Abstract
Objective
The natural history of patients with Metabolic Syndrome (MetS) undergoing hemodialysis access placement is unknown. MetS has previously been found as a risk factor for poor outcomes for vascular surgery patients undergoing other interventions. The aim of this is study is to describe the outcomes of MetS patients undergoing primary hemodialysis access placement.
Methods
The medical records of all patients undergoing arteriovenous fistula placement between 1999 and 2009 at the VA Connecticut Healthcare system were reviewed (n=187). Survival, primary patency, and secondary patency were evaluated using the Gehan-Breslow test for survival. MetS was defined as the presence of three or more of the following: blood pressure ≥130 mmHg/≥90 mmHg; triglycerides ≥150 mg/dl; high-density lipoproteins (HDL) ≤50 mg/dl for women and ≤40 mg/dl for men; Body Mass Index (BMI) ≥30 kg/m2; fasting blood glucose ≥110 mg/dl.
Results
One hundred and eighty-seven patients underwent hemodialysis access placement. One hundred and fifteen (61%) of the patients were identified to have MetS. The distribution of MetS factors among all patients were: 98% were hypertensive.; 58% patients were diabetic; 39% patients had elevated triglycerides; 60% of patients had decreased HDL; 36% of patients had an elevated BMI. 39% of patients were currently receiving hemodialysis. The mean age of patients was 66 years. The median length of follow-up was 4.2 years. The forearm was site of fistula placement in 53% of patients; no difference existed between groups (57% for MetS, 50% for No MetS, p = 0.388). The median time to primary failure was 0.46 years for all patients (0.555 years[No MetS], 0.436 years [MetS]; p = 0.255). Secondary patency was 50% at 1.18 years for all patients (1.94 years [No MetS], 0.72 years [MetS]; p = 0.024). Median survival duration for all patients was 4.15 years (5.07 years [No MetS], 3.63 years [MetS]; p = 0.019).
Conclusion
MetS is prevalent among patients undergoing hemodialysis access placement. Patients with MetS have equivalent primary patency rates, however their survival and cumulative patency rates are significantly lower compared to those patients without MetS. Patients with MetS form a high-risk group of patients that need intensive surveillance protocols.
Keywords: Hemodialysis access, renal insufficiency, metabolic syndrome
Introduction
Renal disease in the United States is prevalent, morbid, costly, and associated with significant mortality.i,ii Autogenous arteriovenous (AV) fistula placement affords the most durable and preferred mode of access for hemodialysis. However, despite the superior results of AV fistulae compared to the alternatives, AV fistulae fail to mature in 20–50% of cases and posses early failure rates with 1-year primary patency of 60–65%.iii
Metabolic Syndrome (MetS) consists of atherogenic dyslipidemia, hypertension, hyperglycemia, a prothrombotic state, and a proinflammatory stateiv, and MetS affects 20–30% of the overall population.v The Framingham Heart Study found the prevalence of MetS doubled over a ten-year period and was associated with a 2.36-fold increased rate of cardiovascular events and a 3-fold increased risk in mortality.vi Patients with MetS have been shown to have unfavorable perioperative outcomes for cardiovascularvii and peripheral vascular interventionsviii,ix, as well as non-vascular operations.x MetS increases the risk of both postoperative saphenous vein graft occlusion and development of a significant lesion after coronary artery bypass grafting.xi
Although patients with lipid abnormalitiesxii and diabetes mellitusxiii have been identified as having greater rates of access dysfunction, surprisingly, the influence of MetS on hemodialysis access placement has not been specifically described in the literature. The primary objective of this study is to analyze the short-term and long-term outcomes of patients undergoing AV fistula placement in the presence of MetS. We hypothesize metabolic syndrome will negatively effect long-term hemodialysis access patency.
Methods
Study design
A database of patients undergoing primary hemodialysis access placement from 1999 to 2009 at the VA Connecticut Healthcare System, West Haven, CT, was queried. If a patient had two or more access placement attempts during the study period, only data pertaining to the initial operation was utilized. The study excluded access sites placed in a patient’s lower extremity. Medical records were reviewed through December 2011 for follow-up data. The Social Security Death Index was utilized to assist in determining post-operative mortality.
For each patient, demographics, comorbidities, and perioperative outcomes were identified. Demographics included age, gender, and race. Comorbidities included the preoperative presence of myocardial infarction history, congestive heart failure, atrial fibrillation, hypertension, diabetes mellitus, hypothyroidism, cancer history, prior tobacco usage, current tobacco usage, current hemodialysis, HIV positive, intravenous drug abuse, and hepatitis C. Patient’s medical records were reviewed for serum triglyceride, high-density lipoprotein cholesterol, total cholesterol, hemoglobin A1c, height, and weight.
Procedures
Selection of hemodialysis access location (forearm versus upper arm) and conduit choice (autogenous versus non-autogenous) was left to the discretion of the surgeon.
