Abstract
Isoprene synthase is the enzyme responsible for the foliar emission of the hydrocarbon isoprene (2-methyl-1,3-butadiene) from many C3 plants. Previously, thylakoid-bound and soluble forms of isoprene synthase had been isolated separately, each from different plant species using different procedures. Here we describe the isolation of thylakoid-bound and soluble isoprene synthases from a single willow (Salix discolor L.) leaf-fractionation protocol. Willow leaf isoprene synthase appears to be plastidic, with whole-leaf and intact chloroplast fractionations yielding approximately equal soluble (i.e. stromal) and thylakoid-bound isoprene synthase activities. Although thylakoid-bound isoprene synthase is tightly bound to the thylakoid membrane (M.C. Wildermuth, R. Fall [1996] Plant Physiol 112: 171–182), it can be solubilized by pH 10.0 treatment. The solubilized thylakoid-bound and stromal isoprene synthases exhibit similar catalytic properties, and contain essential cysteine, histidine, and arginine residues, as do other isoprenoid synthases. In addition, two regulators of foliar isoprene emission, leaf age and light, do not alter the percentage of isoprene synthase activity in the bound or soluble form. The relationship between the isoprene synthase isoforms and the implications for function and regulation of isoprene production are discussed.
Isoprene (2-methyl-1,3-butadiene) is a volatile hydrocarbon emitted from the leaves of a variety of C3 plants (Zimmerman, 1979; Guenther et al., 1994). Interest in leaf isoprene emission spans a number of disciplines: tropospheric chemistry, plant physiology, and metabolism. Biogenic isoprene emissions may dominate anthropogenic hydrocarbon emissions for a given region, altering its tropospheric chemistry. For example, in southeastern U.S. cities oak forest isoprene emissions are thought to contribute more to ozone formation than hydrocarbons from automobile exhaust (Chameides et al., 1988). From a physiological perspective, leaf isoprene emission is intriguing in that a significant portion of a plant's fixed carbon, 1 to 8%, may be emitted as isoprene (Monson and Fall, 1989), and yet the function of isoprene production remains uncertain. Finally, isoprenoid metabolism occurs in all living organisms, yielding a vast array of important primary and secondary compounds and providing precursors for protein prenylation (Bach, 1995; McGarvey and Croteau, 1995). Isoprenoid metabolism is most diverse and specialized in plants (Chappell, 1995; McGarvey and Croteau, 1995), and the production of isoprene from DMAPP, a channeling substrate from the plastidic isoprenoid pathway at its inception, may represent a significant control point for the plastidic pathway.
The enzyme responsible for leaf isoprene emission, isoprene synthase, catalyzes the conversion of DMAPP to isoprene and pyrophosphate (Silver and Fall, 1991, 1995). Isoprene synthase was first discovered as a soluble enzyme from aspen leaves using a whole-leaf extraction process in which leaves were ground in liquid nitrogen, followed by extraction of soluble proteins in PEB (Silver and Fall, 1991). Using this extraction procedure, soluble isoprene synthases have also been isolated from leaves of velvet bean (Mucuna sp.) (Kuzma and Fall, 1993) and oak (Quercus petrae) (Schnitzler et al., 1996). In contrast, a thylakoid-bound isoprene synthase was isolated from willow (Salix discolor L.) using a leaf-fractionation protocol in which leaves were homogenized in a blender and chloroplasts were isolated and ruptured to yield thylakoids (Wildermuth and Fall, 1996). The discovery of soluble and thylakoid-bound forms of isoprene synthase in different plant species and using different extraction protocols led us to question whether both soluble and thylakoid-bound forms of the enzyme exist in vivo and what the relationship between these two forms may be.
Here we present two procedural advances that enable us to address these questions: a method for isolating soluble and thylakoid-bound isoprene synthases from the same willow leaf preparation, and a procedure for solubilizing active thylakoid-bound isoprene synthase from willow. We demonstrate that stromal and thylakoid-bound plastidic isoprene synthases exist within a given leaf preparation and present initial characterization of these isoforms. The implications of our findings for the function and regulation of isoprene production are discussed.
MATERIALS AND METHODS
Willow (Salix discolor L.) branches were collected from a naturally growing population in Boulder, Colorado, during the summer season. Willow clones from this population were propagated and grown in 10-gallon plastic containers in Agro Mix No. 2 (American Clay, Denver, CO) and fertilized weekly with Peters Professional Soluble Plant Food–General Purpose Special (Peters Fertilizer Products, Fogelsville, PA). These plants were grown in a greenhouse with supplemental lighting (500 μmol m−2 s−1) from low-pressure sodium vapor lamps (General Electric) for a 16-h photoperiod. Temperatures ranged from 21°C (night) to 27°C (day). Healthy, mature willow leaves from the naturally growing population were used in experiments during the summer season, and leaves from greenhouse clones were used the rest of the year.
Reagents
DMAPP was synthesized, purified, confirmed, and stored as previously described (Wildermuth and Fall, 1996). Enzymes and other reagents were purchased from Sigma unless otherwise specified.
Assays
Isoprene Synthase
Isoprene production was assayed in 4.8-mL glass vials sealed with Teflon-lined septa. After a 10-min incubation at 35°C, 1 mL of headspace was analyzed for isoprene by GC with a reduction gas detector, as described previously (Silver and Fall, 1991; Greenberg et al., 1993). Unless otherwise specified, isoprene synthase activity was assayed with 10 mm DMAPP and 8 mm MgCl2. For each sample, background levels of isoprene, produced by the nonenzymatic conversion of DMAPP to isoprene, were assessed using buffer in place of the plant fraction. All samples were run at least in duplicate and within a linear range of activity.
Leaf Isoprene Emission
Leaf isoprene emission was determined by incubating 0.785-cm2 leaf discs taken from the vertical center of the leaf next to the midrib in a 4.8-mL vial (as described above) with 1 mL of distilled H2O. After a 20-min incubation at 35°C, 1 mL of headspace was analyzed for isoprene as described above.
Leaf Area
Leaf area was determined by tracing the leaf perimeter onto paper, cutting out the leaf area, and weighing the leaf area cutout. Comparison with the weight of the paper standard areas enabled leaf-area determination.
NADP-GAPD
The chloroplast stromal marker NADP-GAPD was quantified using the method of Ferri et al. (1978) as described by Wildermuth and Fall (1996).
Chl and Protein
Chl was determined using 80 or 100% acetone, according to Lichtenthaler (1987). Protein concentrations were determined by the Bradford assay (Bradford, 1976) with BSA as the standard protein.
