Abstract
Neuropeptide Y (NPY) is a potent hypothalamic orexigenic peptide. Within the hypothalamus, Npy is primarily expressed in the arcuate nucleus (ARC) and the dorsomedial hypothalamus (DMH). While the actions of ARC NPY in energy balance control have been well studied, a role for DMH NPY is still being unraveled. In contrast to ARC NPY that serves as one of downstream mediators of actions of leptin in maintaining energy homeostasis, DMH NPY is not under the control of leptin. Npy gene expression in the DMH is regulated by brain cholecystokinin (CCK) and other yet to be identified molecules. The findings of DMH NPY overexpression or induction in animals with increased energy demands and in certain rodent models of obesity implicate a role for DMH NPY in maintaining energy homeostasis. In support of this view, adeno-associated virus (AAV)-mediated overexpression of NPY in the DMH causes increases in food intake and body weight and exacerbates high-fat diet-induced hyperphagia and obesity. Knockdown of NPY in the DMH via AAV-mediated RNAi ameliorates hyperphagia, obesity and glucose intolerance of Otsuka Long-Evans Tokushima Fatty rats in which DMH NPY overexpression has been proposed to play a causal role. NPY knockdown in the DMH also prevents high-fat diet-induced hyperphagia, obesity and impaired glucose homeostasis. A detailed examination of actions of DMH NPY reveals that DMH NPY specifically affects nocturnal meal size and produces an inhibitory action on within meal satiety signals. In addition, DMH NPY modulates energy expenditure likely through affecting brown adipocyte formation and thermogenic activity. Overall, the recent findings provide clear evidence demonstrating critical roles for DMH NPY in energy balance control, and also imply a potential role for DMH NPY in maintaining glucose homeostasis.
Keywords: Neuropeptide Y, dorsomedial hypothalamus, nucleus of solitary tract, food intake, energy expenditure, brown adipogenesis, glucose homeostasis, obesity, adeno-associated virus, primate
1. Introduction
The dorsomedial hypothalamic nucleus (DMH) plays an important role in maintaining energy homeostasis. Beginning with the work of Bernardis and colleagues (Bernardis et al., 1963), we have appreciated that electrolytic or excitotoxic lesions of the DMH result in hypophagia, hypodipsia, reduced body weight and decreased linear growth (Bellinger and Bernardis, 2002). Chemical stimulation or disinhibition of neurons in the DMH provokes nonshivering thermogenesis and elevates core body temperature (Dimicco and Zaretsky, 2007; Morrison and Nakamura, 2011). These data indicate the importance of the DMH in the control of energy balance through affecting both aspects of food intake and energy expenditure. Within the DMH, a number of neuropeptides such as neuropeptide Y (NPY), cholecystokinin (CCK), corticotrophin-releasing factor (CRF), and receptors such as CCK-1, melanocortin (MC) 4, Y1, Y5, and leptin receptors (LepRb) have been found, and their roles in controlling energy balance have been investigated (Bellinger and Bernardis, 2002; Bi, 2007). Relevant to this review, we have recently established a critical role for DMH NPY in the control of energy balance (Chao et al., 2011; Yang et al., 2009).
NPY is a 36-amino acid neuropeptide that was discovered by Tatemoto and colleagues in 1982 (Tatemoto et al., 1982) and belongs to the pancreatic polypeptide family that includes peptide tyrosine-tyrosine (PYY) and pancreatic polypeptide (PP) (Tatemoto et al., 1982) (Tatemoto, 1982). NPY is ubiquitously distributed in both central and peripheral nervous systems. Central NPY is most prevalent in cortical, limbic, and hypothalamic regions (Adrian et al., 1983) (Allen et al., 1983) and peripheral NPY is produced in the sympathetic nervous system (SNS) and co-operates with norepinephrine to affect sympathetic functions (Lundberg et al., 1982). NPY exhibits a variety of biological and physiological actions including modulation of feeding, thermoregulation, locomotor activity, cardiovascular function, cognition and memory, and stress-related behaviors (Bi, 2007; Colmers and Wahlestedt, 1993; Gray and Morley, 1986). This review will outline our present understanding of the actions of DMH NPY in the control of energy balance and underscore DMH NPY as a potential target for combatting obesity and related metabolic disorders.