Definitions
MetS was defined based on the presence of three or more of the following five criteria: hypertension (systolic blood pressure >140 mm Hg or diastolic pressure >90mmHg on three occasions during a 6-month period), reduced high-density lipoprotein cholesterol (<40 mg/dL for men, <50 mg/dL for women), elevated triglycerides (>150 mg/dL), impaired glucose control (>110 mg/dl fasting serum glucose), and a body mass index (BMI), >30.0 kg/m2.
Primary patency, secondary patency, and survival were defined using current Society of Vascular Surgery criteria.xiv Primary patency was defined as the interval from the time of access placement until any intervention designed to maintain or reestablish patency, access thrombosis, or the time of measurement of patency. Secondary patency was defined as the interval from the time of access placement until access abandonment, thrombosis, or the time of patency measurement including interventions performed for reestablishing functionality of thrombosed access. Patients with hemodialysis access that failed to mature were included in the final analysis.
Statistical Analysis
Measured values are reported as percentages or means +/− standard deviations (SDs). Rates for comorbidities, complications, and 30-day outcomes were compared between patients with MetS and without MetS (No-MetS) by χ2 test. Survival, primary patency, and secondary patency were calculated using Kaplan-Meier analysis using the Gehan-Breslow test. Standard errors are reported in Kaplan-Meier analyses. Cox proportional hazards model was used to examine the associations between MetS and primary patency, secondary patency, and survival. Analyses were performed using JMP software version 9.0 (SAS Institute, Cary, North Carolina).
Results
Patient Population
One hundred and eight-seven hemodialysis access sites were placed during the study period (Table I). One hundred and eighty-four (98.4%) of the patients were male. The mean age was 66 ±12 years (range: 35 to 90 years). One hundred and thirty-one patients (70%) were Caucasian. The median follow-up was 4.2 years.
Table I.
Patient Demographics and Comorbidities.
| All Patients | No-MetS | MetS | P value | |
|---|---|---|---|---|
| Demographics | ||||
| Patients | 187 | 72 | 115 | - |
| Male | 185 | 99% | 98% | 0.85 |
| Age (years) | 66.1 ±11.6 | 66.9 ±12.4 | 65.7 ±11.0 | 0.47 |
| Caucasian | 70% | 63% | 75% | 0.075 |
| Comorbidities | ||||
| Myocardial Infarction History | 19% | 11% | 24% | 0.025 |
| Congestive Heart Failure | 25% | 21% | 28% | 0.29 |
| Atrial Fibrillation | 12% | 10% | 14% | 0.40 |
| COPD | 12% | 11% | 12% | 0.83 |
| Hypertension | 98% | 96% | 99% | 0.13 |
| IDDM | 11% | 10% | 12% | 0.61 |
| NIDDM | 47% | 17% | 65% | <0.0001* |
| DM | 57% | 26% | 76% | <0.0001* |
| Hypothyroidism | 7% | 7% | 8% | 0.83 |
| Past Tobacco Usage | 33% | 38% | 30% | 0.26 |
| Current Tobacco Usage | 16% | 21% | 12% | 0.11 |
| Cancer History | 17% | 15% | 17% | 0.71 |
| Current Hemodialysis | 39% | 40% | 38% | 0.79 |
| HIV Positivity | 4% | 1% | 6% | 0.12 |
| IV Drug Abuse | 7% | 7% | 8% | 0.83 |
| Hepatitis C | 13% | 13% | 14% | 0.78 |
| Surgical Variables | ||||
| Prosthetic Conduit | 6% | 7% | 6% | 0.82 |
| Forearm Location | 53% | 57% | 50% | 0.39 |
MetS, Metabolic Syndrome; No-MetS, No Metabolic Syndrome; COPD, Chronic Obstructive Pulmonary Disease; IDDM, Insulin-Dependent Diabetes Mellitus; NIDDM, Non Insulin-Dependent Diabetes Mellitus; DM, Diabetes Mellitus.
Significant.
Comorbidities
Patients with MetS had a significantly higher rate of myocardial infarction history (24% versus 11%, P = 0.025; Table I). The two groups (MetS versus No-MetS) had equivalent rates of congestive heart failure (28% versus 21%, P = 0.286), atrial fibrillation (14% versus 10%, P = 0.396), hypertension (99% versus 96%, P = 0.129), and chronic obstructive pulmonary disease (11% versus 12%, P = 0.826). MetS patients were more likely to have diabetes mellitus (76% versus 26%, P < 0.0001). Both groups had equivalent rates of hypothyroidism (78% versus 69%, P = 0.824), documented past tobacco usage (30% versus 38%, P = 0.26), documented current tobacco usage (12% versus 21%, P = 0.111), and cancer history (17% versus 15%, P = 0.705). No difference was identified with respect to HIV positivity (6% versus 1%, P = 0.122), prior intravenous drug abuse (8% versus 7%, P = 0.824), and hepatitis C (14% versus 13%, P = 0.782). The two groups had equivalent rates of current hemodialysis usage (38% versus 40%, P = 0.783) as well as prior peritoneal dialysis usage (0% versus 3%, P = 0.072).
