cytokine, IL-12. In addition to generalized immune activation that occurs during HIV infection, immune dysregulation has been characterized by imbalances in Th1 versus Th2-type immune responses18–20. IL-12 is a key regulatory cytokine that has a central role in the differentiation of naïve CD4 + T cells towards the Th1 pathway to generate anti-viral cytotoxic T cells and IFNγ secretion21–23. IL-12 also activates the innate immune system via enhancement of natural killer (NK) cell cytotoxicity23. Given the critical role mDC play during the induction and skewing of adaptive responses, impaired secretion of IL-12 by these cells during HIV infection may have a profound impact on the formation of effective immune responses to HIV, opportunistic pathogens, and vaccines. Data regarding IL-12 secretion by mDC during HIV infection have been mixed. Certain studies have described decreases in IL-12 secretion by mDC in response to stimuli; primarily CD40L, TLR4 and TLR7/8 agonists5, 7, 11, 14, though these data have been inconsistent16, 24. Reports examining peripheral blood mononuclear cells (PBMCs) and monocytes from HIV-infected individuals have also revealed lower IL-12 production25–32, with such decreases found attributable to direct effects of HIV proteins gp120 and vpr33, 34.
The aim of our study was to further characterize the impact of HIV infection on mDC function by determining whether circulating plasma factors during chronic HIV infection impair their ability to secrete IL-12 and skew Th1 CD4+ T cell responses. In order to address these questions, we employed in vitro systems in which monocyte-derived DC (moDC) from uninfected donors were exposed to plasma from both cART suppressed and untreated HIV-infected individuals and subsequently activated by TLR stimulation. This approach has allowed us to investigate the contribution of individual plasma factors that may lead to suppression of mDC function, with a focus on the virus itself. We chose to primarily study the TLR3 agonist, Poly I:C, which is a synthetic dsRNA complex that is under investigation as a promising vaccine adjuvant35, 36, in part due to its ability to induce potent secretion of bioactive IL-12 (IL- 12p70) by mDC37–39. We observed that plasma factors during chronic HIV infection significantly impair moDC function via mechanism(s) that are not directly mediated by HIV, despite mitigation of this suppression with combination anti-retroviral therapy (cART).
Methods
Study population
The HIV-infected subjects enrolled in this cross-sectional study who donated plasma samples were recruited through New York University AIDS Clinical Trial Unit and the Center for AIDS Research. Healthy donors served as controls. Chronically infected subjects on therapy (cART, N=10) were taking cART for ≥1 year without detectable viremia at the time of sampling (median CD4+ T cell count 578.5 cells/ml, median age 50.4 years). Chronically infected, untreated subjects (untreated, N=10) were infected for ≥1 year (median viral load 18,600 copies/ml, median CD4+ T cell count 275 cells/ml, median age 44.5 years). Subjects were sex-matched (overall 83% male) (Table 1). The subjects enrolled in the study for viral propagation were recruited through Center for HIV/AIDS Vaccine Immunology (CHAVI 012). These were HIV-infected subjects whom were not receiving therapy at the time of leukapheresis. Informed consent was obtained from all study participants in accordance with the Declaration of Helsinki. This study was reviewed and approved by the Institutional Review Board of Bellevue Hospital, New York University School of Medicine, the University of North Carolina Chapel Hill, Aaron Diamond AIDS Research Center, and CHAVI.
Table 1.
Patient Characteristics
| Patient Group | |||
|---|---|---|---|
| cART(n=10) | untreated (n=10) | control (n=10)* | |
| Age (range, mean) | 37–58, 50.4 | 35–53, 44.3 | 24–52, 36 |
| CD4+ T cells in cells/ml (range, mean) | 228–953, 621.2 | 160–810, 342.1 | N/A |
| Viral load in copies/ml (range, mean) | <50 | 2220–202,000, 44,202.2 | N/A |
| Sex (#) | |||
| male | 8 | 9 | 7 |
| female | 2 | 1 | 2 |
| unknown | 0 | 0 | 1 |
| Race (#) | |||
| white | 0 | 2 | 2 |
| hispanic | 4 | 2 | 3 |
| black | 5 | 4 | 0 |
| other or unknown | 1 | 1 | 5 |
Data listed for controls represents plasma samples used for cytokine secretion assays presented in Figure 1A+B. Due to specimen availability, additional control donor plasma was substituted in certain experiments
Dendritic cell preparation, plasma exposure, and stimulation
Peripheral blood mononuclear cells (PBMCs) were purified from buffy coats from uninfected donors purchased from the New York Blood Center (Queens, NY) and plated at 50×106 cells/10 ml/dish in RPMI with 5% PHS. Cells were allowed to adhere for 1–2 hours at 37°C. Nonadherent cells were removed by washing with RPMI. The adherent monocyte-enriched fraction was supplemented with 100 UI/ml rhGM-CSF and 300 UI/ml rhIL-4 (R&D Systems) on days 0 and 2. On day 4 of culture, moDC were harvested, washed, and resuspended in RPMI supplemented with 10% plasma from each donor along with IL-4 and rhGM-CSF for 24 hours. On day 5, moDC were stimulated with Poly (I:C) (Amersham) at 5μg/ml/106 moDC overnight (15–18 hours). Secretion of IL-12p70, TNFα, IL-6, IL-10, IL-1β, and IL-8 was measured in moDC supernatants using the Human Inflammatory Cytokine Cytometric Bead Array (BD Pharmingen). For each individual experiment, moDC generated from a single uninfected donor were exposed to plasma from 28–30 donors (plasma from both treated and untreated HIV-infected subjects in addition to control plasma donors) prior to stimulation with Poly I:C. This was independently repeated on three moDC donors. In select experiments, circulating mDC were enriched from control PBMC (EasySep™ Human Myeloid DC Enrichment Kit, Stem Cell) and exposed to 10% plasma from each group (N=3 per plasma donors per group) followed by Poly IC stimulation. Additional moDC stimulations were performed following exposure to plasma from HIV-infected subjects (N=7 per plasma donors per group) using the TLR7/8 ligand, resiquimod (R848) at 10μM. To assess moDC phenotype and viability following plasma exposure and stimulation, moDC were stained with conjugated fluorescent antibodies to CD11c (BD Pharmingen), CD86 (BD Pharmingen), HLA DR (BD Pharmingen), DC-SIGN (BD Pharmingen), PDL-1 (ebiosciences), and Annexin V (FITC, BD Pharmingen).