2. Arcuate NPY in energy balance control
Within the hypothalamus, NPY plays a pivotal role in the regulation of food intake and body weight. Central administration of NPY via intracerebroventricular (Clark et al., 1984; Levine and Morley, 1984) or intrahypothalamic injection (Stanley and Leibowitz, 1985) (Stanley et al., 1986) causes robust increases in food intake and body weight and, with chronic administration, can eventually produce obesity (Zarjevski et al., 1993). Hypothalamic NPY-expressing neurons are primarily identified in the arcuate nucleus (ARC) and the DMH (Bi et al., 2003; White and Kershaw, 1990). A role for ARC NPY in the control of energy balance has been well studied. The ARC contains two distinct populations of neurons: orexigenic neuropeptide NPY/agouti-related protein (AgRP) neurons and anorexigenic proopiomelanocortin (POMC) neurons. Both types of neurons contain LepRbs. Leptin, a hormone produced by adipose tissue (Friedman and Halaas, 1998), acts on these neurons to down-regulate Npy/Agrp gene expression and up-regulates Pomc gene expression (Schwartz et al., 2000). We now appreciate that these two neural systems integrate adiposity signals (such as leptin) and nutrient signals as well as other hormonal signals (such as ghrelin) to modulate food intake and energy balance (Cone, 2006; Elmquist et al., 1999; Friedman and Halaas, 1998; Nakazato et al., 2001; Schwartz et al., 2000; Spiegelman and Flier, 2001). Anatomically, these ARC peptide containing neurons project to the paraventricular nucleus (PVN) and the lateral hypothalamus (LH), to act on local neurons to affect food intake and energy homeostasis (Elmquist et al., 1999). Although data from mouse models with targeted disruption of NPY [either knock-out (Erickson et al., 1996a) or transcriptional alterations through doxycycline-regulated system (Ste Marie et al., 2005)] have failed to demonstrate significant effects on food intake or body weight, the deletion of NPY does attenuate a hyperphagic and obese phenotype of leptin-deficient ob/ob mice (Erickson et al., 1996b) and modulation of ARC NPY signaling in adult animals also significantly impacts energy balance. Genetic ablation of neurons expressing NPY/AgRP in adult mice results in a lean and hypophagic phenotype (Bewick et al., 2005; Gropp et al., 2005). In addition, NPY/AgRP neurons co-release GABA (γ-amino butyric acid) (Horvath et al., 1997) that also contributes to the actions of NPY/AgRP neurons in feeding control through affecting hypothalamic and extrahypothalamic neuronal signaling (Pu et al., 1999; Wu and Palmiter 2011). Furthermore, adeno-associated virus (AAV)-mediated expression of antisense Npy cRNA in the ARC of adult rats decreases NPY expression and results in decreased food intake and body weight (Gardiner et al., 2005). Consistent with ARC NPY mediation of food deprivation-induced feeding, knockdown of NPY in the ARC via AAV-mediated RNA interference (RNAi) attenuates the feeding response to food deprivation (Yang et al., 2009). Together, these data identify ARC NPY serves as an important neuromodulator in the controls of food intake and energy balance.
3. Regulation of DMH NPY expression
Although NPY-expressing neurons in the DMH have long been noted (White and Kershaw, 1990) and alterations in Npy gene expression in the DMH have been reported in various rodent models of obesity, the study of the importance of DMH NPY in the control of energy balance is just beginning. Evidence has indicated that the regulation or control of Npy gene expression in the ARC and the DMH differs. While ARC NPY is under the control of circulating leptin, the controls of DMH NPY are leptin-independent (Bi et al., 2003). DMH NPY neurons do not contain LepRbs although LepRbs are abundant in the DMH (Bi et al., 2003). Dual in situ hybridization histochemistry revealed that while Npy and LepRbs are co-expressed in ARC neurons, DMH Npy-expressing neurons do not co-express LepRbs (Bi et al., 2003). Gene expression determination further revealed that Npy gene expression is increased in the ARC in response to acute food deprivation, a time when circulating leptin levels are significantly decreased, whereas DMH Npy expression is only significantly increased in rats with chronic food restriction (Bi et al., 2003). Moreover, Npy gene expression is elevated or induced in the DMH of certain rodent models of obesity including the lethal yellow agouti (Ay) (Kesterson et al., 1997), MC4R knockout (Kesterson et al., 1997), diet-induced obese (Guan et al., 1998a), tubby (Guan et al., 1998b), and brown adipose tissue-deficient obese mice (Tritos et al., 1998) and Otsuka Long-Evans Tokushima Fatty (OLETF) rats (Bi et al., 2001), but such elevation or induction is not evident in leptin deficient ob/ob mice (Kesterson et al., 1997). In fact, while Npy gene expression is significantly increased in the ARC of obese animals with leptin signaling deficiency (Beck, 2006; Sanacora et al., 1990; Wilding et al., 1993), obese animals with DMH NPY overexpression generally have significantly decreased Npy expression in the ARC (Bi et al., 2001; Guan et al., 1998a; Kesterson et al., 1997).
To investigate the potential molecules that regulate Npy expression in the DMH, we have examined hypothalamic gene expression in OLETF rats, an obesity model with a congenital deletion of CCK1Rs (Takiguchi et al., 1997). We found that Npy gene expression is significantly elevated in the DMH in both adult pair-fed and pre-obese young OLETF rats (Bi et al., 2001). Based on these observations, we hypothesized that DMH NPY overexpression in OLETF rats is resulted from CCK1R deficiency. To test this hypothesis, we conducted dual immunohistochemistry in DMH areas with anti-NPY and anti-CCK1R antibodies. We found that while NPY and CCK1R are co-localized in DMH neurons in lean control rats, DMH NPY neurons are not co-stained with CCK1Rs in OLETF rats (Bi et al., 2004). These data suggest that brain CCK directly acts on DMH NPY neurons to regulate DMH Npy expression and that a lack of CCK1Rs results in a deficit in the control of DMH NPY signaling, such that the resultant increased Npy expression in the DMH causes the hyperphagia and obesity of OLETF rats. In support of this view, parenchymal administration of CCK into the DMH decreases Npy mRNA levels in the DMH and inhibits food intake in intact rats (Bi et al., 2004). Knockdown of NPY in the DMH via AAV-mediated RNAi ameliorates the hyperphagia, obesity and diabetes of OLETF rats (Yang et al., 2009), whereas viral mediated NPY overexpression causes increased food intake and body weight and exacerbates high-fat diet-induced hyperphagia and obesity (Yang et al., 2009). Thus, these findings demonstrate the importance of DMH CCK-NPY signaling pathway in energy balance control although the source of brain CCK that acts on DMH NPY neurons through interacting with DMH CCK1Rs remains to be determined.