The most common etiology for renal failure among all patients was a combination of hypertension and diabetes mellitus (42%), followed by hypertension alone (33%), diabetes mellitus alone (12%), and polycystic kidney disease (5%). The remaining etiologies for renal failure included one to two occurrences of the following: lithium toxicity, contrast-induced nephropathy, HIV nephropathy, ciprofloxacin toxicity, cyclosporine toxicity, lupus, IgA nephropathy, focal segmental glomerulosclerosis, and membranoproliferative glomerulonephritis.
A difference between groups did exist with respect to the etiologies of renal failure. As expected, the MetS patients had higher rates of DM-induced renal failure (15.7% vs 5.5%, p = 0.029), as well as the combination of hypertension- & DM-induced renal failure (53.9% vs 22.2%, p = <0.001). On the other hand, the No-MetS group had a higher rate of hypertension alone-induced renal failure (59.7% vs 15.7%, p <0.001). No difference existed between groups with respect to polycystic kidney disease or any of the other etiologies that existed in only 1–2 patients.
Metabolic Syndrome Status
One hundred and fifteen (61%) of the patients were found to have MetS (Table II). The distribution of MetS scores was: 5 (10%), 4 (23%), 3 (28%), 2 (25%), 1 (12%), and 0 (1%). Of the five factors determining MetS, hypertension was the most prevalent, 98% of patients. Sixty percent of patients had serum HDL levels less than 40 mg/dl. Fifty-eight percent of patients had an elevated fasting blood sugar. Thirty-nine percent of the patients had serum triglyceride levels greater than 150 mg/dl. Thirty-six percent of patients had a BMI greater than 30 kg/m2. Between groups, the MetS group exhibited greater rates of serum triglyceride >150mg/dl (54% versus 14%, P < 0.0001), serum HDL <40 mg/dl (85% versus 19%, P < 0.0001), BMI >30 kg/m2 (54% versus 7%, P < 0.0001), and elevated fasting blood glucose (78% versus 26%, P < 0.0001). However, the two groups had equivalent rates of hypertension, MetS with 99% and No-MetS with 96%, P = 0.129. Of those patients identified as having metabolic syndrome, the most common triad of factors, which was present in 64% of the MetS patients, was hypertension (99%), decreased HDL cholesterol (85%), and elevated fasting blood glucose (78%).
Table II.
Components of Metabolic Syndrome.
| All Patients | No-MetS | MetS | P value | |
|---|---|---|---|---|
| Serum Triglyceride | ||||
| Mean (mg/dl) | 155.2 ±7.2 | 110.7 ±7.8 | 182.2 ±9.7 | <0.0001* |
| >150 mg/dl | 39% | 14% | 54% | <0.0001* |
| Serum HDL Cholesterol | ||||
| Mean (mg/dl) | 40.2 ±1.3 | 51.2 ±2.6 | 33.6 ±0.9 | <0.0001* |
| <40, 50 mg/dl | 60% | 19% | 85% | <0.0001* |
| Hypertension | 98% | 96% | 99% | .010 |
| Elevated Blood Glucose | 58% | 26% | 78% | <0.0001* |
| BMI >30kg/m2 | 36% | 7% | 54% | <0.0001* |
| Total MetS Score | 2.9 ±0.09 | 1.6 ±0.06 | 3.7 ±0.07 | <0.0001* |
MetS, Metabolic Syndrome; No-MetS, No Metabolic Syndrome; HDL, High Density Lipoprotein; BMI, Body Mass Index.
Significant.
Procedures
One hundred and seventy-five (94%) of the hemodialysis access sites were autogenous, where as 6% were prosthetic. No difference in conduit type was identified between groups (MetS, 6% versus No-MetS, 7%, P = 0.817; Table III). Ninety-nine (53%) of the access sites placed were located in the forearm. No difference in upper extremity location was identified between groups (MetS, 57% versus No-MetS, 50%, P = 0.388).
Table III.
Surgical Variables and Outcomes.
| All Patients | No-MetS | MetS | P value | |
|---|---|---|---|---|
| 30-Day Outcomes | ||||
| Primary Patency Failure | 5% | 4% | 5% | 0.75 |
| Secondary Patency Failure | 4% | 4% | 3% | 0.81 |
| Death Event | 0.5% | 1% | 0% | 0.21 |
MetS, Metabolic Syndrome; No-MetS, No Metabolic Syndrome.