Naive CD4+ T cell co-culture
T cell proliferation and IFNγ secretion assays were performed through co-culture of CFSE labeled allogeneic naïve CD4+ T cells with moDC that had been exposed to plasma from a subset of HIV-infected subjects (N=7 per group) and stimulated with Poly I:C as described above. Naïve CD4+ T cells were purified by magnetic cell sorting (Naïve CD4+ T cell Isolation Kit II, Miltenyi Biotec) from uninfected donor PBMCs and stained with CFSE (1μM) for 10 minutes prior to incubation with moDC at a ratio of 1:10 (moDC/Tcell). After 6 days, CFSE dilution was analyzed by FACS and culture supernatants were assayed for IFNγ using the Human Th1/Th2/Th17 Cytokine Cytometric Bead Array (BD Pharmingen).
DC exposure to viruses
To assess the direct effect of HIV on moDC cytokine production, moDC from uninfected donors were exposed to varying doses (100–1000pg/ml) of laboratory strains of HIV (HIV-1MN (X4-tropic) and HIV-1ADA (R5-tropic))(AIDS Vaccine Program (AVP), National Cancer Institute(NCI)) or to patient-derived strains (10–1000pg/ml) of HIV from untreated donors in 10% uninfected control plasma for 24 hours prior to stimulation with Poly I:C. Patient-derived HIV was propagated from CD3 antibody-activated, (eBioscience) autologous CD4+ T cells supplemented with 100IU/ml rhIL-2 (R&D systems). The doses of exogenous patient-derived HIV that were added to cultures were estimated to be in the physiologic range based on the viral loads of our plasma cohort40. Culture supernatants were harvested every 3 days and assayed for viral content with HIV-1 p24 antigen capture kits (AVP, NCI). As controls, supernatant from activated CD4+ T cell cultures from uninfected donors was applied to moDC. In separate experiments, HIV was removed from untreated donor plasma via ultracentrifugation (100,000 G for 57 minutes) and supernatants assayed for the absence of virus with HIV-1 p24 antigen capture kits (AVP, NCI). MoDC were exposed to whole plasma versus supernatant for 24 hours prior to stimulation with Poly I:C.
Milliplex analysis
Luminex analysis was performed on plasma samples from HIV-infected subjects versus control plasma donors, and on supernatants from an moDC:naïve CD4 T cell co-culture experiment (MILLIPLEX MAP Human Cytokine/Chemokine Panel I, Millipore, http://www.millipore.com/bmia/flx4/bmia_immunology#tab1=2). Analytes assayed included EGF, Eotaxin, FGF-2, Flt-3 ligand, Fractalkine, G-CSF, GM-CSF, GRO, IFN-α2, IFN-γ, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17, IL-1ra, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IP-10, MCP-1, MCP-3, MDC (CCL22), MIP-1α, MIP-1β, TGF-α, TNF-α, TNF-β, VEGF, sCD40L, sIL-2Rα. Samples were analyzed according to the manufacturer’s protocol.
qRT-PCR
RNA from HIV versus control plasma-exposed moDC (N=3 per group) was isolated using RNAeasy Mini Kit (Qiagen) and converted to cDNA using RT2 Strand Kit (SABiosciences). Expression of 84 genes central to TLR-mediated signal transduction were analyzed using the Human Toll-Like Receptor (TLR) Signaling Pathway RT2 Profiler PCR Array (SABiosciences) per manufacturer’s protocol. (see http://www.sabiosciences.com/rt_pcr_product/HTML/PAHS-018A.html for complete list of genes). Reactions were conducted using a BioRad icycler IQ5 RT-PCR detection system. All data and statistics were analyzed via software provided by the manufacturer (http://www.sabiosciences.com/pcrarraydataanalysis.php).
Western blots
IKKβ was detected by western blot using rabbit polyclonal antibody (Cell Signaling). HRP-linked anti-rabbit IgG (Cell Signaling) was used as secondary antibody before chemiluminescent detection (ECL Plus, Amersham Biosciences).
Statistical Analysis
Statistical analyses were performed on individual experiments and on combined data when applicable using SAS 9.1 or GraphPad Prism 4. The data displayed in Figures 1 and 2 were logarithmically transformed for statistical analyses that assume normality of data. Mixed effects models were used for repeated measures analyses comparing values from the different moDC donors among the three treatment groups. The mixed model included treatment group and moDC donor as fixed effects, with repeated observations for plasma donor. Although there were significant effects for both treatment group and moDC donor, no interaction was seen. Therefore, pairwise contrasts were performed among treatment groups for the combined data from moDC donors without adjustments for multiple comparisons. For the remaining experiments, analyses of variance were performed for an overall comparison if ≥3 groups were present, and t tests were then used for pairwise comparisons. Relationship of IL-12p70 secretion by moDC following HIV-plasma exposure and Poly I:C stimulation with CD4 count, viral load, and age were evaluated via linear regression. In all cases, P values < 0.05 using two-sided tests were considered significant.
Figure 1. Suppression of moDC cytokine secretion is partially mitigated by suppressive cART.