Isoprene Synthase-Extraction Protocols
Leaf-Grind Protocol
This method involves grinding whole leaves in liquid nitrogen and extracting soluble proteins in extract buffer. Willow leaves were harvested and used in the morning, after a few hours of exposure to light. Leaves (30 g) were cut and rinsed in distilled H2O, 2% (v/v) bleach, 0.05% (v/v) Nonidet P-40 detergent solution, and again (three to four times) in distilled H2O to remove surface microorganisms and debris. Exclusion of the bleach/detergent wash did not alter results. The leaves were then blotted dry and ground in liquid nitrogen using a mortar and pestle. The frozen, ground leaves were added to 300 mL of PEB consisting of 50 mm Tris (pH 8.0 at 4°C), 5% glycerol, 20 mm MgCl2, with 10% (w/v) PVP (average Mr, 40,000), 1 mm PMSF, 1 mm Benz-HCl, and 10 mm DTT added before use. The plant mixture was then filtered through cheesecloth and Miracloth (Calbiochem) and centrifuged at 12,000g for 20 min. The supernatant was centrifuged at 39,000g for 20 min, and the protein of the resulting supernatant was precipitated using either 30 or 40% PEG 3350, stirred for 1 h, and centrifuged for 20 min at 12,000g to pellet the protein. The pellet was resuspended in approximately 7 mL of PEB (with DTT) and treated with an equal volume of CM-Sepharose “fast-flow” resin (equilibrated with PEB/DTT). After it was stirred for 15 min, the mixture was centrifuged at 12,000g for 5 min to pellet the resin, and the supernatant was collected. All procedures were performed with prechilled buffers and at 4°C.
Concentration of the soluble protein was attempted using ammonium sulfate (25–100% saturation), soluble absorbing matrix concentration, and PEGs with a variety of average Mrs at varying concentrations. Ammonium sulfate precipitation was not successful because of the presence of secondary compounds, which caused much of the protein to be associated in a mucus-like film on top of the solution. The responsible constituents may be phenolic compounds such as phenolic glucosides and (+)-catechin and polymeric phenolics, which are common in willow (e.g. Julkunen-Tiitto et al., 1993) and are known to preferentially bind proteins, carbohydrates, and metal ions (see Hemingway and Karchesy, 1988). PEG precipitation with an average Mr of 3350 at 30 to 40% was successful in precipitating soluble protein. The precipitated protein, however, was very viscous. A number of methods were used, without success, to remove the interfering secondary compounds and reduce the viscosity of the final protein precipitate, including gel filtration, ultrafiltration with membranes of various Mr cutoffs, and electroelution. A variety of batch resin treatments were then tested in an attempt to either (a) specifically bind isoprene synthase, but not the viscous material, or (b) specifically bind the viscous material, but not isoprene synthase. CM-Sepharose batch treatment was successful in binding most of the viscous material, whereas the isoprene synthase activity remained exclusively in solution.
Leaf-Fractionation Protocol
In this method leaves are cut and homogenized (using a blender) in chloroplast extract buffer, and fractionation yields both thylakoid membranes and soluble proteins. Willow leaves (10 g) were harvested and washed as described for the leaf-grind protocol. They were then cut into small pieces (of approximate area 2 cm2) using sharp scissors and directly added to 100 mL of prechilled (on ice) GB. GB consisted of 0.5 m sorbitol, 50 mm Tricine (pH 7.8), 1 mm EDTA, 5 mm MgCl2, and 1 mm DTT, 1 mm PMSF, 1 mm Benz-HCl, and 0.1% defatted BSA (Calbiochem), which were added just before use. All solutions and equipment coming into contact with the plant solution were prechilled, plant fractions were kept on ice, and all centrifugation and other steps were performed at 4°C. The leaves were then homogenized using a Waring Blendor with a small volume attachment three times for 2 s each. This solution was filtered through cheesecloth and a 202-μm nylon filter (Tekto, Inc., Briarcliff Manor, NY).
The crude homogenate was centrifuged for 15 min at 12,000g. The supernatant (SN1) was then centrifuged at 35,000g for 20 min, yielding SN2 and P2. This pellet (P2) was combined with the pellet from the original spin (P1), resuspended in 17 mL of GB (no sorbitol and no BSA) to rupture intact chloroplasts, and centrifuged at 6,000g for 10 min. This rupturing step was repeated and these supernatants were added to SN2. The combined supernatant fraction was centrifuged at 35,000g for 30 min and the pellet (P3, containing thylakoids and other membranes) was resuspended in 2 to 6 mL of GB (no sorbitol and no BSA), depending on the experimental design. To maintain consistency among similar experiments, the pellets were resuspended in equal volumes at comparable protein concentrations. Protein in the final supernatant fraction (SN3) was precipitated using 40% PEG 3350, stirred for 1 h, and centrifuged at 12,000g for 20 min. This pellet (SNP) was resuspended in the same volume of GB (no sorbitol and no BSA) that was used to resuspend P3. Typically, inclusion of the CM-Sepharose batch treatment to reduce the viscosity of this resuspended pellet was not necessary (see above procedure). When larger preparations were required, 30 g of willow leaves was used with the volumes in the protocol described above adjusted proportionally.
This procedure allowed us to isolate total soluble and membrane-bound isoprene synthase activities. To minimize variation in extractable isoprene synthase activities, clonal plants were used, growth conditions were standardized (as above), and experiments were performed within a 2-week period (and on consecutive days when possible) for a given set of results.
Chloroplast-Fractionation Protocol
Intact chloroplasts were prepared from 30 g of willow leaves by homogenizing the leaves in a blender, pelleting crude chloroplasts, and then pelleting purified, intact chloroplasts (P2) using a 40% Percoll gradient. The details of this protocol are given by Wildermuth and Fall (1996); typical contamination of P2 by mitochondria and peroxisomes as a percentage of their total enzyme marker activity was 2 and 10%, respectively. Recoveries of the plastidic markers Chl and NADP-GAPD in the P2 fraction were typically 30% of total activity. Intactness was ≥ 60%, estimated visually at 1000× using a microscope.
The intact chloroplast pellet (P2) was then ruptured by resuspending it in 50 mL of GB (no sorbitol) and was centrifuged at 12,000g for 10 min. This rupturing procedure was repeated and the supernatants were pooled. Protein in the supernatant was precipitated using 40% PEG as described above, and the pellet was resuspended in approximately 2 mL of GB (no sorbitol). The thylakoid pellet was also resuspended in approximately 2 mL of GB (no sorbitol). The inclusion of defatted BSA in the rupturing buffer facilitated the precipitation of active stromal isoprene synthase. Ultracentrifugation of the pooled supernatants at 100,000g for 60 min (to ensure that no residual membrane fragments were in the supernatant) did not alter the results. All procedures were performed with prechilled buffers and at 4°C.