Outcomes
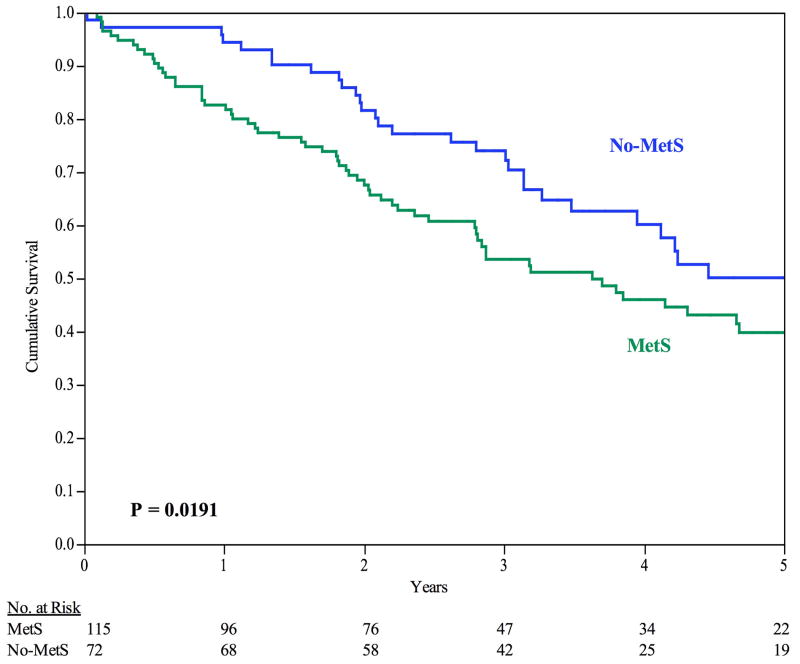
The 30-day survival rate was 99%, 2 deaths. The median time until death was 4.15 years (95% CI: 3.19 to 5.07 years). Patients with MetS exhibited increased mortality during long-term follow up with a median survival time of 3.63 years versus 5.07 years (P = 0.019), MetS versus No-MetS, respectively, see Figure I. The 6-, 12-, and 24-month survival rates were 91%, 83%, and 69% for MetS patients, respectively, and 97%, 94%, and 82% for No-MetS patients, respectively (P = 0.019).
Figure I.
Survival. For all patients, the 6-month, 12-month, and 24-month survival rates are 93%, 87%, and 73%, respectively. By Kaplan-Meier analysis, MetS patients experienced decreased freedom from secondary patency failure. Error bars are omitted for clarity. Standard error did not exceed 10% at all time intervals analyzed.
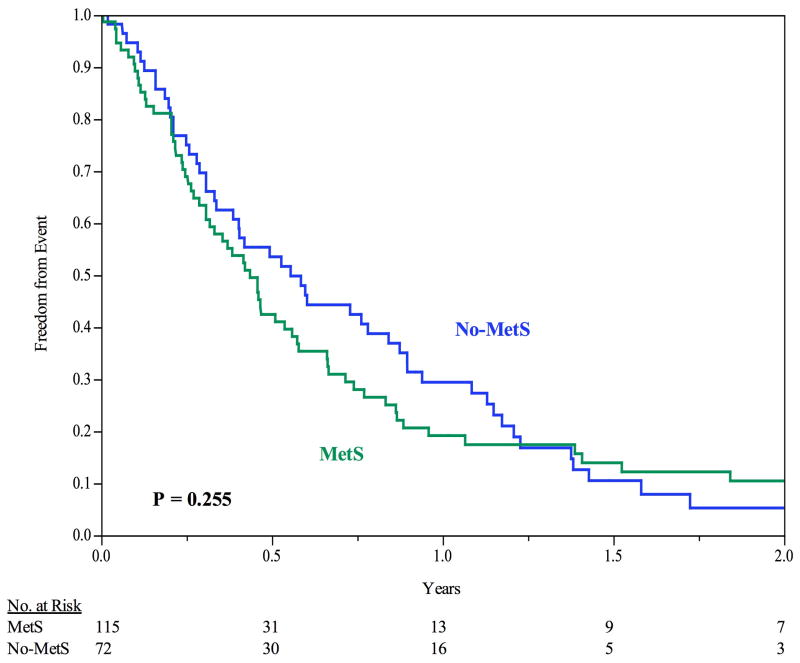
The 30-day primary patency rate was 93.1%. The median time until failure of primary patency was 0.460 years (95% CI: 0.37 to 0.58 yrs). No difference was demonstrated between groups with respect to primary patency, median time to primary patency failure was 0.436 years for MetS and 0.555 years for No-MetS, P = 0.26), see Figure II. The 6-, 12-, and 24-month freedom from primary patency failure was 43%, 19%, and 9%, respectively, for MetS, and 54%, 29%, and 5%, respectively, for No-MetS (P = 0.255). The majority of access failures occurred in the venous outflow (91%), with 18% of failures having an arterial lesion and 9% having both an arterial and a venous lesion; there was no difference between patients with and without MetS.
Figure II.
Freedom from Primary Patency Failure. For all patients, the 6-month, 12-month, and 24-month freedom from primary patency failure is 47%, 24%, and 8%, respectively. No difference identified between groups. Error bars are omitted for clarity. Standard error did not exceed 10% at all time intervals analyzed.