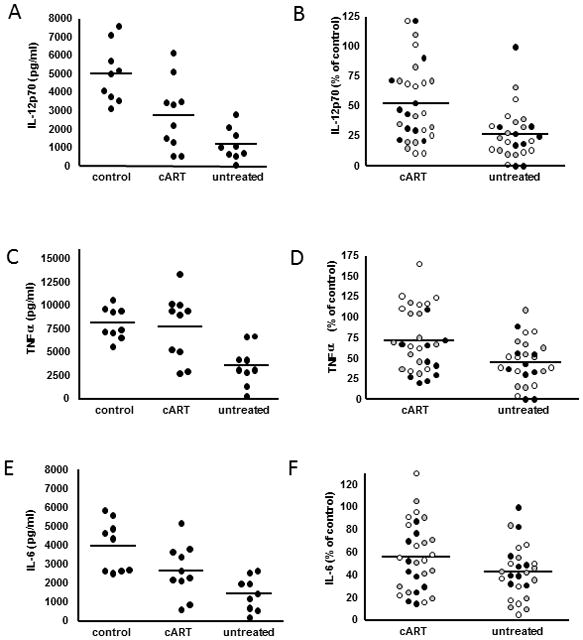
Measurement of cytokine secretion by moDC from uninfected donors was performed following exposure to plasma from either control or HIV-infected subjects and subsequent stimulation with the TLR3 agonist, Poly I:C. Each experiment was repeated on a total of 3 moDC donors and p values were generated using a mixed effects model (MEM) for repeat measures. Raw cytokine data from a single representative moDC donor following plasma exposure and stimulation is displayed for IL-12p70 (A), TNFα (C) and IL-6 (E). Each dot represents an individual plasma donor (mean of 2 replicates per donor). (p values generated from MEM: control vs untreated p<0.0001 for all cytokines; control vs cART p=0.007 for IL-12p70, p=0.11 for TNFα, and p=0.0004 for IL-6). The effect of cART on cytokine secretion is displayed by combining all data points from each moDC experiment following normalization of values from HIV-infected donor plasma to the mean of control plasma donors for IL-12p70 (B), TNFα (D), and IL-6 (F)(p values generated from MEM: p= 0.0013 for IL-12p70, p=0.0042 for TNFα, p= 0.073 for IL-6). Different dot shadings represent values from different moDC donors.
Figure 2. Plasma from untreated HIV-infected individuals suppresses moDC Th1-skewing capacity.
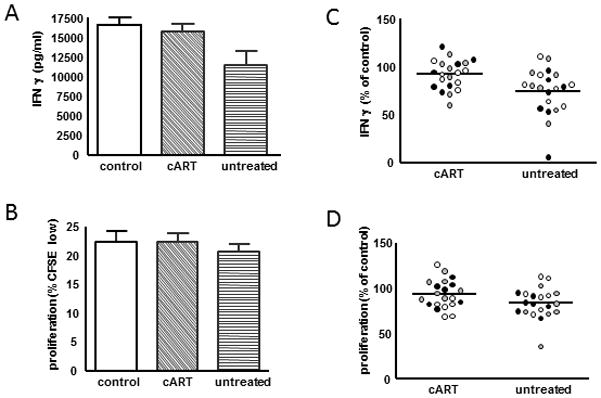
To measure T cell proliferation and IFNγ secretion, moDC from a single donor were exposed to plasma from HIV-infected subjects versus control plasma donors, stimulated with Poly I:C, and then cocultured with CFSE-labeled allogeneic naïve CD4+T cells. Each experiment was repeated on a total of 3 moDC donors and p values were generated using a mixed effects model (MEM) for repeat measures. (A + B) IFNγ secretion (top) and T cell proliferation (bottom) from a single representative experiment. (p values generated from MEM: control vs untreated p=0.007 for CFSE, p=0.005 for IFNγ, control vs cART p=0.27 for CFSE, p=0.49 for IFNγ) (C + D) The effect of cART on IFNγ secretion (top) and T cell proliferation (bottom) is graphically displayed by combining data points from each experiment following normalization of values from HIV-infected donor plasma to controls (p values generated from MEM: p=0.0595 for CFSE, p=0.002 for IFNγ). Different dot shadings represent values from different moDC donors
Results
Plasma from untreated HIV-infected donors suppresses cytokine secretion and Th1-skewing capacity; an effect partially mitigated by cART
Exposure of moDC to plasma samples from HIV-infected individuals resulted in significant suppression of IL-12p70 secretion upon Poly I:C stimulation compared with controls (control vs untreated p<0.0001, control vs cART p=0.007; p values generated from mixed effects model (MEM) for repeat measures)(Figure 1A). Of note, in experiments where moDC were exposed to plasma free conditions (but together with GM-CSF and IL-4) as an additional control prior to stimulation, IL-12p70 secretion was also abrogated compared to all plasma groups (data not shown). Upon examination of the treatment effect, it was found that the suppression of IL-12p70 was significantly less in the cART group compared with the untreated group (cART vs untreated p=0.0013) (Figure 1B) When primary circulating mDC were exposed to plasma from HIV-infected donors prior to Poly I:C stimulation, suppression of IL-12p70 secretion was comparable to that observed by moDC (Supplementary Digital Content, Figure 1A). Additionally, IL-12p70 secretion was similarly lower by moDC exposed to plasma from a subset of HIV-infected subjects following stimulation with the TLR7/8 agonist, R848 (Supplemental Digital Content, Figure 1B).
Exposure of moDC to plasma samples from HIV-infected individuals also resulted in significantly lower production of TNFα and IL-6 upon Poly I:C stimulation, with the exception of TNFα secretion in the cART group (control vs untreated p<0.0001 for TNFα and IL-6; control vs cART p=0.11 for TNFα and p=0.0004 for IL-6)(Figure 1C and 1E). The suppression of TNFα was significantly less in the cART group compared with the untreated group, and a similar trend was noted for IL-6 (cART vs untreated p=0.0042 for TNFα, and p=0.073 for IL-6) (Figure 1D and 1F). IL-8 levels were not consistently different amongst control and HIV groups. IL-1β secretion was generally very low by moDC in all plasma groups, however, when 10 measurable it followed a similar trend as IL-12p70, TNFα, and IL-6 (data not shown). When moDC were left unstimulated following plasma exposure, no differences in IL-12p70, TNFα or IL-6 secretion were observed between groups (data not shown). The phenotype of moDC following plasma exposure and Poly I:C stimulation was not consistently different between groups in terms of expression of CD86, HLA DR, DC-SIGN, or PDL-1 (Supplemental Digital Content, Figure 2A). Cell viability following exposure to HIV plasma was not significantly lower via FACS analysis of both annexin V staining and forward scatter/side scatter characteristics (Supplemental Digital Content, Figure 2B).