Solubilization of Thylakoid-Bound Isoprene Synthase by pH 10.0 Treatment
Willow thylakoids were isolated from 10 g of leaves, as described by Wildermuth and Fall (1996). The thylakoids were then washed with 17 mL of 2 m NaBr solution (2 m NaBr, 50 mm Tricine [pH 7.8], 1 mm PMSF, 1 mm Benz-HCl, 1 mm DTT, and 1% PEG 400) and stirred for 10 min on ice to remove peripheral membrane proteins. Centrifugation at 6,000g for 10 min followed, with resuspension of the washed thylakoids in 3 mL of pH 10.0 solubilization buffer (100 mm 2-[N-cyclohexylamino]ethanesulfonic acid [pH 10.0], 8.8 mm MgCl2, 1 mm Benz-HCl, 1 mm DTT, and 1% PEG 400), stirring for 10 min on ice, and centrifugation at 35,000g for 20 min. This final spin was sufficient to pellet all membranous material, since ultracentrifugation at 100,000g for 60 min did not alter the solubilized isoprene synthase recovery. Typically, the supernatant was then adjusted to approximately pH 8 with Tricine (1 m, pH 8.0). Often the adjusted supernatant would then be concentrated using ammonium sulfate precipitation (70% saturation) and dialyzed against GB or another appropriate buffer.
Biochemical Characterization of Isoprene Synthases
Mg2+ Dependence
Concentrated, solubilized thylakoid-bound isoprene synthase was resuspended in PEB/5 mm DTT, and then dialyzed extensively against PEB (without Mg2+) before use. Leaf soluble isoprene synthase was prepared as described above, except that PEB had 1 mm Mg2+, not 20 mm Mg2+; for the leaf-soluble experiments, the Mg2+ profile started at 1 mm Mg2+. Isoprene synthase activity was assayed using 100 μL of enzyme preparation, 10 mm DMAPP, and increasing concentrations of Mg2+ (0–25 mm).
pH Dependence
The solubilized thylakoid-bound isoprene synthase was extensively diafiltrated (10,000 Mr cutoff, Filtron Technology Corp., Northborough, MA) into an equivalent final volume of 5 mm Tricine (pH 7.8), 10 mm MgCl2, 1 mm DTT, 1% PEG 400, and 1 mm Benz-HCl. Isoprene production was determined using 100 μL of diafiltrated solution, 100 mm of the specified pH buffer, 8 mm MgCl2, and 10 mm DMAPP. One hundred microliters of leaf-soluble and stromal isoprene synthases (from leaf-grind and -fractionation protocols, respectively), was assayed with 100 mm of the specified pH buffers, 16 mm MgCl2, and 10 mm DMAPP. The buffers used were: Mes, pH 5.0, 6.0, and 7.0; Tricine, pH 7.0, 8.0, and 9.0; 2-(N-cyclohexylamino)ethanesulfonic acid, pH 9.0 and 10.0; and 3-(cyclohexylamino)-1-propanesulfonic acid, pH 11.0.
Apparent Km
Fifty microliters of concentrated, solubilized thylakoid-bound isoprene synthase in PEB/5 mm DTT was assayed for isoprene synthase activity with 16 mm MgCl2 and varying concentrations of DMAPP (0–20 mm). Leaf-soluble isoprene synthase (100 μL) was also assayed with 16 mm MgCl2 and varying concentrations of DMAPP (0–20 mm).
Inhibition Experiments
Solubilized thylakoid-bound and leaf-soluble isoprene synthases were isolated as detailed above in the solubilized thylakoid-bound isoprene synthase and the leaf-grind protocols, with the pellets resuspended as necessary for each experiment.
For the Pi and pyrophosphate experiments, 100 μL of plant extract resuspended in PEB/5 mm DTT was incubated with 10 μL of inhibition solution or double-distilled H2O and 12 μL of 100 mm DMAPP, and assayed for isoprene production as described above. A few of these experiments were performed with 50 μL of plant extract and one-half of the other reaction components. The addition of the sodium phosphate or pyrophosphate solutions (pH 8.0) did not result in observable precipitate. These experiments were performed similarly to those done with soluble aspen (Populus tremuloides Michx.) leaf isoprene synthase (Silver, 1994).
Experiments involving the covalent modification of Cys, His, and Arg residues of isoprene synthase were modeled on those by Rajaonarivony et al. (1992b) and Savage et al. (1995), with technical tips taken from Lundblad (1991). For cysteinyl-directed inhibition experiments using NEM, the plant extracts (stored as aliquots resuspended in PEB/5 mm DTT) were diluted 1:3 into 50 mm Tricine (pH 7.8) with 5% glycerol and then dialyzed against that solution for 1 to 2 h to remove Mg2+ and DTT. DTT can prevent the covalent modification of proteins by NEM. NEM was prepared in 50 mm Tricine (pH 7.8). Ten microliters of NEM or Tricine/glycerol buffer was added to 100 μL of plant extract and incubated at 35°C for 0, 10, or 20 min. Two microliters of 1 m DTT, 2 μL of 1 m MgCl2, and 12 μL of 100 mm DMAPP were then added and isoprene production was assessed. For the substrate-protection experiments, the MgCl2 and DMAPP were first incubated with the plant extract for 5 or 10 min before the NEM was added for the specified incubation time.
For the histidyl-directed inhibition experiments using DEPC, the plant extract was diluted 1:3 in 50 mm Tricine (pH 7.0) with 5% glycerol and dialyzed against this buffer with 1 mm DTT for 1 to 2 h. Tricine buffer at pH 7.0 was used because Tris buffers react with DEPC, and pH 7.0 increases the specificity of DEPC for histidyl residues. Immediately before use DEPC was diluted into anhydrous ethanol and kept on ice throughout the experiment. One hundred microliters of plant extract was incubated with 2 μL of DEPC solution or anhydrous ethanol and 3 μL of the Tricine/glycerol buffer for 0 to 30 min at room temperature, followed by the addition of 2 μL of 100 mm MgCl2 and 12 μL of 100 mm DMAPP for the isoprene synthase assay. The substrate-protection experiments were performed in an analogous manner to that detailed above. In addition, to confirm that DEPC was covalently modifying His residues, resulting in inactivation, 3 μL of His solution (4.4 mm final) was incubated with the plant extract for 10 min at room temperature before the addition of DEPC.
For the arginyl-directed inhibition experiments using PG, the plant extract was diluted into 50 mm Tricine (pH 7.8) with 5% glycerol and then dialyzed against that buffer with 5 mm DTT. Fifty microliters of plant extract was treated with 11 μL of phenylglyoxal or buffer solution for 30 min at room temperature, transferred to a vial, and incubated for 10 min at 35°C with 7 μL of 100 mm DMAPP and 1.1 μL of 1 m MgCl2. For the substrate-protection experiments, the DMAPP and MgCl2 were first added to the plant extract, incubated for 10 min at 35°C, and then treated with PG. In all experiments, a final concentration of 50 mm PG was used for the 30-min treatment.