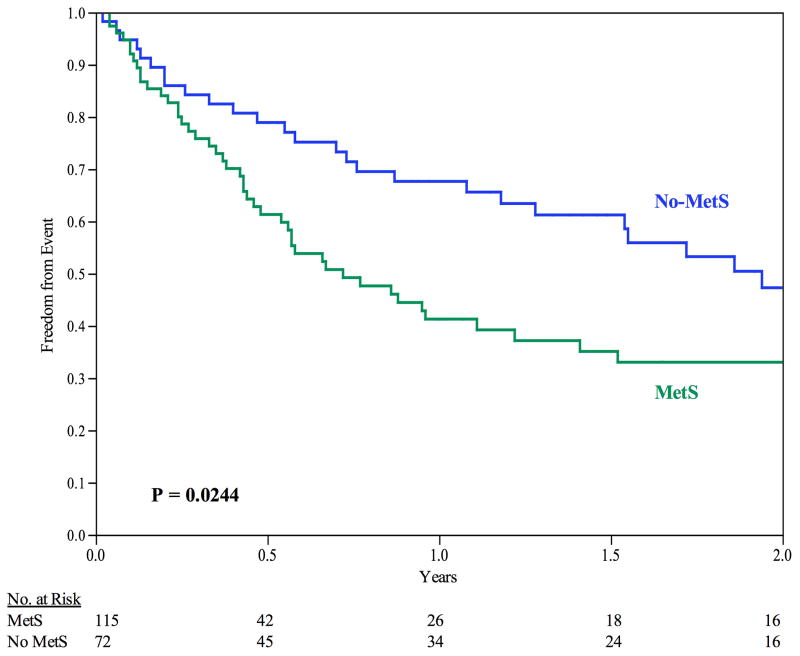
The 30-day secondary patency rate was 94.7%. The median time until failure of secondary patency was 1.180 years (95% CI: 0.73 to 1.86 years). Patients with MetS demonstrated failure of secondary patency at an earlier time point (0.720 years) than compared to those with No-MetS (1.940 years), P = 0.024, see Figure III. The 6-, 12-, and 24-month freedom from secondary patency failure was 61%, 41%, and 33%, respectively for MetS, and 79%, 68%, and 47%, respectively for No-MetS (P = 0.024).
Figure III.
Freedom from Secondary Patency Failure. For all patients, the 6-month, 12-month, and 24-month freedom from primary patency failure is 69%, 53%, and 38%, respectively. By Kaplan-Meier analysis, MetS patients experienced decreased freedom from secondary patency failure. Error bars are omitted for clarity. Standard error did not exceed 10% at all time intervals analyzed.
Influence of MetS Score
The severity of MetS score was associated with unfavorable outcomes. Patients with a MetS score of 4 or 5 exhibited decreased survival rates compared to those with a score of 3 or 0–2; the median survival time was 2.87 yrs, 4.31 yrs, and 5.07 yrs, respectively (P = 0.0143), and the median time to failure of secondary patency was 0.58 yrs, 2.67 yrs, and 1.94 yrs, respectively (P = 0.0065). No difference existed between groups with respect to primary patency.
Hazard Analysis
Of the patient demographics, comorbidities, and surgical variables, only one factor was identified as having an effect on primary patency: hepatitis C, HR: 0.47 (95% CI: 0.21– 0.96), see Table IV. For secondary patency, only the presence of metabolic syndrome was identified as having an effect, HR: 1.65 (95% CI: 1.05 – 2.62), see Table V. For overall survival, the presence of metabolic syndrome (HR: 1.53, 95% CI: 1.01 – 2.35), congestive heart failure (HR: 1.61, 95% CI: 1.05 – 2.44), and atrial fibrillation (HR: 1.83, 95% CI: 1.03 – 3.08) to be deleterious, while history of IV drug abuse (HR: 0.34, 0.10 – 0.88) and hypertension (HR: 0.21, 95% CI: 0.07 – 0.89) were protective, see Table VI.
Table IV.
Cox Proportional Hazard Analysis: Factors Affecting Primary Patency Failure.
| P value | Hazard Ratio | 95.0% CI for Hazard Ratio | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Metabolic Syndrome Present | 0.65 | 1.09 | 0.74 | 1.61 |
| Myocardial Infarction History | 0.89 | 0.96 | 0.55 | 1.61 |
| Congestive Heart Failure | 0.20 | 1.34 | 0.85 | 2.06 |
| Atrial Fibrillation | 0.50 | 1.22 | 0.67 | 2.08 |
| COPD | 0.97 | 0.99 | 0.53 | 1.72 |
| HIV Positivity | 0.11 | 2.33 | 0.80 | 5.69 |
| IV Drug Abuse | 0.81 | 0.90 | 0.38 | 2.02 |
| Hepatitis C | 0.04* | 0.47 | 0.21 | 0.96 |
| Hypertension | 0.65 | 1.32 | 0.45 | 5.68 |
| Cancer History | 0.31 | 0.77 | 0.46 | 1.26 |
| Upper Arm | 0.23 | 0.79 | 0.54 | 1.16 |
| AV Graft | 0.88 | 0.94 | 0.42 | 1.94 |
COPD, Chronic Obstructive Pulmonary Disorder; HIV, Human Immunodeficiency Virus; IV, Intravenous; AV, Arteriovenous;
Significant.