When plasma-exposed moDC were cocultured with allogeneic CFSE-labeled naïve CD4+ T cells, less IFNγ secretion and small decreases in proliferation were observed in the untreated group compared with controls (p=0.005, p=0.007), but not in the cART group compared with controls (p=0.27,p=0.49)(p values generated from MEM) (Figures 2A and 2B). Upon direct comparison between the cART and untreated groups, lower IFNγ was seen in the untreated group, with a trend toward lower proliferation (p=0.002, p=0.0595) (Figure 2C and 2D). T cells alone were cultured with HIV versus control plasma +/− stimulation with antibodies to CD3/CD28 and no differences in proliferation were observed amongst groups (data not shown). In addition to IFNγ, luminex analysis of co-culture supernatants revealed significant decreases in several cytokines/chemokines in the HIV plasma groups including MIP-1β, IL-6, IFNα2, IL-1α, eotaxin, IL-7, TNFα, IL-12p40, and IL-12p70 (Supplemental Digital Content, Figure 3).
No evidence for direct viral mediated suppression of moDC function
When laboratory strains of HIV (100pg/ml) were added to moDC cultures in 10% uninfected control plasma prior to stimulation, no significant decrease in IL-12p70 secretion was demonstrated (Figure 3A). Dose titration was performed with addition of log higher doses of laboratory strains of HIV without suppression of IL-12p70 secretion (data not shown). Furthermore, when patient-derived HIV strains (100pg/ml) from four separate donors were added to moDC cultures in 10% uninfected control plasma prior to stimulation, no decrease in IL-12p70 secretion was demonstrated (Figure 3B). Again, dose titration was performed without suppression of IL-12p70 secretion when log higher and lower doses of patient-derived HIV were added (data not shown). When HIV was removed from the plasma from 4 untreated HIV infected individuals via ultracentrifugation, there was no significant effect on IL-12p70 secretion overall (Figure 3C). Within the individual moDC experiments performed using these 4 untreated plasma donors, occasional partial decreases and increases in IL-12p70 secretion were observed following ultracentrifugation of plasma (Figure 3C left). However, upon combining the data from each moDC donor (N=3) following normalization to whole plasma IL-12p70 secretion, no overall effect was detected in any of the four untreated HIV donors tested (Figure 3C right). No correlation of IL-12p70 levels (data displayed in Figure 1B) with viral load levels or CD4+T cell count of plasma donors was demonstrated via linear regression analysis (Figure 3D). The lack of correlation was consistent when data from each treatment group was analyzed separately. Additionally, no correlation with age or race (black vs non-black) with IL-12p70 secretion was found.
Figure 3. No evidence for direct viral-mediated suppression of moDC function.
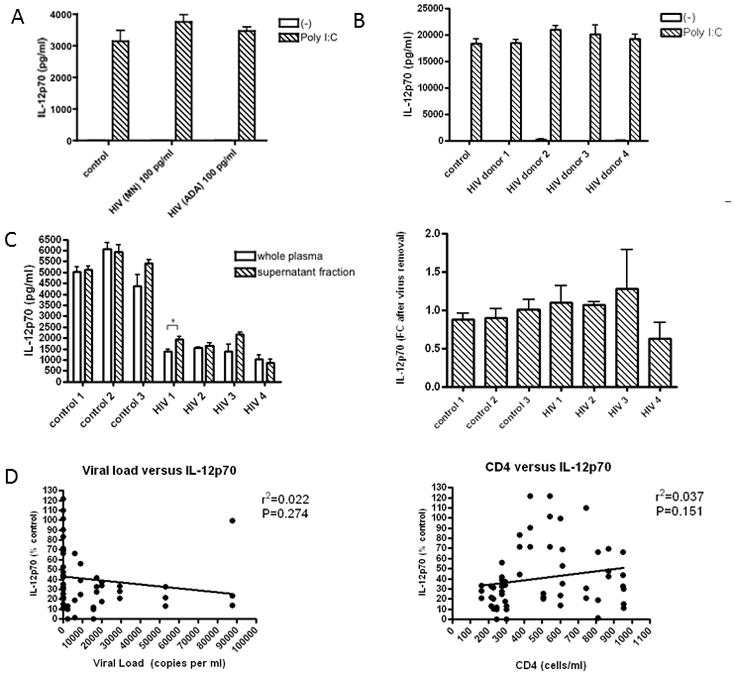
(A) MoDC from a single donor were exposed to laboratory strains of HIV (HIV-1MN (X4-tropic) and HIV-1ADA (R5-tropic)(100pg/ml) in 10% control donor plasma prior to stimulation with Poly I:C. (B) MoDC from a single donor were exposed to patient-derived strains of HIV (100pg/ml) from 4 untreated donors in 10% control donor plasma prior to stimulation. Data displayed in A and B are representative of experiments performed on 3–4 separate moDC donors. (C) HIV was removed from the plasma of 4 untreated HIV-infected donors via ultracentrifugation, and moDC from a single donor (left) were exposed to whole plasma versus the virus-free supernatant fraction from each plasma donor prior to stimulation (only stimulated conditions are shown). The asterisks indicate p values: *p < .05. This was repeated on 3 separate moDC donors and pooled data are displayed as fold change (FC) of IL-12p70 secretion of supernatant compared with whole plasma (right). (D) Correlation of IL-12p70 secretion from HIV plasma-exposed DC following Poly I:C stimulation (values displayed in Figure 1b) with viral load and CD4+ T cell count was evaluated via linear regression.