RESULTS
Isolation of Soluble and Thylakoid-Bound Isoprene Synthases from Intact Chloroplasts and Leaves of Willow
As described in the introduction, soluble and thylakoid-bound isoprene synthases have been isolated and characterized from different plant species using different extraction techniques. To determine whether both soluble and bound forms of the enzyme exist in vivo, it was first necessary to determine whether the two enzyme forms could be detected in one protocol. Wildermuth and Fall (1996) localized enzymatic isoprene biosynthesis to the chloroplast using intact chloroplasts from willow. In addition, a thylakoid-bound isoprene synthase was discovered and characterized. However, the possibility of a soluble isoprene synthase either of plastidic or cytosolic origin could not be discounted.
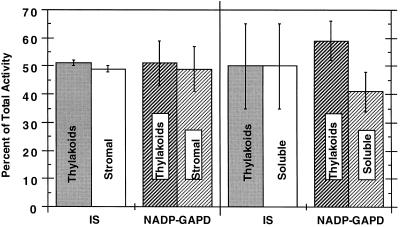
We are now able to detect both thylakoid-bound and stromal isoprene synthase activities from intact willow chloroplasts. Concentration of the stromal willow chloroplast fraction by 40% PEG 3350 precipitation facilitated the detection of the stromal isoform. Previous attempts to detect enzyme activity in the willow stroma concentrated by ammonium sulfate precipitation or microconcentration had been unsuccessful (Wildermuth and Fall, 1996). As shown in Figure 1 (left), willow chloroplast fractionation yielded approximately equal soluble and thylakoid-bound isoprene synthase activities. NADP-GAPD activity in the willow chloroplasts was similarly distributed. The significant proportion of NADP-GAPD associated with the thylakoids was surprising at first because NADP-GAPD has been considered to be a stromal enzyme. However, recent reports indicate that roughly one-half of plastidic NADP-GAPD is associated with the thylakoids (e.g. Adler et al., 1993; Anderson et al., 1996).
Figure 1.
Percentage of total isoprene synthase and NADP-GAPD activities associated with thylakoid membranes or as soluble enzymes isolated from intact willow chloroplasts (left) and whole willow leaves (right). Intact chloroplast- and leaf-fractionation protocols and enzymatic assays are detailed in Methods. Values presented for the chloroplast fractionations are an average of two separate experiments performed on consecutive days; total isoprene synthase and NADP-GAPD activities are 220 ± 37 pmol isoprene min−1 and 1.70 ± 0.31 μmol NADP converted min−1, respectively. Total values are given for a 10-g leaf preparation with adjustment of the chloroplast results by 3.3-fold to reflect the 30% recovery of intact chloroplasts from whole leaves on a Chl and NADP-GAPD basis. Values presented for leaf fractionations (10 g) are from three separate experiments performed on consecutive days; total isoprene synthase and NADP-GAPD activities are 4630 ± 1990 pmol isoprene min−1 and 7.46 ± 1.81 μmol NADP converted min−1, respectively. IS, Isoprene synthase.
Whole-leaf fractionations were then performed to separate membrane-associated (thylakoid) and soluble enzymatic activities, as shown in Figure 1 (right). These experiments also resulted in about equal soluble and thylakoid-bound isoprene synthase and NADP-GAPD activities. The increased variability of isoprene synthase activity from leaf fractionations compared with chloroplast fractionations is likely the result of differences in the fractionation protocols. In particular, increased exposure of the thylakoid and soluble fractions to extraplastidic enzymes occurs during the leaf-fractionation procedure.
The similar distribution of thylakoid-bound and soluble isoprene synthase activities from the fractionation of whole willow leaves and from willow chloroplasts suggests that, like NADP-GAPD, isoprene synthase is plastidic. Support for this conclusion includes the plastidic-localization experiments of Wildermuth and Fall (1996) and the plastidic origin of DMAPP, the substrate for isoprene synthase, via the glyceraldehyde-3-phosphate/pyruvate pathway (Zeidler et al., 1997). The 20-fold difference in total isoprene synthase activity from the leaf versus the intact chloroplast fractionation is somewhat disturbing. It is likely the result of (a) differences in leaf-growth environments for the two sets of experiments and (b) differences in the enzyme-isolation protocols. The leaf-fractionation experiments were performed with leaves grown during the summer months, whereas the chloroplast fractionations used winter-grown leaves. As much as 10-fold variability has been observed in total isoprene synthase activity extracted from leaves in experiments performed many months apart. To ascertain whether the leaf-growth environment could account for the majority of this 20-fold difference, a chloroplast fractionation was performed just subsequent to the leaf-fractionation experiments of Figure 1. In this experiment total isoprene synthase activity from the chloroplast fractionations accounted for 25% of the leaf-fractionation totals (a 4-fold difference). Because the typical variation in total isoprene synthase activity isolated using a given protocol varies approximately 2-fold for experiments performed within a 2-week period (see Table I), the 4-fold discrepancy in totals observed for the chloroplast- versus leaf-fractionation protocols is reasonable. Finally, differences in the enzyme-isolation protocols (leaf versus intact chloroplast fractionation) may influence the activation states of the isoprene synthases or the recovery of isoprene synthase activity.
Table I.
Thylakoid-bound and soluble isoprene synthase activity from leaf fractionations performed within a 2-week period
| Experiment | Isoprene
Synthase Activity
|
|||||
|---|---|---|---|---|---|---|
| Thylakoid bound | Percent | Soluble | Percent | Total | Percent | |
| pmol isoprene min−1 | pmol isoprene min−1 | pmol isoprene min−1 | ||||
| 1 | 1146 | 45 | 1418 | 55 | 2564 | 100 |
| 2 | 740 | 47 | 821 | 53 | 1561 | 100 |
| 3 | 1368 | 44 | 1742 | 56 | 3110 | 100 |
| 4 | 840 | 25 | 2583 | 75 | 3423 | 100 |
| Average | 1024 | 40 | 1641 | 60 | 2665 | 100 |
| sd | 287 | 11 | 735 | 11 | 817 | |
Mature willow leaves (10 g) were fractionated into total thylakoid-bound and soluble isoprene synthase activities, as described in Methods. Each value is the average of duplicate samples.