Our subsequent studies revealed that exercise normalizes food intake and body weight of OLETF rats as well as limits DMH Npy expression in these rats even in the absence of CCK signaling (Bi et al., 2005). These findings indicate that additional factor(s) or molecule(s) other than brain CCK also play an important role in regulating Npy gene expression in the DMH. Consistent with this view, induction of Npy gene expression in the DMH has been noted in various mouse models of obesity (Guan et al., 1998a; Guan et al., 1998b; Kesterson et al., 1997; Tritos et al., 1998) where CCK1Rs are actually not present in the DMH of wild-type mice (Bi et al., 2004). Thus, important future studies will be needed to identify these additional factor(s) that contribute to dysregulation of DMH Npy expression in these animals and determine whether their signaling pathways play a role in modulation of DMH NPY actions to affect food intake and energy balance.
4. Effects of DMH NPY on food intake
Previous studies have shown that DMH lesions result in hypophagia characterized by a specific reduction in meal size (Bellinger et al., 1986), suggesting that the primary outputs of the DMH in feeding control are orexigenic, likely through affecting within-meal satiety signals. To examine the feeding effects of DMH NPY, we characterized the effects of DMH NPY knockdown on meal patterns in OLETF rats. We found that while OLETF rats consume larger meals during both dark and light periods, DMH NPY knockdown completely normalizes meal sizes during the dark, a time when the rodents consume a majority of food, leading to ameliorating the hyperphagia and obesity of OLETF rats (Yang et al., 2009). Thus, these data demonstrate that the dysregulation of DMH NPY plays a causal role in disordered energy balance in OLETF rats and also provide evidence suggesting that DMH NPY is an important output of the DMH for modulating within-meal satiety signals.
We next examined the potential pathway for DMH NPY in feeding control using intact Sprague Dawley rats. Consistent with the findings in OLETF rats, DMH NPY knockdown produces a nocturnal meal size-specific effect (Yang et al., 2009). Moreover, in support of the view of an interaction of DMH NPY projections with systems that mediate within-meal feedback signaling, we found that DMH NPY neurons project to the nucleus of solitary tract (NTS)/dorsal motor nucleus of vagus (DMV), brain relays integrating gastrointestinal satiety signals (such as CCK) to control the size of individual meals (Moran and Kinzig, 2004). Unilateral knockdown of NPY in the DMH suppressed Npy mRNA expression in the ipsilateral DMH and led to decreased NPY fiber staining in the ipsilateral NTS (Yang et al., 2009). Consistent with these findings, DMH NPY knockdown enhanced a feeding inhibitory effect of peripheral exogenous CCK and increased CCK-induced c-Fos activation in the NTS (Yang et al., 2009). Together, these data demonstrate that DMH NPY modulates within-meal satiety signals in the NTS to affect food intake and energy balance.
Although AAV-mediated overexpression of NPY in the PVN has been shown to result in increased food intake via increased meal frequency (Tiesjema et al., 2007a; Tiesjema et al., 2007b), suggesting that the feeding effect of PVN NPY is different from that of DMH NPY, we cannot completely exclude the possibility of the projection of DMH NPY neurons to the PVN to affect food intake because a majority of DMH neuronal projections is to the PVN (Thompson et al., 1996). Thus, whether DMH NPY affects neuronal activity in the PVN and, in that way, modulates food intake and energy balance remains to be determined. Also, whether DMH NPY neurons project to other hypothalamic areas and how these neural circuits contribute to energy balance control merits further investigation.
5. Effects of DMH NPY on adiposity and thermogenesis
Two types of fat, white adipose tissue (WAT) and brown adipose tissue (BAT), exist in mammals including adult humans (Cypess et al., 2009; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009). WAT consists of unilocular adipocytes that act as an energy reservoir to store excess calories and supply the stored lipid once a body is starved or during periods of energy shortage. BAT is comprised of multilocular and mitochondrial-rich adipocytes that dissipate chemical energy to produce heat via nonshivering thermogenesis as a defense against cold and the potential for combating obesity. Both types of fat are innervated by the sympathetic nervous system (SNS) (Bartness and Bamshad, 1998; Cannon and Nedergaard, 2004). Activation of the sympathetic innervation induces lipolysis in WAT (Fredholm and Karlsson, 1970; Weiss and Maickel, 1968) and produces thermogenesis through mitochondrial uncoupling protein 1 (UCP1) in BAT (Cannon and Nedergaard, 2004). Although prior data have shown that intracerebroventricular administration of NPY increases WAT lipoprotein lipase activity (suggesting increased lipid storage) and decreases BAT GDP binding activity (indicating decreased thermogenic activity) in addition to its orexigenic effect (Billington et al., 1991) and that central administration of NPY suppresses sympathetic activity in interscapular BAT in rats (Egawa et al., 1991), the source of central NPY that normally underlies these actions is undetermined. Through examining the effects of DMH NPY knockdown on adiposity, we noted that DMH NPY knockdown specifically promotes white into brown adipocyte transformation in inguinal fat and increases lipolysis of inguinal fat (Chao et al., 2011). Denervation of sympathetic nerve innervating inguinal fat depot prevents this brown adipocyte formation (Chao et al., 2011). In addition, we found that DMH NPY knockdown results in increased Ucp1 expression in the interscapular BAT. Together, these findings suggest that DMH NPY likely modulates SNS signaling to influence adiposity and energy homeostasis and that knockdown of NPY in the DMH results in increases in peripheral sympathetic tone selectively in the inguinal fat and interscapular brown fat, and overall, leads to increased thermogenesis and energy expenditure.