Decreased IKKβ expression in moDC exposed to plasma from untreated donors
In moDC exposed to control versus minimally suppressive cART donor plasma (N=3 per group), TLR pathway-specific qRT-PCR arrays revealed no significant differences in expression levels of the 84 pathway-specific genes assayed (fold change cut-off of >+/−3.0). In contrast, upon comparing untreated donors with controls, IKKβ (IKBKB) expression was lower via qRT-PCR (−5.37, p=0.029) in these suppressive plasma donors. No other significant differences were observed between controls and untreated donors of the remaining genes assayed (fold change cut-off of >+/−3.0). Decreased levels of IKKβ by moDC exposed to plasma from untreated donors compared to both control and the cART groups were confirmed by western blot (p<0.01) (Figure 4).
Figure 4. Decreased IKKβ expression in moDC exposed to plasma from untreated donors.
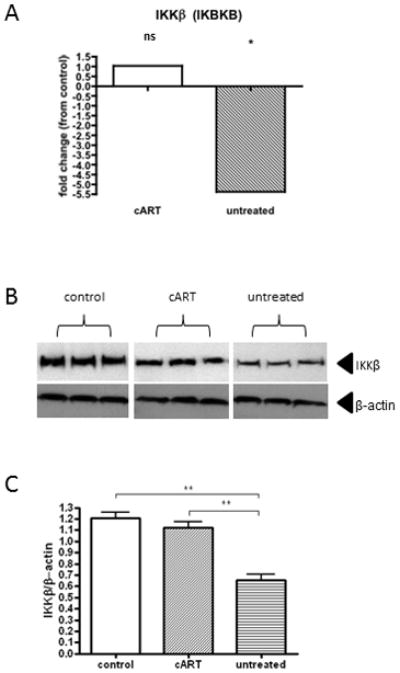
(A) TLR pathway-specific qRT-PCR array analysis of moDC exposed to control versus cART versus untreated plasma groups (N=3 per group) revealed that expression of IKKβ was significantly decreased in the untreated group, but not in cART group, compared with controls. (B+C) Western blot analysis of moDC exposed to control versus cART versus untreated plasma groups (N=3 per group) revealed that levels of IKKβ were significantly decreased in the untreated group compared with control and cART groups using analysis of variance followed by pairwise comparisons with two-tailed t-tests. The asterisks indicate p values: **p < .01, *p < .05.
Discussion
We report the presence of circulating plasma factors during chronic HIV infection that profoundly impair the secretion of IL-12 by moDC following TLR stimulation. In keeping with our finding of lower IL-12 secretion, we also observed impaired skewing of Th1 CD4+ T cells and small decreases in T cell proliferation by moDC exposed to plasma from untreated HIV-infected individuals. Due to the central role IL-12 plays in activation of both NK cells and type I cell mediated immunity21–23, this defect in expression likely impacts the formation of innate and adaptive immune responses during HIV infection not only to the virus itself, but to opportunistic pathogens and vaccines. Furthermore, these findings bear particular relevance to ongoing research regarding therapeutic vaccines for HIV that aim to target and stimulate myeloid dendritic cells in order to enhance T cell effector functions. Such vaccines, if successful, not only may be a means to improve viral control, but ultimately may comprise an important component of eradication strategies41. The importance of IL-12 in the formation of effective HIV-specific immunity has been previously elucidated in multiple studies. Enhancement of ex vivo HIV-specific CD4+ and CD8+ T cell responses 42–44, as well as inhibition of CD4+ T cell apoptosis from HIV-infection individuals has been demonstrated by the addition of IL-1245. Utilization of non-human primate models of SIV revealed that administration of IL-12 during infection increases NK cell numbers and lytic function, and can partially restore SIV-specific cytotoxic T lymphocyte function46, 47, whereas, preconditioning with IL-12 prior to SIV challenge lowered viral load set point and delayed progression of disease 48.
We found that inhibition of moDC was partially alleviated in patients receiving cART, but, surprisingly, no direct correlation to plasma donor CD4+ T cell count or viral load level was observed. Unfortunately, the relatively small sample size and diversity in cART regimens did not permit the evaluation of specific drug effects within this group. In keeping with the lack of correlation with viral load, we did not find evidence in our system that moDC dysfunction is directly mediated by the virus itself. The addition of both laboratory strains and patient-derived strains of HIV to control donor plasma did not suppress cytokine secretion, nor did the removal of virus from plasma from untreated HIV-infected donors reverse the suppression. This is in sharp contrast to previous reports that HIV directly modulates mDC responses to TLR ligands via binding to certain C-type lectins, including DC-SIGN, or that HIV proteins, such as gp120 and vpr, can inhibit mDC function9, 11, 13, 14, 17. Rather, our findings suggest that HIV infection indirectly inhibits mDC function possibly through increases in circulating inhibitory factors, or, alternatively, through depletion of factors necessary for mDC function. While it remains unclear why our findings differ from these cited studies, several key differences in experimental design may have contributed including genetic differences in the viruses, DC stimuli used, and cytokines assayed. Though these studies also evaluated the effect of HIV on human moDC derived from control donors, a prominent difference is our exposure of these cells to plasma from HIV-infected donors and patient-derived strains of HIV, whereas the cited work utilized laboratory strains of HIV or HIV proteins.
Some studies have shown that HIV and/or its proteins increases expression of IL-10, an antiinflammatory cytokine49, 50, leading to mDC suppression8, 11, 13, 17. High expression of IL-10 has been implicated in the suppression of IL-12 during HIV infection 5, 7, 26, though others have found IL-12 levels to be independent of IL-1025, 30. Consistent with these latter studies, we did not find evidence to support a role of IL-10 in our system. The plasma from HIV-infected individuals did not stimulate the production of IL-10 by mDC, nor were levels of IL-10 elevated in the plasma samples from HIV-infected donors (Supplemental Digital Content, Figure 4A and 4B). On Luminex analysis of the plasma samples, of the few factors where significant differences were detected between HIV groups versus controls, only a small increase in sCD40L and GRO were seen in the untreated donor plasma compared with cART (data not shown). The effects of these are unlikely to be responsible for the DC suppression observed. It remains possible that subtle differences amongst plasma factors were not found to be significant given the relatively small sample size.