The increased variability in the percentage of total isoprene synthase activity associated with the thylakoids in the leaf-fractionation experiments led us to question whether nonphysiological proteolysis of thylakoid-bound isoprene synthase might occur. For example, when thylakoid-bound Cyt f is extracted from charlock or turnip leaves, proteolytic cleavage of a C-terminal domain essential for anchoring solubilizes the enzyme (Gray et al., 1994). The isoprenoid enzyme squalene synthase may also be artificially solubilized when exposed to proteases during extraction (Shechter et al., 1992). Therefore, we tried to inhibit proteolysis by using an array of protease inhibitors that are active on many classes of proteases (5 mm amino-N-caproic acid, 1 μm antipain, 1 mm Benz-HCl, 1 mm p-hydroxymercuribenzoate, 1 μm leupeptin, 10 μm pepstatin A, and 1 mm PMSF; Gegenheimer, 1990). These protease inhibitors included those used to prevent cleavage of squalene synthase from rat hepatic microsomal membranes (Shechter et al., 1992). Willow leaf fractionations were performed in parallel using the standard buffers, which included 1 mm PMSF and 1 mm Benz-HCl, and the standard buffer with additional protease inhibitors (see above). Total thylakoid-bound and soluble isoprene synthase activities were obtained and the ratio of thylakoid-bound to soluble activity was determined; neither was significantly altered by the inclusion of any of the protease inhibitors (data not shown). In addition, the substitution of 10 mm EGTA for 1 mm EDTA did not influence isoprene synthase activities.
We then sought to promote the putative proteolysis of the thylakoid-bound isoprene synthase into a soluble enzyme using exposure experiments. Exposure of thylakoid-bound isoprene synthase to the leaf-fractionation supernatant fraction (isolated as usual or without PMSF, Benz-HCl, and EDTA) during the course of 2 h did not result in the solubilization of active thylakoid-bound isoprene synthase or in the loss of thylakoid-bound isoprene synthase activity. Because neither inhibition nor promotion of proteolysis altered the activities of the two enzyme forms, it appears that proteolytic cleavage of thylakoid-bound isoprene synthase during the extraction process is not responsible for the presence of the soluble enzyme.
Irreversible Solubilization of Thylakoid-Bound Isoprene Synthase Suggests the Involvement of a Lipid Tail in Membrane Association
Numerous previous attempts at solubilizing active thylakoid-bound isoprene synthase from willow were unsuccessful (Wildermuth and Fall, 1996). However, we have now developed two methods of solubilizing active thylakoid-bound isoprene synthase: pH 10.0 solubilization and 1.0% (w/v) octanoyl-N-methyl glucamide detergent treatment (data not shown). The pH 10.0 solubilization procedure was used throughout the experiments described here, since it avoids the complications associated with the inclusion of detergent.
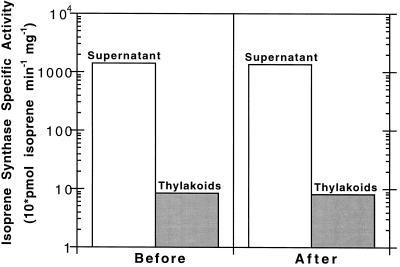
Solubilization of the enzyme at pH 10.0 occurred rapidly, typically with 60% of the thylakoid-bound activity in soluble form after a 10-min exposure. Centrifugation at 100,000g for 1 h did not sediment this form of the enzyme, confirming its solubility. The combined activity of the solubilized thylakoid-bound isoprene synthase and the remaining bound isoprene synthase may be up to 4-fold greater than the original thylakoid-bound activity (data not shown).
Once pH 10.0 solubilization was discovered, we used it to probe the nature of the thylakoid-bound isoprene synthase membrane association. This solubilization could result from the disruption of ionic and hydrogen-bonding interactions required for membrane association (e.g. Hager and Holocher, 1994), or cleavage of an acyl tail responsible for anchoring (e.g. Linder et al., 1993), or both. To examine these possibilities, solubilization of thylakoid-bound isoprene synthase at pH 10.0, adjustment to pH 7.8, and rebinding to thylakoids at pH 7.8 was undertaken. The experimental design was based on the pH-dependent rebinding experiments of Hager and Holocher (1994) for violaxanthin de-epoxidase. As shown in Figure 2, pH 10.0-solubilized thylakoid-bound isoprene synthase did not reassociate with the thylakoid membranes at pH 7.8. The exclusion of 1% PEG 400 in the solubilization solution, in case it preferentially stabilized the solubilized enzyme, did not alter this result. Therefore, it is possible that isoprene synthase protein interactions with the thylakoid membrane and its component enzymes may not be fully responsible for the tightly associated thylakoid-bound isoprene synthase. Perhaps an alkaline-sensitive lipid tail is involved in this membrane association. Thioester linkages (such as those used in palmitoylation) are labile at pH 10.0 (Linder et al., 1993). To determine whether a thioester-linked acyl tail was involved, hydroxylamine treatment of willow thylakoid-bound isoprene synthase at neutral pH was undertaken at 4 and 37°C (as described by Pepperberg et al., 1995; Zeng and Weigel, 1996). These experiments were inconclusive, however, since hydroxylamine inhibited isoprene synthase activity, and we were only capable of assaying active enzyme.
Figure 2.
Attempts to rebind solubilized isoprene synthase to thylakoid membranes. Washed willow thylakoid membranes underwent pH 10.0 solubilization to release active isoprene synthase. This solubilized isoprene synthase was then adjusted to approximately pH 8 and dialyzed against the pH 7.8 solution to obtain the “Before” supernatant fraction. Addition of this fraction to the willow thylakoid membrane fraction, followed by centrifugation to separate the supernatant and pellet, resulted in the “After” supernatant and pellet fractions. The Before pellet fraction was obtained in a similar manner to the After pellet fraction, but instead of solubilized enzyme, only the pH 7.8 buffer was used. Both pellets were resuspended in pH 7.8 solution. All volumes were kept constant throughout. Shown is the result of a typical experiment, repeated several times. A logarithmic scale was used to present the supernatant and pellet isoprene synthase activities within the same graph.
Thylakoid-Bound, Solubilized Thylakoid-Bound, and Stromal Isoprene Synthases Have Similar Catalytic Properties
Stromal and thylakoid-bound isoprene synthases may differ only in their ability to associate with the thylakoid membrane. If this were the case, we would expect solubilized thylakoid-bound isoprene synthase and soluble isoprene synthase to have similar or identical catalytic properties, such as pH optima, Mg2+ optima, and Km for DMAPP.
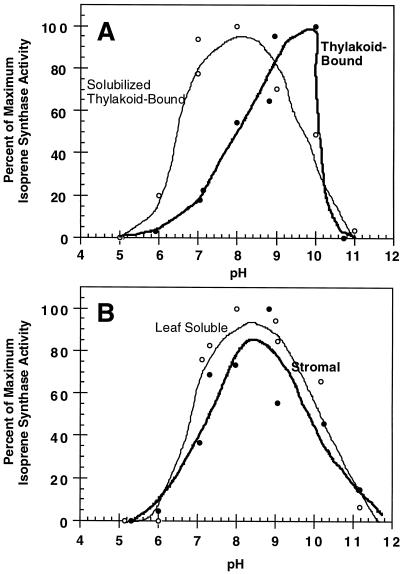
The solubilization of thylakoid-bound isoprene synthase by pH 10.0 treatment shifted the optimal activity of the enzyme from pH 10.0 to 8.0, as depicted in Figure 3A. In fact, the pH optimum for the thylakoid-bound enzyme is likely to be 8.0. At pH 10.0, a portion of the thylakoid-bound enzyme would be solubilized. Because pH 10.0 solubilization increases isoprene synthase activity, it could account for the nonphysiological pH optimum of 10.0 for the thylakoid-bound enzyme. As detailed by Wildermuth and Fall (1996), the pH 10.0 optimum for the thylakoid-bound isoprene synthase was confirmed using two different buffers and two separate pH-treatment protocols. The lack of activity exhibited by the bound and solubilized forms at pH 11.0 is probably the result of denaturation of the enzyme, thus accounting for the rapid decline in thylakoid-bound isoprene synthase activity.