Although the neural signaling pathway underlying the effects of DMH NPY on inguinal adiposity remains to be determined, evidence from the study of viral transsynaptic retrograde tracing provides support for the idea of DMH NPY modulation of SNS innervation in inguinal WAT. Bamshad and colleagues (Bamshad et al., 1998) found less viral tracer in the DMH in animals receiving epididymal viral injection than in those receiving inguinal injection, implying that the central nervous control of inguinal WAT is more DMH related than that of epididymal WAT, i.e., DMH NPY may serve as a central modulator influencing SNS outflow to inguinal WAT. Nevertheless, the detailed neural signaling pathways and molecular mechanisms underlying this adipocyte transformation merit fully investigation.
As mentioned above, DMH NPY knockdown increases Ucp1 gene expression in the interscapular BAT (Chao et al., 2011), indicating that DMH NPY also contributes to the thermogenic regulation of interscapular BAT activity. Previous evidence has shown an important role for the DMH in regulating sympathetic nerve activity to interscapular BAT and thermogenesis. Stimulation or disinhibition of neurons in the DMH by parenchymal microinjection of glutamate or GABAA receptor antagonist results in significant increases in sympathetic nerve activity to interscapular BAT and increases in BAT and core body temperature (Dimicco and Zaretsky, 2007; Morrison and Nakamura, 2011). The DMH has been proposed as an intermediate relay receiving the inputs from the hypothalamic preoptic area (POA), a center of integrating centrally and peripherally thermal signals, and sending the outputs to the rostral raphe pallidus (rRP) in the medulla, the area containing premotor neurons that innervate SNS to interscapular BAT (Dimicco and Zaretsky, 2007; Morrison and Nakamura, 2011). Moreover, using a model of LepRbEGFP reporter mice, Zhang and colleagues (Zhang et al., 2011) have recently identified LepRb-expressing neurons in the DMH and dorsal hypothalamic area (DHA) involved in these sympathetic BAT circuits. They found that (1) DMH/DHA LepRb neurons projected and synaptically coupled to rRP neurons; (2) retrograde viral tracer were detected in DMH/DHA LepRb neurons of LepRbEGFP reporter mice receiving interscapular BAT injection of viral tracer; and (3) acute cold exposure induced c-Fos activation in DMH/DHA LepRb neurons (Zhang et al., 2011). Since DMH NPY neurons do not contain LepRbs and the regulation of DMH NPY is not under the control of leptin (Bi et al., 2003), it is less likely that DMH NPY modulation of interscapular BAT thermogenesis is through DMH leptin signaling pathway. Thus, whether and how DMH NPY signaling contributes to these well-studied neural circuits of POA-DMH-rRP-SNS in DMH thermoregulation of interscapular BAT activity remains to be determined.
6. Effects of DMH NPY on glucose homeostasis
Previous studies have shown that the DMH contains both glucoreceptive and glucose-sensitive neurons and lesions of the DMH alter feeding response to exogenous glucose and insulin (Bellinger and Bernardis, 2002), implicating this region in the regulation of glucose homeostasis. We found that DMH NPY knockdown enhanced insulin sensitivity, improved glucose tolerance, and prevented high-fat diet-induced hyperglycemia and hyperinsulinemia (Chao et al., 2011). DMH NPY knockdown also ameliorated the hyperglycemia and hyperinsulinemia of OLETF rats (Yang et al., 2009), an animal model of non-insulin dependent diabetes mellitus (NIDDM) (Kawano et al., 1992). These results suggest an important role for DMH NPY in the regulation of glucose homeostasis. Recent evidence has demonstrated that BAT plays a significant role in triglyceride clearance and glucose disposal (Nedergaard et al., 2011). We have found that DMH NPY knockdown promotes white into brown adipocyte transformation in inguinal fat and increases BAT activity in both inguinal and interscapular BAT (Chao et al., 2011). DMH NPY knockdown also causes lipomobilization from lipogenesis to lipolysis in inguinal fat (Chao et al., 2011). Thus, these data suggest that the effects of DMH NPY on BAT activity may contribute to its actions on glucose homeostasis. In addition, a role for central NPY in hepatic insulin resistance has been implicated. Singhal et al. have reported that central resistin induces hepatic insulin resistance via NPY (Singhal et al., 2007). ICV administration of NPY induces hepatic insulin resistance via sympathetic innervation (van den Hoek et al., 2008). Despite these observations, the origins of the NPY-containing neurons contributing to these effects have yet to be determined. Our findings of glycemic effects of DMH NPY may provide additional evidence suggesting that DMH NPY also serves as a central neuromodulator, such as modulating hepatic insulin actions or sensitivity, to regulate glucose homeostasis.