Due to the disruption of immunity that occurs in the gut during HIV infection, much attention has been focused upon the potential immunomodulatory effects that elevated circulating products of microbial translocation, including LPS, may have on pathogenesis and disease progression51–54. In a tumor model, we have previously shown that TLR4 engagement on moDC can lead to decreases in cytokine secretion upon TLR3 stimulation37. However, we did not find LPS to be responsible for the suppression of cytokine secretion by moDC as we were unable to reverse the suppressive effect of HIV plasma with the addition of the LPS inhibitor, polymyxin B (Supplemental Digital Content, Figure 4C).
Taking into account our findings with the existing data, it is likely that mDC dysregulation during chronic HIV infection is multi-factorial, with a contribution from as yet unindentified factor(s). Several disturbances in plasma composition that exist during the course of HIV infection could potentially play a role including circulating immune complexes, acute phase reactants including activated complement, or upregulation of other inhibitory cytokines 55, 56. It also remains possible that HIV infection may result in the relative depletion of certain plasma factors that are necessary for mDC function. Future exploratory studies utilizing plasma fractionation systems may be useful in identifying the factor(s) that are involved in mDC suppression. Though our efforts continue to more clearly define these circulating factor(s), on a transcriptional level, we have identified that lower expression of IKKβ, a central molecule in the TLR/NF-kappaB signaling pathway that regulates secretion of inflammatory cytokines57, 58, corresponds with lower cytokine secretion by mDC. Based on these findings, further investigation of the role of IKKβ on mDC dysregulation during HIV infection and characterization of the circulating factor(s) leading to its suppression is warranted as targeted therapeutic strategies may be of use. Future efforts should be placed on the identification of adjuvants that are able to overcome the suppression of IL-12 secretion in mDC during HIV infection, as this will likely be a central issue for the success of therapeutic vaccination endeavors. Further elucidation of the mechanisms that underlie this suppression will aid in the rational development of these agents.
Supplementary Material
Acknowledgments
We would like to acknowledge Jeffrey Lifson (AIDS Vaccine Program, Frederick, Maryland, USA) for providing HIV-1 MN and HIV-1 ADA, Luis Vargas (NYU Aids Clinical Trial Unit) for study recruitment, and Judith Aberg (NYU Aids Clinical Trial Unit) for guidance. This work has been supported by National Institutes of Health [K08 AI84578 to E.A.M., R37 AI044628 to N.B., and U01 A1067854]; Bill and Melinda Gates Foundation [Collaboration for AIDS Vaccine Discovery Grant ID: 38645]; Center for AIDS Research [P01AI057127]; and the New York University Langone Medical Center Grunebaum AIDS Research Fund and Saul Farber Scholar Fund.
Footnotes
Conflicts of Interest
Possible conflict of interested declared by N.B. for receipt of small royalty (<$2000 annually) for a patent related to virus preparation. For the remaining authors none were declared.
Presentations
A portion of this data was presented at the Conference for Retroviruses and Opportunistic Infections (CROI) in 2011 (Boston) and 2012 (Seattle).
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998 Mar 19;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Almeida M, Cordero M, Almeida J, Orfao A. Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection. Aids. 2005 Feb 18;19(3):261–271. [PubMed] [Google Scholar]
- 3.Almeida M, Cordero M, Almeida J, Orfao A. Persistent abnormalities in peripheral blood dendritic cells and monocytes from HIV-1-positive patients after 1 year of antiretroviral therapy. J Acquir Immune Defic Syndr. 2006 Apr 1;41(4):405–415. doi: 10.1097/01.qai.0000209896.82255.d3. [DOI] [PubMed] [Google Scholar]
- 4.Almeida M, Cordero M, Almeida J, Orfao A. Abnormal cytokine production by circulating monocytes and dendritic cells of myeloid origin in ART-treated HIV-1+ patients relates to CD4+ T-cell recovery and HCV co-infection. Curr HIV Res. 2007 May;5(3):325–336. doi: 10.2174/157016207780636524. [DOI] [PubMed] [Google Scholar]
- 5.Buisson S, Benlahrech A, Gazzard B, Gotch F, Kelleher P, Patterson S. Monocyte-derived dendritic cells from HIV type 1-infected individuals show reduced ability to stimulate T cells and have altered production of interleukin (IL)-12 and IL-10. J Infect Dis. 2009 Jun 15;199(12):1862–1871. doi: 10.1086/599122. [DOI] [PubMed] [Google Scholar]
- 6.Chang JJ, Lacas A, Lindsay RJ, et al. Differential regulation of TLR pathways in acute and chronic HIV-1 infection. Aids. 2012 Dec 29; doi: 10.1097/QAD.0b013e32834f3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan Z, Huang XL, Kalinski P, Young S, Rinaldo CR., Jr Dendritic cell function during chronic hepatitis C virus and human immunodeficiency virus type 1 infection. Clin Vaccine Immunol. 2007 Sep;14(9):1127–1137. doi: 10.1128/CVI.00141-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granelli-Piperno A, Golebiowska A, Trumpfheller C, Siegal FP, Steinman RM. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proc Natl Acad Sci U S A. 2004 May 18;101(20):7669–7674. doi: 10.1073/pnas.0402431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodges A, Sharrocks K, Edelmann M, et al. Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nat Immunol. 2007 Jun;8(6):569–577. doi: 10.1038/ni1470. [DOI] [PubMed] [Google Scholar]