Figure 3.
pH dependence of different forms of isoprene synthase with activity normalized to maximal activity. A, Profiles for thylakoid-bound isoprene synthase and solubilized thylakoid-bound isoprene synthase. The maximal isoprene synthase-specific activities for the thylakoid-bound and solubilized thylakoid-bound isoprene synthases are 30.8 and 209 pmol isoprene min−1 mg−1, respectively. B, pH curves for soluble isoprene synthase from a willow leaf-fractionation experiment (stromal) and for leaf-soluble isoprene synthase (obtained by grinding whole leaves in liquid nitrogen). The maximal isoprene synthase-specific activities for these samples are 75.3 and 166 pmol isoprene min−1 mg−1 for the stromal and leaf soluble isoprene synthases, respectively. Details for the thylakoid-bound isoprene synthase pH experiments are given in Wildermuth and Fall (1996). pH profiles for the other isoprene synthases were conducted similarly, as described in Methods. Results above are from a typical experiment, repeated several times.
The solubilized thylakoid-bound isoform exhibited a pH optimum and profile similar to that of soluble isoprene synthase from willow leaf fractionations (i.e. “stromal” isoprene synthase) and from willow leaf grinds (i.e. “leaf-soluble” isoprene synthase), as shown in Figure 3B. The comparison with leaf-soluble isoprene synthase is significant in that previously isolated, soluble isoprene synthases (aspen and velvet bean [Mucuna sp.]) were obtained using this method, extracting soluble proteins after leaves were ground in liquid nitrogen. Previous attempts at isolating leaf-soluble isoprene synthase from willow using the leaf-grind protocol were unsuccessful because secondary compounds in willow leaves prevented ammonium sulfate precipitation of the enzyme; the use of 40% PEG 3350 precipitation followed by CM-Sepharose batch treatment enabled willow leaf-soluble isoprene synthase to be isolated in a manner similar to aspen and other leaf-soluble isoprene synthases, but with a different concentration step. The similarities between the solubilized thylakoid-bound isoprene synthase, stromal isoprene synthase, and leaf-soluble isoprene synthase pH profiles and optima suggest that solubilized thylakoid-bound isoprene synthase is representative of soluble isoprene synthase from willow. In addition, the stromal and leaf-soluble results from willow correspond with those of soluble isoprene synthases from aspen and velvet bean leaves (Silver and Fall, 1991; Kuzma and Fall, 1993).
A comparison of the additional catalytic properties and Km and Mg2+ optima for the various forms of willow isoprene synthase (Table II) also supports the hypothesis that the solubilized thylakoid-bound and soluble forms of isoprene synthase are catalytically similar. Each form of isoprene synthase required Mg2+ for activity and exhibited optimal activity at 10 to 20 mm MgCl2, similar to previously reported results for soluble isoprene synthases from aspen and velvet bean (Silver and Fall, 1991; Kuzma and Fall, 1993). The apparent Km for DMAPP of the willow thylakoid-bound, solubilized thylakoid-bound, and soluble isoprene synthases were all in the 1 to 8 mm range, and were similar to the Km values of the aspen and velvet bean enzymes (Kuzma, 1995; Silver and Fall, 1995). The variation in Km values reported for a given isoprene synthase form is due to slightly different results obtained for experiments performed months apart. This variation is not the result of any difference in DMAPP preparation, quality, or storage. A similar variation in Km values (1–9 mm) was found for velvet bean leaf-soluble isoprene synthase (Kuzma, 1995). We are not yet able to explain the range of Km values obtained for different experiments but propose that it reflects slight changes in the activation state of the enzyme caused by unknown factors.
Table II.
Comparison of catalytic properties for thylakoid-bound, solubilized thylakoid-bound, and soluble isoprene synthases from willow leaves
| Catalytic Property | Thylakoid-Bound Isoprene Synthase | Solubilized Thylakoid-Bound Isoprene Synthase | Soluble (Stromal) Isoprene Synthase |
|---|---|---|---|
| Mg2+ optimum | 10–20 mm | 10–15 mm | 15–20 mm |
| pH optimum | 10 | 8 | 8 |
| Km (DMAPP) | 1 and 8 mm | 1 mm | 1 and 4 mm |
Thylakoid-bound and leaf-soluble isoprene synthase were prepared as described in Methods. pH 10.0 Solubilization of thylakoid-bound isoprene synthase followed by ammonium sulfate precipitation and dialysis into the appropriate buffer yielded solubilized thylakoid-bound isoprene synthase. Assays of catalytic properties are detailed in Methods. Results for thylakoid-bound isoprene synthase are from Wildermuth and Fall (1996), with the exception of the Km value of 1 mm obtained in a subsequent experiment.
Solubilized Thylakoid-Bound and Stromal Isoprene Synthases Exhibit Similar Inhibition by Small Molecules
To further examine whether solubilized thylakoid-bound and soluble isoprene synthases are similar, product-based inhibition and amino acid-directed inhibition were performed. Table III presents these results, which are best compared qualitatively, because the experiments were performed using partially purified enzymes. Product-based inhibitors were chosen based on the work of Silver (1994) in which pyrophosphate, a reaction product, but not phosphate was shown to inhibit soluble isoprene synthase from aspen. Solubilized thylakoid-bound and soluble isoprene synthases from willow were similarly inhibited by up to 69 to 100% by pyrophosphate, but not by phosphate.
Table III.
Comparison of product-based and amino acid-directed inhibition of solubilized thylakoid-bound and soluble isoprene synthases from willow leaves
| Inhibition | Inhibition of Isoprene
Synthase Activity
|
|
|---|---|---|
| Solubilized thylakoid-bound isoprene synthase | Soluble (stromal) isoprene synthase | |
| % | ||
| Product-based inhibition | ||
| 1 mm NaPi | 3 | 0 |
| 2.5 mm NaPi | 3 | naa |
| 1 mm NaPPi | 23 | 76 |
| 2.5 mm NaPPi | 69 | 100 |
| Amino acid-directed inhibition | ||
| Cys | ||
| 1.1 mm NEM | 100 | 100 |
| Substrate protected | 45 | 49 |
| His | ||
| 1.1 mm DEPC | 59 | 34 |
| Substrate protected | 71 | 0 |
| Arg | ||
| 50 mm PG | 100 | 100 |
| Substrate protected | 60 | 69 |
Inhibition results are displayed as percentages of inhibition of isoprene synthase activity relative to the corresponding uninhibited control set at 100. Isoprene production for the uninhibited controls ranged from 11.1 to 28.9 pmol isoprene min−1. Substrate protection experiments include a preincubation with 10 mm DMAPP and 8 mm Mg2+ before inhibition. Solubilized thylakoid-bound and soluble isoprene synthase-extraction protocols are given in Methods. Inhibition experiments are also detailed in Methods. Values presented are the average of two to three inhibition assays.
na, Not assayed.