7. NPY expression in the DMH of nonhuman primate
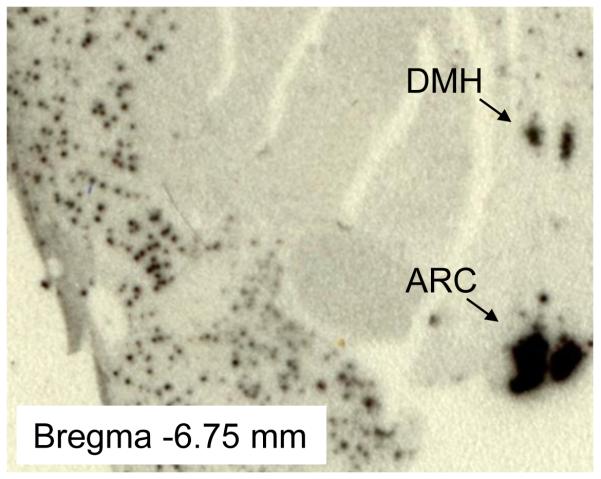
As mentioned above, DMH NPY plays a critical role in energy balance control and may serve as an important neuromodulator to affect glucose homeostasis in the rat. Induction of DMH Npy expression in several mouse models of obesity implies that DMH NPY may also play an important role in mice even though Npy gene expression is undetectable in the DMH of normal growing mice. It remains an important question whether DMH NPY is also important for energy homeostasis in primates. To begin to address this question, we have examined whether Npy gene is expressed in the DMH in the non-human primate brain. Consistent with the pattern of Npy gene expression in the rat hypothalamus (Bi et al., 2003), we found that Npy gene is strongly expressed in both the ARC and the DMH of adult rhesus monkey brain using in situ hybridization determination with human Npy mRNA antisense probe (Fig 1, Paxinos et al., 2009). In fact, Dudas and colleagues have recently reported that NPY-containing neurons are scattered throughout the human hypothalamus including the DMH using immunohistochemistry (Dudas et al., 2000; Merchenthaler et al., 2010). Together, these data underscore the potential importance of DMH NPY in humans.
Figure 1.
In situ hybridization with [35S]-labeled antisense riboprobe of human Npy shows Npy gene expression in the arcuate nucleus (ARC) and the dorsomedial hypothalamus (DMH) in adult rhesus monkey brain.
8. Conclusions and perspectives
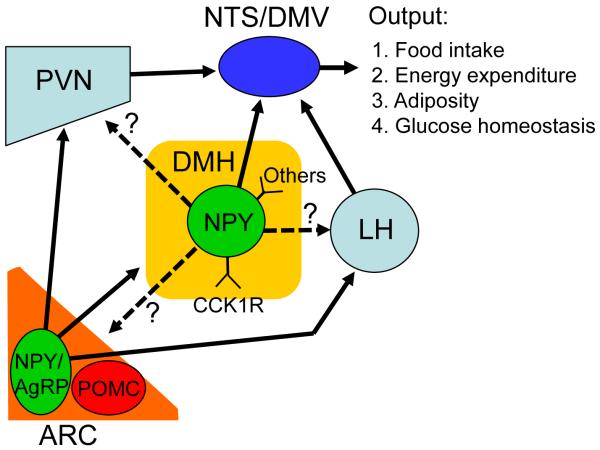
Our understanding of a role for hypothalamic NPY in energy balance control has increased enormously since the discovery of the orexigenic effects of central NPY in 1984. Within the hypothalamus, NPY-containing neurons are primarily present in the ARC and the DMH (Figure 2). ARC NPY neurons project to the PVN, the LH, and the DMH (Bai et al., 1985; Chronwall et al., 1985; Elmquist et al., 1999), and in cooperation with melanocortin systems, the projections of ARC NPY to the PVN and the LH play an important role in maintaining energy homeostasis (Cone, 2006; Elmquist et al., 1999; Nakazato et al., 2001; Schwartz et al., 2000; Spiegelman and Flier, 2001). Although the projection of ARC NPY to the DMH has been documented (Bai et al., 1985; Chronwall et al., 1985; Wang et al., 1997), its detailed neural circuits and actions remain to be determined.
Figure 2.
Projections of hypothalamic NPY-containing neurons. ARC NPY neurons project to the paraventricular nucleus (PVN), lateral hypothalamus (LH) and DMH. The projections of ARC NPY to the PVN and LH act on local neurons to affect food intake and energy balance. The actions of the projection of ARC NPY to the DMH remain undetermined. DMH NPY neurons project to the nucleus of solitary tract (NTS) and dorsal motor nucleus of vagus (DMV) and produce inhibitory effects on within meal satiety signals to modulate food intake. DMH NPY neurons may also project to other hypothalamic nuclei such as the PVN, LH and ARC. The detailed neural circuits underlying the overall actions of DMH NPY in energy balance control remain to be determined.
Recent evidence indicates that DMH NPY also serves as an important neuromodulator in modulating energy balance. In contrast to ARC NPY, DMH NPY is not under the control of leptin (Bi et al., 2003). DMH NPY signaling is modulated by brain CCK and other not yet identified molecules (Figure 2). DMH NPY-expressing neurons project to brainstem NTS/DMV and affect food intake essentially through modulating within meal satiety signals (such as CCK) (Yang et al., 2009)(Figure 2). In addition, DMH NPY plays an important role in regulating energy expenditure through affecting adiposity and BAT-thermogenesis. DMH NPY also likely has an important action in maintaining glucose homeostasis. But, the detailed neural circuits underlying these actions have yet to be characterized.