- 10.Lester RT, Yao XD, Ball TB, et al. Toll-like receptor expression and responsiveness are increased in viraemic HIV-1 infection. Aids. 2008 Mar 30;22(6):685–694. doi: 10.1097/QAD.0b013e3282f4de35. [DOI] [PubMed] [Google Scholar]
- 11.Majumder B, Janket ML, Schafer EA, et al. Human immunodeficiency virus type 1 Vpr impairs dendritic cell maturation and T-cell activation: implications for viral immune escape. J Virol. 2005 Jul;79(13):7990–8003. doi: 10.1128/JVI.79.13.7990-8003.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabado RL, O’Brien M, Subedi A, et al. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. 2010 Nov 11;116(19):3839–3852. doi: 10.1182/blood-2010-03-273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shan M, Klasse PJ, Banerjee K, et al. HIV-1 gp120 mannoses induce immunosuppressive responses from dendritic cells. PLoS Pathog. 2007 Nov;3(11):e169. doi: 10.1371/journal.ppat.0030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smed-Sorensen A, Lore K, Walther-Jallow L, Andersson J, Spetz AL. HIV-1-infected dendritic cells up-regulate cell surface markers but fail to produce IL-12 p70 in response to CD40 ligand stimulation. Blood. 2004 Nov 1;104(9):2810–2817. doi: 10.1182/blood-2003-07-2314. [DOI] [PubMed] [Google Scholar]
- 15.Yonkers NL, Rodriguez B, Asaad R, Lederman MM, Anthony DD. Systemic immune activation in HIV infection is associated with decreased MDC responsiveness to TLR ligand and inability to activate naive CD4 T-cells. PLoS One. 2011;6(9):e23884. doi: 10.1371/journal.pone.0023884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chougnet C, Cohen SS, Kawamura T, et al. Normal immune function of monocyte-derived dendritic cells from HIV-infected individuals: implications for immunotherapy. J Immunol. 1999 Aug 1;163(3):1666–1673. [PubMed] [Google Scholar]
- 17.Gringhuis SI, den Dunnen J, Litjens M, van Het Hof B, van Kooyk Y, Geijtenbeek TB. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity. 2007 May;26(5):605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Clerici M, Shearer GM. A TH1-->TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993 Mar;14(3):107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 19.Maggi E, Mazzetti M, Ravina A, et al. Ability of HIV to promote a TH1 to TH0 shift and to replicate preferentially in TH2 and TH0 cells. Science. 1994 Jul 8;265(5169):244–248. doi: 10.1126/science.8023142. [DOI] [PubMed] [Google Scholar]
- 20.Meroni L, Trabattoni D, Balotta C, et al. Evidence for type 2 cytokine production and lymphocyte activation in the early phases of HIV-1 infection. Aids. 1996 Jan;10(1):23–30. doi: 10.1097/00002030-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 22.D’Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993 Sep 1;178(3):1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf SF, Temple PA, Kobayashi M, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991 May 1;146(9):3074–3081. [PubMed] [Google Scholar]
- 24.Martinson JA, Roman-Gonzalez A, Tenorio AR, et al. Dendritic cells from HIV-1 infected individuals are less responsive to toll-like receptor (TLR) ligands. Cell Immunol. 2007 Nov-Dec;250(1–2):75–84. doi: 10.1016/j.cellimm.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chehimi J, Starr SE, Frank I, et al. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J Exp Med. 1994 Apr 1;179(4):1361–1366. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chougnet C, Wynn TA, Clerici M, et al. Molecular analysis of decreased interleukin-12 production in persons infected with human immunodeficiency virus. J Infect Dis. 1996 Jul;174(1):46–53. doi: 10.1093/infdis/174.1.46. [DOI] [PubMed] [Google Scholar]
- 27.Harrison TS, Levitz SM. Role of IL-12 in peripheral blood mononuclear cell responses to fungi in persons with and without HIV infection. J Immunol. 1996 Jun 1;156(11):4492–4497. [PubMed] [Google Scholar]
- 28.Harrison TS, Levitz SM. Priming with IFN-gamma restores deficient IL-12 production by peripheral blood mononuclear cells from HIV-seropositive donors. J Immunol. 1997 Jan 1;158(1):459–463. [PubMed] [Google Scholar]
- 29.Marshall JD, Chehimi J, Gri G, Kostman JR, Montaner LJ, Trinchieri G. The interleukin-12- mediated pathway of immune events is dysfunctional in human immunodeficiency virusinfected individuals. Blood. 1999 Aug 1;94(3):1003–1011. [PubMed] [Google Scholar]
- 30.Meyaard L, Hovenkamp E, Pakker N, van der Pouw Kraan TC, Miedema F. Interleukin-12 (IL-12) production in whole blood cultures from human immunodeficiency virus-infected individuals studied in relation to IL-10 and prostaglandin E2 production. Blood. 1997 Jan 15;89(2):570–576. [PubMed] [Google Scholar]
- 31.Paganin C, Frank I, Trinchieri G. Priming for high interferon-gamma production induced by interleukin-12 in both CD4+ and CD8+ T cell clones from HIV-infected patients. J Clin Invest. 1995 Sep;96(3):1677–1682. doi: 10.1172/JCI118209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanham G, Penne L, Fransen K, Kestens L, De Brabander M. HIV-associated dysfunction of in vitro IL-12 production depends on the nature of the stimulus and on the CD4 T-cell count of the patient. Blood. 2000 Mar 15;95(6):2185–2187. [PubMed] [Google Scholar]
- 33.Mirani M, Elenkov I, Volpi S, Hiroi N, Chrousos GP, Kino T. HIV-1 protein Vpr suppresses IL-12 production from human monocytes by enhancing glucocorticoid action: potential implications of Vpr coactivator activity for the innate and cellular immunity deficits observed in HIV-1 infection. J Immunol. 2002 Dec 1;169(11):6361–6368. doi: 10.4049/jimmunol.169.11.6361. [DOI] [PubMed] [Google Scholar]
- 34.Taoufik Y, Lantz O, Wallon C, Charles A, Dussaix E, Delfraissy JF. Human immunodeficiency virus gp120 inhibits interleukin-12 secretion by human monocytes: an indirect interleukin-10-mediated effect. Blood. 1997 Apr 15;89(8):2842–2848. [PubMed] [Google Scholar]