Amino acid-directed inhibitors against Cys, His, and Arg were used because these residues are essential for a variety of isoprenoid synthases (Rajaonarivony et al., 1992b; Savage et al., 1995). Treatment of the isoprene synthases with 1.1 mm NEM for 10 min resulted in complete inactivation, similar to the finding for leaf-soluble isoprene synthase from aspen (Silver and Fall, 1995) and for various mono-terpene synthases (Savage et al., 1995). Preincubation with DMAPP and Mg2+ afforded significant protection of enzymatic activities (51–55%), suggesting that catalytically important cysteinyl residues reside at a substrate-protectable site of the enzymes. This finding is in agreement with those using monoterpene synthases from angiosperms (Savage et al., 1995).
Incubation of the isoprene synthases with 1.1 mm DEPC for 10 min resulted in 34 to 59% inhibition of activity, with longer incubations resulting in increased inhibition (data not shown). Incubation and assay conditions for His-directed inhibition with DEPC were chosen to favor reaction with His, not Cys (Lundblad, 1991; Rajaonarivony et al., 1992b). Preincubation with 4.4 mm His prevented inactivation of isoprene synthase activity (data not shown), confirming that histidyl residues are essential for activity. This is in agreement with the findings of Rajaonarivony et al. (1992b), who found evidence for an essential histidyl residue in limonene synthase and a variety of other terpene cyclases. We noted the difference in response to preincubation with DMAPP and Mg2+, with no protection of the solubilized thylakoid-bound isoform and complete protection of the stromal enzyme.
Treatment of the willow isoprene synthases with 50 mm phenylglyoxal for 30 min resulted in complete inactivation of the enzymes, with DMAPP and Mg2+ offering some protection (31–40%) in each case. Similarly, monoterpene synthases from both conifers and angiosperms appear to have catalytically important arginyl residues, although they differ in whether these residues appear to be located in the active site (Savage et al., 1995).
Ratios of Thylakoid-Bound to Stromal Isoprene Synthase Activities Do Not Change with Leaf Age or Leaf Illumination
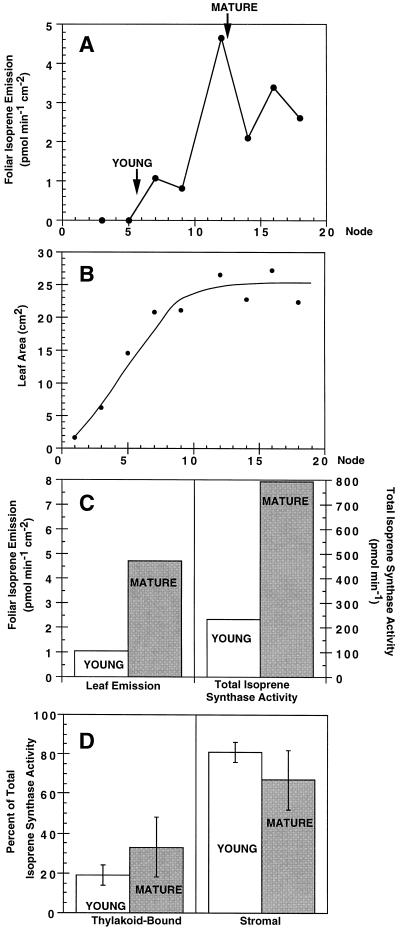
Another means of comparing two enzyme forms is to examine whether their activities are differentially regulated. Willow leaf fractionations were used to examine regulation of thylakoid-bound and stromal isoprene synthases by the foliar regulators leaf age and light. Foliar isoprene emission and soluble isoprene synthase activity have been shown to be dependent on leaf age, with induction as leaves mature (Kuzma and Fall, 1993). In the experiments shown in Figure 4, we first confirmed these findings for willow leaves. Willow foliar isoprene emission also demonstrated a developmental dependence (Fig. 4A), with full leaf expansion (Fig. 4B) required for maximal leaf isoprene emission. Willow leaves from node 6 were then chosen to represent young leaves because they were the youngest leaves to emit detectable levels of isoprene, and leaves from node 12 were chosen to represent mature leaves because they emitted maximal isoprene and were fully expanded. As shown in Figure 4C, leaf discs from pools of mature willow leaves (node 12) emitted 4.5-fold more isoprene than did those from young willow leaves (node 6), and pooled mature leaves had 3.4-fold higher total extractable isoprene synthase activity than did young leaves on a gram fresh weight basis. When the total isoprene synthase activity was expressed on a leaf-area basis for direct comparison with leaf-disc emission, mature leaves contained 3.9-fold more total isoprene synthase activity than did young leaves. Kuzma (1995) also found that, although leaf isoprene emission and isoprene synthase activity were tightly correlated with leaf development, leaf isoprene emission increased more than soluble isoprene synthase activity on a leaf-area basis. Figure 4D indicates that although total isoprene synthase activities changed dramatically with development, the percentage of activity in thylakoid-bound and stromal forms (approximately 25 and 75%, respectively, for these experiments) did not change significantly with leaf age.
Figure 4.
Effects of willow leaf age on isoprene emission and isoprene synthase isoforms. A, Foliar isoprene emission as a function of nodal position (leaf age), average of duplicate samples. B, Leaf area as a function of nodal position, average of duplicate samples. Leaves from node 6 (YOUNG) and node 12 (MATURE) from different willow stems were then pooled. Foliar isoprene emission and total isoprene synthase activity for these pooled samples (of 10 g fresh weight) are portrayed in C. The percentages of total isoprene synthase activity in thylakoid-bound and soluble forms for these pooled samples are shown in D. Results shown in C and D are averages of three separate experiments performed on consecutive days. Leaf isoprene emission rates are shown for leaves at PAR of 100 μmol m−2 s−1. Additional experiments (not shown) utilized PAR of 1000 μmol m−2 s−1 and obtained a similar profile to A with increased emission rates. Further details are given in Methods.