Neuroanatomical study of DMH projections has revealed that DMH projections are largely intrahypothalamic (Thompson et al., 1996). Within the hypothalamus, the most densely innervated areas from the DMH are the PVN and relatively heavy in the perifornical region with a modest projection to the ARC (Thompson et al., 1996). Li and colleagues have reported that lactation-induced Npy-expressing neurons in the DMH project to the PVN, particularly in the parvocellular part of the PVN (Li et al., 1998) even though lactation-induced Npy-expressing neurons were specifically in the dorsal-lateral DMH where NPY neurons are not detected in non-lactation animals. Thus, whether DMH NPY projects to the PVN, LH, and ARC in a normal condition and how these projections contribute to the overall effects of DMH NPY on energy balance merit further investigation. Nevertheless, the present findings of actions of DMH NPY in energy balance control underscore DMH NPY as an important neuromodulator in the regulation of energy homeostasis and also provide the potential target for combatting obesity and related metabolic disorders.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK057609 and DK087888.
Sources of support: This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK057609 and DK087888.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adrian TE, Allen JM, Bloom SR, Ghatei MA, Rossor MN, Roberts GW, Crow TJ, Tatemoto K, Polak JM. Neuropeptide Y distribution in human brain. Nature. 1983;306:584–586. doi: 10.1038/306584a0. [DOI] [PubMed] [Google Scholar]
- Allen YS, Adrian TE, Allen JM, Tatemoto K, Crow TJ, Bloom SR, Polak JM. Neuropeptide Y distribution in the rat brain. Science. 1983;221:877–879. doi: 10.1126/science.6136091. [DOI] [PubMed] [Google Scholar]
- Bai FL, Yamano M, Shiotani Y, Emson PC, Smith AD, Powell JF, Tohyama M. An arcuato-paraventricular and -dorsomedial hypothalamic neuropeptide Y-containing system which lacks noradrenaline in the rat. Brain research. 1985;331:172–175. doi: 10.1016/0006-8993(85)90730-9. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. The American journal of physiology. 1998;275:R291–299. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Bamshad M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. The American journal of physiology. 1998;275:R1399–1411. doi: 10.1152/ajpregu.1998.275.5.R1399. [DOI] [PubMed] [Google Scholar]
- Beck B. Neuropeptide Y in normal eating and in genetic and dietary-induced obesity. Philosophical transactions of the Royal Society of London Series B. Biological sciences. 2006;361:1159–1185. doi: 10.1098/rstb.2006.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiology & behavior. 2002;76:431–442. doi: 10.1016/s0031-9384(02)00756-4. [DOI] [PubMed] [Google Scholar]
- Bellinger LL, Mendel VE, Bernardis LL, Castonguay TW. Meal patterns of rats with dorsomedial hypothalamic nuclei lesions or sham operations. Physiology & behavior. 1986;36:693–698. doi: 10.1016/0031-9384(86)90356-2. [DOI] [PubMed] [Google Scholar]
- Bernardis LL, Box BM, Stevenson JA. Growth following hypothalamic lesions in the weanling rat. Endocrinology. 1963;72:684–692. doi: 10.1210/endo-72-5-684. [DOI] [PubMed] [Google Scholar]
- Bewick GA, Gardiner JV, Dhillo WS, Kent AS, White NE, Webster Z, Ghatei MA, Bloom SR. Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;19:1680–1682. doi: 10.1096/fj.04-3434fje. [DOI] [PubMed] [Google Scholar]
- Bi S. Role of dorsomedial hypothalamic neuropeptide Y in energy homeostasis. Peptides. 2007;28:352–356. doi: 10.1016/j.peptides.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Bi S, Ladenheim EE, Schwartz GJ, Moran TH. A role for NPY overexpression in the dorsomedial hypothalamus in hyperphagia and obesity of OLETF rats. American journal of physiology Regulatory, integrative and comparative physiology. 2001;281:R254–260. doi: 10.1152/ajpregu.2001.281.1.R254. [DOI] [PubMed] [Google Scholar]
- Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. American journal of physiology Regulatory, integrative and comparative physiology. 2003;285:R1030–1036. doi: 10.1152/ajpregu.00734.2002. [DOI] [PubMed] [Google Scholar]
- Bi S, Scott KA, Hyun J, Ladenheim EE, Moran TH. Running wheel activity prevents hyperphagia and obesity in Otsuka long-evans Tokushima Fatty rats: role of hypothalamic signaling. Endocrinology. 2005;146:1676–1685. doi: 10.1210/en.2004-1441. [DOI] [PubMed] [Google Scholar]
- Bi S, Scott KA, Kopin AS, Moran TH. Differential roles for cholecystokinin a receptors in energy balance in rats and mice. Endocrinology. 2004;145:3873–3880. doi: 10.1210/en.2004-0284. [DOI] [PubMed] [Google Scholar]
- Billington CJ, Briggs JE, Grace M, Levine AS. Effects of intracerebroventricular injection of neuropeptide Y on energy metabolism. The American journal of physiology. 1991;260:R321–327. doi: 10.1152/ajpregu.1991.260.2.R321. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Chao PT, Yang L, Aja S, Moran TH, Bi S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell metabolism. 2011;13:573–583. doi: 10.1016/j.cmet.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronwall BM, DiMaggio DA, Massari VJ, Pickel VM, Ruggiero DA, O’Donohue TL. The anatomy of neuropeptide-Y-containing neurons in rat brain. Neuroscience. 1985;15:1159–1181. doi: 10.1016/0306-4522(85)90260-x. [DOI] [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- Colmers WF, Wahlestedt C. The biology of neuropeptide Y and related peptides. Humana Press; New Jersey: 1993. [Google Scholar]