- 35.Trumpfheller C, Caskey M, Nchinda G, et al. The microbial mimic poly IC induces durable and protective CD4+ T cell immunity together with a dendritic cell targeted vaccine. Proc Natl Acad Sci U S A. 2008 Feb 19;105(7):2574–2579. doi: 10.1073/pnas.0711976105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vagenas P, Aravantinou M, Williams VG, et al. A tonsillar PolyICLC/AT-2 SIV therapeutic vaccine maintains low viremia following antiretroviral therapy cessation. PLoS One. 2010;5(9):e12891. doi: 10.1371/journal.pone.0012891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bogunovic D, Manches O, Godefroy E, et al. TLR4 engagement during TLR3-induced proinflammatory signaling in dendritic cells promotes IL-10-mediated suppression of antitumor immunity. Cancer Res. 2011 Aug 15;71(16):5467–5476. doi: 10.1158/0008-5472.CAN-10-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rouas R, Lewalle P, El Ouriaghli F, Nowak B, Duvillier H, Martiat P. Poly(I:C) used for human dendritic cell maturation preserves their ability to secondarily secrete bioactive IL-12. Int Immunol. 2004 May;16(5):767–773. doi: 10.1093/intimm/dxh077. [DOI] [PubMed] [Google Scholar]
- 39.Zobywalski A, Javorovic M, Frankenberger B, et al. Generation of clinical grade dendritic cells with capacity to produce biologically active IL-12p70. J Transl Med. 2007;5:18. doi: 10.1186/1479-5876-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piatak M, Jr, Saag MS, Yang LC, et al. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993 Mar 19;259(5102):1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- 41.Shan L, Deng K, Shroff NS, et al. Stimulation of HIV-1-Specific Cytolytic T Lymphocytes Facilitates Elimination of Latent Viral Reservoir after Virus Reactivation. Immunity. 2012 Mar 23;36(3):491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clerici M, Lucey DR, Berzofsky JA, et al. Restoration of HIV-specific cell-mediated immune responses by interleukin-12 in vitro. Science. 1993 Dec 10;262(5140):1721–1724. doi: 10.1126/science.7903123. [DOI] [PubMed] [Google Scholar]
- 43.Dybul M, Mercier G, Belson M, et al. CD40 ligand trimer and IL-12 enhance peripheral blood mononuclear cells and CD4+ T cell proliferation and production of IFN-gamma in response to p24 antigen in HIV-infected individuals: potential contribution of anergy to HIV-specific unresponsiveness. J Immunol. 2000 Aug 1;165(3):1685–1691. doi: 10.4049/jimmunol.165.3.1685. [DOI] [PubMed] [Google Scholar]
- 44.Landay AL, Clerici M, Hashemi F, Kessler H, Berzofsky JA, Shearer GM. In vitro restoration of T cell immune function in human immunodeficiency virus-positive persons: effects of interleukin (IL)-12 and anti-IL-10. J Infect Dis. 1996 May;173(5):1085–1091. doi: 10.1093/infdis/173.5.1085. [DOI] [PubMed] [Google Scholar]
- 45.Estaquier J, Idziorek T, Zou W, et al. T helper type 1/T helper type 2 cytokines and T cell death: preventive effect of interleukin 12 on activation-induced and CD95 (FAS/APO-1)-mediated apoptosis of CD4+ T cells from human immunodeficiency virus-infected persons. J Exp Med. 1995 Dec 1;182(6):1759–1767. doi: 10.1084/jem.182.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villinger F, Bucur S, Chikkala NF, et al. In vitro and in vivo responses to interleukin 12 are maintained until the late SIV infection stage but lost during AIDS. AIDS Res Hum Retroviruses. 2000 May 20;16(8):751–763. doi: 10.1089/088922200308756. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe N, Sypek JP, Mittler S, et al. Administration of recombinant human interleukin 12 to chronically SIVmac-infected rhesus monkeys. AIDS Res Hum Retroviruses. 1998 Mar 20;14(5):393–399. doi: 10.1089/aid.1998.14.393. [DOI] [PubMed] [Google Scholar]
- 48.Ansari AA, Mayne AE, Sundstrom JB, et al. Administration of recombinant rhesus interleukin-12 during acute simian immunodeficiency virus (SIV) infection leads to decreased viral loads associated with prolonged survival in SIVmac251-infected rhesus macaques. J Virol. 2002 Feb;76(4):1731–1743. doi: 10.1128/JVI.76.4.1731-1743.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aste-Amezaga M, Ma X, Sartori A, Trinchieri G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J Immunol. 1998 Jun 15;160(12):5936–5944. [PubMed] [Google Scholar]
- 50.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006 Dec;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 52.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008 Jan 1;197(1):126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang W, Lederman MM, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009 Apr 15;199(8):1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pandrea I, Gaufin T, Brenchley JM, et al. Cutting edge: Experimentally induced immune activation in natural hosts of simian immunodeficiency virus induces significant increases in viral replication and CD4+ T cell depletion. J Immunol. 2008 Nov 15;181(10):6687–6691. doi: 10.4049/jimmunol.181.10.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta S, Licorish K. Circulating immune complexes in AIDS. N Engl J Med. 1984 Jun 7;310(23):1530–1531. doi: 10.1056/NEJM198406073102312. [DOI] [PubMed] [Google Scholar]
- 56.Kramer HB, Lavender KJ, Qin L, et al. Elevation of intact and proteolytic fragments of acute phase proteins constitutes the earliest systemic antiviral response in HIV-1 infection. PLoS Pathog. 2010 May;6(5):e1000893. doi: 10.1371/journal.ppat.1000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997 Oct 17;91(2):243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 58.Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004 May;5(5):392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