Table V.
Cox Proportional Hazard Analysis: Factors Affecting Secondary Patency Failure.
| P value | Hazard Ratio | 95.0% CI for Hazard Ratio | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Metabolic Syndrome Present | 0.03* | 1.65 | 1.05 | 2.62 |
| Myocardial Infarction History | 0.58 | 0.83 | 0.40 | 1.60 |
| Congestive Heart Failure | 0.80 | 0.93 | 0.54 | 1.55 |
| Atrial Fibrillation | 0.23 | 1.47 | 0.77 | 2.62 |
| COPD | 0.37 | 1.39 | 0.65 | 2.68 |
| HIV Positivity | 0.08 | 0.35 | 0.09 | 1.12 |
| IV Drug Abuse | 0.32 | 1.66 | 0.60 | 4.23 |
| Hepatitis C | 0.07 | 0.44 | 0.16 | 1.06 |
| Hypertension | 0.90 | 1.11 | 0.30 | 7.28 |
| Cancer History | 0.87 | 1.05 | 0.57 | 1.86 |
| Upper Arm | 0.36 | 0.81 | 0.51 | 1.28 |
| AV Graft | 0.32 | 1.57 | 0.63 | 3.49 |
COPD, Chronic Obstructive Pulmonary Disorder; HIV, Human Immunodeficiency Virus; IV, Intravenous; AV, Arteriovenous;
Significant.
Table VI.
Cox Proportional Hazard Analysis: Factors Affecting Survival.
| P value | Hazard Ratio | 95.0% CI for Hazard Ratio | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Metabolic Syndrome Present | 0.045* | 1.53 | 1.01 | 2.35 |
| Myocardial Infarction History | 0.52 | 1.18 | 0.70 | 1.90 |
| Congestive Heart Failure | 0.031* | 1.61 | 1.04 | 2.44 |
| Atrial Fibrillation | 0.040* | 1.83 | 1.03 | 3.08 |
| COPD | 0.51 | 0.81 | 0.41 | 1.46 |
| HIV Positivity | 0.18 | 0.32 | 0.02 | 1.53 |
| IV Drug Abuse | 0.024* | 0.34 | 0.10 | 0.88 |
| Hepatitis C | 0.20 | 0.62 | 0.26 | 1.26 |
| Hypertension | 0.037* | 0.21 | 0.07 | 0.89 |
| Cancer History | 0.69 | 1.11 | 0.65 | 1.82 |
COPD, Chronic Obstructive Pulmonary Disorder; HIV, Human Immunodeficiency Virus; IV, Intravenous;
Significant.
A hazard analysis consisting of only the five individual factors constituting MetS revealed elevated blood glucose and elevated BMI as independent risk factors for either mortality or cumulative patency failure. In particular, diabetes mellitus was found as a significant risk factor for mortality (RR: 1.73; P = 0.008) and cumulative patency failure (RR: 1.62; P = 0.032). An elevated BMI >30 kg per m2 was found as a significant risk factor only for primary patency failure (RR: 1.63; P = 0.029).
Discussion
In this study, we characterized the prevalence of MetS and assessed its relation to the short- and long-term outcomes for 187 patients undergoing first-time hemodialysis access placement in the upper extremity. We found MetS to be highly prevalent in our patient population, 61%, which is similar to the prevalence of MetS found by Gorter et al for patients with peripheral arterial disease.xv To our knowledge, no other study has examined the prevalence of MetS in patients undergoing hemodialysis access placement, or the long-term outcomes in these patients. MetS was found to have no influence upon primary patency rates, however, MetS patients had significantly greater mortality and secondary patency failure events. Patients with No-MetS exhibited a median survival time of 1.44 years greater and a more than double length of time for freedom from secondary patency failure. While a difference existed with respect to long-term patency rates between groups, we did not identify differences between groups with respect to the etiology of access failure.
MetS results in a state of platelet dysfunction, endothelial dysfunction, and a prothomobtic environment. Several inflammatory markers are increased in MetS: MCP-1, TNF-α, IL-6, IL-8, PAI-1.4 Prior to the conception of metabolic syndrome, De Marchi et al found patients who presented with fistula dysfunction post-operatively to have elevated levels of IL-6, MCP-1, PAI-1, Protein C, and Protein S compared to those patients without fistula dysfunction.12 Not surprisingly, these patients were also found to have elevated cholesterol and triglyceride levels and lower HDL levels. As IL-6, MCP-1, PAI-1, and dyslipidemia are hallmark characteristics of MetS, our long-term findings of AV fistula failure are consistent with their short-term fistula dysfunction findings.