- Cone RD. Studies on the physiological functions of the melanocortin system. Endocrine reviews. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. The New England journal of medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. American journal of physiology Regulatory, integrative and comparative physiology. 2007;292:R47–63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- Dudas B, Mihaly A, Merchenthaler I. Topography and associations of luteinizing hormone-releasing hormone and neuropeptide Y-immunoreactive neuronal systems in the human diencephalon. The Journal of comparative neurology. 2000;427:593–603. doi: 10.1002/1096-9861(20001127)427:4<593::aid-cne7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Egawa M, Yoshimatsu H, Bray GA. Neuropeptide Y suppresses sympathetic activity to interscapular brown adipose tissue in rats. The American journal of physiology. 1991;260:R328–334. doi: 10.1152/ajpregu.1991.260.2.R328. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996a;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996b;274:1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Karlsson J. Metabolic effects of prolonged sympathetic nerve stimulation in canine subcutaneous adipose tissue. Acta physiologica Scandinavica. 1970;80:567–576. doi: 10.1111/j.1748-1716.1970.tb04824.x. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Gardiner JV, Kong WM, Ward H, Murphy KG, Dhillo WS, Bloom SR. AAV mediated expression of anti-sense neuropeptide Y cRNA in the arcuate nucleus of rats results in decreased weight gain and food intake. Biochemical and biophysical research communications. 2005;327:1088–1093. doi: 10.1016/j.bbrc.2004.12.113. [DOI] [PubMed] [Google Scholar]
- Gray TS, Morley JE. Neuropeptide Y: anatomical distribution and possible function in mammalian nervous system. Life sciences. 1986;38:389–401. doi: 10.1016/0024-3205(86)90061-5. [DOI] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nature neuroscience. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Trumbauer M, Frazier E, Van der Ploeg LH, Chen H. Induction of neuropeptide Y expression in dorsomedial hypothalamus of diet-induced obese mice. Neuroreport. 1998a;9:3415–3419. doi: 10.1097/00001756-199810260-00015. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Van der Ploeg LH. Evidence of altered hypothalamic pro-opiomelanocortin/ neuropeptide Y mRNA expression in tubby mice. Brain research Molecular brain research. 1998b;59:273–279. doi: 10.1016/s0169-328x(98)00150-8. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Bechmann I, Naftolin F, Kalra SP, Leranth C. Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res. 1997;756:283–286. doi: 10.1016/s0006-8993(97)00184-4. [DOI] [PubMed] [Google Scholar]
- Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- Kesterson RA, Huszar D, Lynch CA, Simerly RB, Cone RD. Induction of neuropeptide Y gene expression in the dorsal medial hypothalamic nucleus in two models of the agouti obesity syndrome. Molecular endocrinology. 1997;11:630–637. doi: 10.1210/mend.11.5.9921. [DOI] [PubMed] [Google Scholar]
- Levine AS, Morley JE. Neuropeptide Y: a potent inducer of consummatory behavior in rats. Peptides. 1984;5:1025–1029. doi: 10.1016/0196-9781(84)90165-7. [DOI] [PubMed] [Google Scholar]
- Li C, Chen P, Smith MS. Neuropeptide Y (NPY) neurons in the arcuate nucleus (ARH) and dorsomedial nucleus (DMH), areas activated during lactation, project to the paraventricular nucleus of the hypothalamus (PVH) Regulatory peptides. 1998;75-76:93–100. doi: 10.1016/s0167-0115(98)00057-3. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Terenius L, Hokfelt T, Martling CR, Tatemoto K, Mutt V, Polak J, Bloom S, Goldstein M. Neuropeptide Y (NPY)-like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta physiologica Scandinavica. 1982;116:477–480. doi: 10.1111/j.1748-1716.1982.tb07171.x. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Rotoli G, Grignol G, Dudas B. Intimate associations between the neuropeptide Y system and the galanin-immunoreactive neurons in the human diencephalon. Neuroscience. 2010;170:839–845. doi: 10.1016/j.neuroscience.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Moran TH, Kinzig KP. Gastrointestinal satiety signals II. Cholecystokinin. American journal of physiology Gastrointestinal and liver physiology. 2004;286:G183–188. doi: 10.1152/ajpgi.00434.2003. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Frontiers in bioscience: a journal and virtual library. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. New powers of brown fat: fighting the metabolic syndrome. Cell metabolism. 2011;13:238–240. doi: 10.1016/j.cmet.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Petrides M, Toga AW. The rhesus monkey brain in stereotaxic coordinates. Ed 2 Elsevier Inc; 2009. [Google Scholar]