Several definitions of MetS are currently in use in the literature.xvi,xvii,xviii,xix As we have previously described8, we substituted BMI >30 kg/m2 instead of truncal obesity. Due to the retrospective design of our study, we were unable to obtain abdominal circumference measurements from each patient, as they are not routinely obtained pre-operatively. We are confident with our identification of No-MetS status in the seventy-two patients is due to a true absence of the syndrome, as opposed to inadequate data collection, since the VA electronic record allows capture and easy access to all of the patients’ laboratory values. For instance, of the one-hundred and eight-seven patients, 180 (96.3%) patients had pre-operative serum triglyceride values, 179 (95.7%) patients had pre-operative HDL values, and 181 (96.8%) patients had a pre-operative BMI calculated. One hundred percent of our patients were identified as having the presence or absence of elevated plasma glucose (from serum glucose of HgBA1c measurements) and hypertension. Of those patients with missing data points, only four patients (2.1%) were found not to have MetS. Therefore, the absence of MetS can be assumed to result from a lack of the syndrome, rather than unavailable data.
Huber et al conducted a 34-study systematic review for primary and secondary patency outcomes for both autogenous and prosthetic conduits. The primary patency for autogenous only conduits at 6- and 18-months was 72% and 51%, respectively, and secondary patency rates of 86% and 77%, respectively.xx Both primary and secondary patency outcomes are superior to our primary patency outcomes of 48% and 12% for autogenous-only conduits at 6- and 18-months, respectively, and secondary patency outcomes of 70% and 47% for autogenously only conduits, respectively. It is possible that our reduced rates of patency may reflect our study reporting an exclusively veteran population, with its high rate of comorbidities as well as the observation that half of our patients are not receiving their dialysis – and access care – within the VA system.21,22 Additionally, with only 6% of our patients receiving prosthetic conduits during AV fistula creation, we were unable to compare our patency outcomes for AV grafts alone due to the low sample size and increased standard error.
Our study was conducted at a Veterans Affair Medical center, which in prior investigationsxxi,xxii has been identified as having a “high risk” surgical patient population for vascular surgery interventions, which is consistent with our observed high mortality rate (Figure III). Snyder et al conducted a retrospective review examining the 1- and 2-year primary and secondary patency outcomes in 64 AV grafts and 50 AV fistulae performed at a Veterans Affair Medical Center.xxiii For AV fistulae alone, the 1- and 2-year primary patency outcomes were 44% and 37%, respectively, and secondary patency outcomes of 75% and 72%, respectively. It is possible that the large number of comorbid conditions associated with the veteran population requires both diagnostic and therapeutic use of the patients’ veins, diminishing their suitability for access.
The data presented in this study represents a retrospective review of patients undergoing hemodialysis access at a single institution over a ten-year period, and such has all the problems associated with retrospective reviews. A potential further limitation of this study is the continual improvement in the medical management of patients with metabolic syndrome, which has evolved during our study period. Future studies examining the outcomes of hemodialysis access in patients with metabolic syndrome may define the presumed benefits due to medical management of this disease.
Conclusion
MetS is prevalent among patients undergoing hemodialysis access placement. MetS is associated with several proinflammatory and prothrombotic factors, which have previously been shown to contribute to AV fistula dysfunction. Patients undergoing hemodialysis access placement in the presence of MetS experience greater mortality and decreased secondary patency rates. We believe that patients with MetS form a high-risk group of patients that need intensive surveillance protocols.
Footnotes
Declaration: No competing interests declared.
Presented at the 40th annual symposium of the Society for Clinical Vascular Surgery, Las Vegas, Nevada (14 March 2012).
Author Contributions.
Conception and Design: CP, AD
Analysis and Interpretation: CP, AD
Statistical Analysis: CP
Writing of Article: CP, AJ, PV, AD
Critical Revision of the Article: CP, AJ, PV, AD
Final Approval of the Article: CP, AJ, PV AD
Overall Responsibility: CP
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- i.Center for Disease Control and Prevention. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States. Atlanta, GA: US Department of Health and Human Services; 2010. [Google Scholar]
- ii.United States Renal Data System. USRDS 2010 Annual Data Report. 2010. [Google Scholar]