- Pu S, Jain MR, Horvath TL, Diano S, Kalra PS, Kalra SP. Interactions between neuropeptide Y and gamma-aminobutyric acid in stimulation of feeding: a morphological and pharmacological analysis. Endocrinology. 1999;140:933–940. doi: 10.1210/endo.140.2.6495. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Kershaw M, Finkelstein JA, White JD. Increased hypothalamic content of preproneuropeptide Y messenger ribonucleic acid in genetically obese Zucker rats and its regulation by food deprivation. Endocrinology. 1990;127:730–737. doi: 10.1210/endo-127-2-730. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Singhal NS, Lazar MA, Ahima RS. Central resistin induces hepatic insulin resistance via neuropeptide Y. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:12924–12932. doi: 10.1523/JNEUROSCI.2443-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Kyrkouli SE, Lampert S, Leibowitz S. Neuropeptide Y chronically injected into the hypothalamus: a powerful neurochemical inducer of hyperphagia and obesity. Peptides. 1986;7:1189–1192. doi: 10.1016/0196-9781(86)90149-x. [DOI] [PubMed] [Google Scholar]
- Stanley BG, Leibowitz SF. Neuropeptide Y injected in the paraventricular hypothalamus: a powerful stimulant of feeding behavior. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:3940–3943. doi: 10.1073/pnas.82.11.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ste Marie L, Luquet S, Cole TB, Palmiter RD. Modulation of neuropeptide Y expression in adult mice does not affect feeding. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18632–18637. doi: 10.1073/pnas.0509240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiguchi S, Takata Y, Funakoshi A, Miyasaka K, Kataoka K, Fujimura Y, Goto T, Kono A. Disrupted cholecystokinin type-A receptor (CCKAR) gene in OLETF rats. Gene. 1997;197:169–175. doi: 10.1016/s0378-1119(97)00259-x. [DOI] [PubMed] [Google Scholar]
- Tatemoto K. Neuropeptide Y: complete amino acid sequence of the brain peptide. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:5485–5489. doi: 10.1073/pnas.79.18.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y--a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Canteras NS, Swanson LW. Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. The Journal of comparative neurology. 1996;376:143–173. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Tiesjema B, Adan RA, Luijendijk MC, Kalsbeek A, la Fleur SE. Differential effects of recombinant adeno-associated virus-mediated neuropeptide Y overexpression in the hypothalamic paraventricular nucleus and lateral hypothalamus on feeding behavior. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007a;27:14139–14146. doi: 10.1523/JNEUROSCI.3280-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiesjema B, la Fleur SE, Luijendijk MC, Brans MA, Lin EJ, During MJ, Adan RA. Viral mediated neuropeptide Y expression in the rat paraventricular nucleus results in obesity. Obesity. 2007b;15:2424–2435. doi: 10.1038/oby.2007.288. [DOI] [PubMed] [Google Scholar]
- Tritos NA, Elmquist JK, Mastaitis JW, Flier JS, Maratos-Flier E. Characterization of expression of hypothalamic appetite-regulating peptides in obese hyperleptinemic brown adipose tissue-deficient (uncoupling protein-promoter-driven diphtheria toxin A) mice. Endocrinology. 1998;139:4634–4641. doi: 10.1210/endo.139.11.6308. [DOI] [PubMed] [Google Scholar]
- van den Hoek AM, van Heijningen C, Schroder-van der Elst JP, Ouwens DM, Havekes LM, Romijn JA, Kalsbeek A, Pijl H. Intracerebroventricular administration of neuropeptide Y induces hepatic insulin resistance via sympathetic innervation. Diabetes. 2008;57:2304–2310. doi: 10.2337/db07-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. The New England journal of medicine. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. Functional brown adipose tissue in healthy adults. The New England journal of medicine. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Wang Q, Bing C, Al-Barazanji K, Mossakowaska DE, Wang XM, McBay DL, Neville WA, Taddayon M, Pickavance L, Dryden S, et al. Interactions between leptin and hypothalamic neuropeptide Y neurons in the control of food intake and energy homeostasis in the rat. Diabetes. 1997;46:335–341. doi: 10.2337/diab.46.3.335. [DOI] [PubMed] [Google Scholar]
- Weiss B, Maickel RP. Sympathetic nervous control of adipose tissue lipolysis. International journal of neuropharmacology. 1968;7:395–403. doi: 10.1016/0028-3908(68)90023-3. [DOI] [PubMed] [Google Scholar]
- White JD, Kershaw M. Increased hypothalamic neuropeptide Y expression following food deprivation. Molecular and cellular neurosciences. 1990;1:41–48. doi: 10.1016/1044-7431(90)90040-b. [DOI] [PubMed] [Google Scholar]
- Wilding JP, Gilbey SG, Bailey CJ, Batt RA, Williams G, Ghatei MA, Bloom SR. Increased neuropeptide-Y messenger ribonucleic acid (mRNA) and decreased neurotensin mRNA in the hypothalamus of the obese (ob/ob) mouse. Endocrinology. 1993;132:1939–1944. doi: 10.1210/endo.132.5.7682936. [DOI] [PubMed] [Google Scholar]
- Wu Q, Palmiter RD. GABAergic signaling by AgRP neurons prevents anorexia via a melanocortin-independent mechanism. Eur J Pharmacol. 2011;660:21–27. doi: 10.1016/j.ejphar.2010.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Scott KA, Hyun J, Tamashiro KL, Tray N, Moran TH, Bi S. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:179–190. doi: 10.1523/JNEUROSCI.4379-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarjevski N, Cusin I, Vettor R, Rohner-Jeanrenaud F, Jeanrenaud B. Chronic intracerebroventricular neuropeptide-Y administration to normal rats mimics hormonal and metabolic changes of obesity. Endocrinology. 1993;133:1753–1758. doi: 10.1210/endo.133.4.8404618. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, Jones JC, Rhodes C, Munzberg H. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:1873–1884. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]