- iii.Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int. 2002;62(4):1109–1124. doi: 10.1111/j.1523-1755.2002.kid551.x. [DOI] [PubMed] [Google Scholar]
- iv.Vykoukal D, Davies MG. Vascular biology of metabolic syndrome. J Vasc Surg. 2011;54(3):819–31. doi: 10.1016/j.jvs.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- v.Franchini M, Targher G, Montagnana M, Lippi G. The metabolic syndrome and the risk of arterial and venous thrombosis. Thromb Res. 2008;122(6):727–735. doi: 10.1016/j.thromres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- vi.Franco OH, Massaro JM, Civil J, Cobain MR, O’Malley B, D’Agostino RB. Trajectories of Entering the Metabolic Syndrome. Circulation. 2009;120(20):1943–1950. doi: 10.1161/CIRCULATIONAHA.109.855817. [DOI] [PubMed] [Google Scholar]
- vii.Echahidi N, Pibarot P, Després JP, Daigle JM, Mohty D, Voisine P, Baillot R, Mathieu P. Metabolic syndrome increases operative mortality in patients undergoing coronary artery bypass grafting surgery. J Am Coll Cardiol. 2007;50(9):843–851. doi: 10.1016/j.jacc.2007.04.075. [DOI] [PubMed] [Google Scholar]
- viii.Protack CD, Bakken AM, Xu J, Saad WA, Lumsden AB, Davies MG. Metabolic syndrome: a predictor of adverse outcomes after carotid revascularization. J Vasc Surg. 2008;49(5):1172–1180. doi: 10.1016/j.jvs.2008.12.011. [DOI] [PubMed] [Google Scholar]
- ix.Davies MG, Saad WE, Bismuth J, Naoum JJ, Peden EK, Lumsden AB. Impact of metabolic syndrome on the outcomes of percutaneous renal angioplasty and stenting. J Vasc Surg. 2010 Apr;51(4):926–32. doi: 10.1016/j.jvs.2009.09.042. [DOI] [PubMed] [Google Scholar]
- x.Lohsiriwat V, Pongsanguansuk W, Lertakyamanee N, Lohsiriwat D. Impact of metabolic syndrome on the short-term outcomes of colorectal cancer surgery. Dis Colon Rectum. 2010;53(2):186–91. doi: 10.1007/DCR.0b013e3181bdbc32. [DOI] [PubMed] [Google Scholar]
- xi.Yilmaz MB, Guray U, Guray Y, Biyikoglu SF, Tandogan I, Sasmaz H, Korkmaz S. Metabolic syndrome negatively impacts early patency of saphenous vein grafts. Coron Artery Dis. 2006;17(1):41. doi: 10.1097/00019501-200602000-00007. [DOI] [PubMed] [Google Scholar]
- xii.De Marchi S, Falleti E, Giacomello R, Stel G, Cecchin E, Sepiacci G, Bortolotti N, Zanello F, Gonano F, Bartoli E. Risk factors for vascular disease and arteriovenous fistula dysfunction in hemodialysis patients. J Am Soc Nephrol. 1996;7(8):1169–77. doi: 10.1681/ASN.V781169. [DOI] [PubMed] [Google Scholar]
- xiii.Kalman PG, Pope M, Bhola C, Richardson R, Sniderman KW. A practical approach to vascular access for hemodialysis and predictors of success. J Vasc Surg. 1999;30(4):727–733. doi: 10.1016/s0741-5214(99)70112-6. [DOI] [PubMed] [Google Scholar]
- xiv.Sidawy AN, Gray R, Besarab A, Henry M, Ascher E, Silva M, Miller A, Scher L, Trerotola S, Grergory RT, Rutheford R, Kent KC. Recommended stanards for reports dealing with arteriovenous hemodialysis access. J Vasc Surg. 2002;35:603–10. doi: 10.1067/mva.2002.122025. [DOI] [PubMed] [Google Scholar]
- xv.Gorter PM, Olijhoek JK, van der Graaf Y, Algra A, Rabelink TJ, Visseren FL SMART Study Group. Prevalence of the metabolic syndrome in patients with coronary heart disease, cerebrovascular disease, peripheral arterial disease or abdominal aortic aneurysm. Atherosclerosis. 2004;173:363–9. doi: 10.1016/j.atherosclerosis.2003.12.033. [DOI] [PubMed] [Google Scholar]
- xvi.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, et al. American Heart Assoc. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- xvii.Einhorn D, Reaven GM, Cobin RH, Ford E, Ganda OP, Handelsman Y, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract. 2003;9:237–52. [PubMed] [Google Scholar]
- xviii.Executive Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholestexrol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- xix.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- xx.Huber TS, Carter JW, Carter RL, Seeger JM. Patency of autogenous and polytetrafluoroethylene upper extremity arteriovenous hemodialysis accesses: a systematic review. J Vasc Surg. 2003;38(5):1005–1011. doi: 10.1016/s0741-5214(03)00426-9. [DOI] [PubMed] [Google Scholar]
- xxi.Fitzgerald TN, Popp C, Federman DG, Dardik A. Success of carotid endarterectomy in veterans: high medical risk does not equate with high surgical risk. J Am Coll Surg. 2008;207(2):219–226. doi: 10.1016/j.jamcollsurg.2008.02.033. [DOI] [PubMed] [Google Scholar]
- xxii.Weiss JS, Dumas P, Cha C, Gusberg RJ, Dardik A. Safety of carotid endarterectomy in a high-risk population: lessons from the VA and Connecticut. J Am Coll Surg. 2006;203(3):277–282. doi: 10.1016/j.jamcollsurg.2006.05.015. [DOI] [PubMed] [Google Scholar]
- xxiii.Snyder DC, Clericuzio CP, Stringer A, May W. Comparison of outcomes of arteriovenous grafts and fistulas at a single Veterans’ Affairs medical center. Am J Surg. 2008;196(5):641–646. doi: 10.1016/j.amjsurg.2008.07.013. [DOI] [PubMed] [Google Scholar]