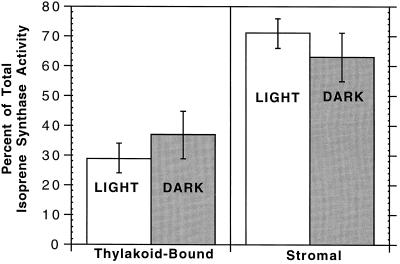
Because foliar isoprene production is light dependent, light could modulate the ratio of thylakoid-bound and stromal isoprene synthase activities. Light is a known stimulant of palmitoylation (e.g. D1 polypeptide; Mattoo et al., 1993), which may be sufficient for membrane anchoring (Bhatnagar and Gordon, 1997). As illustrated in Figure 5, the percentage of isoprene synthase activity in each form (35% bound and 65% soluble) was independent of leaf light-extraction conditions. In addition, total enzyme activity did not change significantly with leaf light-extraction conditions, similar to results obtained with leaf-soluble isoprene synthase from velvet bean (M. Wildermuth, unpublished data).
Figure 5.
Percentage of total isoprene synthase activity in thylakoid-bound and stromal forms from whole-leaf isolations performed with 10 g of willow leaves left in the dark overnight (DARK) and fractionated in the dark, or leaves exposed to 800 μmol m−2 s−1 for 2 h (LIGHT) and fractionated under low light (50–100 μmol m−2 s−1). Details are given in Methods. Shown is an average of two separate experiments performed on consecutive days. Average total isoprene synthase activities for the DARK and LIGHT leaf fractionations are 909 ± 381 and 1635 ± 278 pmol isoprene min−1, respectively.
DISCUSSION
Detection of Plastidic Stromal and Thylakoid-Bound Forms of Isoprene Synthase
Here we report the detection of soluble and thylakoid-bound isoprene synthase activities from willow leaves and intact chloroplasts. Soluble isoprene synthase activity accounts for 50 to 75% of total activity, with thylakoid-bound isoprene synthase activity representing 25 to 50% of total activity. As discussed in “Results,” these activities appear to be plastidic in origin. However, the existence of a cytosolic isoprene synthase has not been specifically disproved. Both soluble and thylakoid-bound activities are enzymatic, because they are protease and heat sensitive and are inhibited by small molecules that covalently interact with specific amino acids (see Table III).
In vivo occurrence of the stromal and thylakoid-bound isoprene synthases was examined from two vantage points. First, we wanted to establish that the two enzyme activities were also present in other isoprene-emitting species and not due to the false activity of another enzyme (e.g. prenyltransferase) capable of converting DMAPP to isoprene when exposed to nonphysiological levels of DMAPP. Using the protocol we developed for willow, we were able to detect soluble and thylakoid-bound isoprene synthase activities in aspen leaves (M. Wildermuth and R. Fall, unpublished data). Therefore, the presence of the soluble and bound forms is not a species-specific result limited to willow. In addition, fractionation of spinach leaves (which do not emit isoprene) did not result in detectable soluble or bound isoprene synthase activities.
Second, we wanted to determine if the two isoprene synthase forms are artifacts of the extraction process. The stromal or thylakoid-bound isoprene synthase activity could result from an alteration of normal hydrogen bonding or ionic interactions. Either false dissociation of the thylakoid-bound isoprene synthase, yielding a soluble activity, or artificial association of the soluble enzyme, resulting in bound activity, may occur. For example, if solely thylakoid-bound isoprene synthase exists in vivo, soluble activity could result from dissociation of the thylakoid-bound form under the ionic/pH conditions of the isolation. A number of isoprenoid enzymes are weakly associated with membranes and may be released during the isolation process. For example, DMAPP:umbelliferone dimethylallyltransferase is released from membranes at low ionic strengths (Dhillon and Brown, 1976). Alternatively, thylakoid-bound enzymes may be part of a bound multienzyme complex, which can be solubilized at high ionic strength (e.g. Calvin-cycle enzymes; Suss et al., 1993). Finally, the association of soluble Rubisco with thylakoids has been correlated with the pH and ionic strength of the chloroplast lysate buffer; however, under conditions of maximal binding, only 8% of Rubisco activity was thylakoid bound (Makino and Osmond, 1991). Thylakoid-bound isoprene synthase accounts for approximately one-half of total isoprene synthase activity and is tightly bound to the membrane. It is not released by low- or high-ionic-strength treatments or a variety of other solubilization protocols (Wildermuth and Fall, 1996). Therefore, it is unlikely that either the stromal or the thylakoid-bound isoform results from an artificial dissociation or association. As discussed in “Results,” during the leaf-extraction process, nonphysiological proteolysis may result in a thylakoid-bound enzyme being artificially solubilized. Attempts to inhibit or promote proteolysis of thylakoid-bound isoprene synthase were unsuccessful, suggesting that nonphysiological proteolysis of thylakoid-bound isoprene synthase is not responsible for the soluble isoform.
Biochemical Characterization of Stromal and Thylakoid-Bound Isoprene Synthases
Are stromal and thylakoid-bound isoprene synthases similar or nearly identical enzymes? To address this question, biochemical characterization of the enzyme forms was undertaken. Experiments were focused on catalytic properties because differences in catalytic properties can aid in identifying the degree of similarity between two enzymes. As shown in Table II, soluble and thylakoid-bound isoprene synthases have similar Mg2+ optima and Km values for DMAPP, but differ in their pH optima. However, as discussed earlier, it is likely that the pH optimum (10.0) for thylakoid-bound isoprene synthase is the result of solubilization of the enzyme into a more active form.
In addition to the similar catalytic properties discussed above, the two enzyme forms are comparably inhibited by product-based and amino acid-directed compounds. As shown in Table III, stromal and solubilized thylakoid-bound isoprene synthases are inhibited by the reaction product pyrophosphate, but not by phosphate, similar to leaf soluble isoprene synthase (Silver, 1994). Amino acid-directed inhibitors indicated that both soluble and solubilized thylakoid-bound isoprene synthase contain essential Cys, His, and Arg residues, as do other isoprenoid synthases (Rajaonarivony et al., 1992b; Savage et al., 1995). The partial substrate protection afforded to the isoprene synthases for arginyl-directed reagents implicates an essential Arg residue(s) in the active site of the enzyme. Isoprenoid synthases characterized to date contain a conserved DDXXD sequence and essential Arg implicated in binding the isoprenyl diphosphate/Mg2+ complexes (Chen et al., 1994; Tarshis et al., 1994; Savage et al., 1995). Similar to monoterpene synthases from angiosperms (Savage et al., 1995), both forms of willow isoprene synthase are sensitive to the sulfhydryl reagent NEM, implicating a Cys residue in catalysis. The variation in response to substrate protection for the histidyl-directed reagent DEPC suggests that the solubilized thylakoid-bound isoprene synthase is not identical to the stromal enzyme. Perhaps the solubilized thylakoid-bound isoprene synthase has a slightly altered conformation as a result of solubilization or it is a monomer, whereas the soluble isoform is a dimer.
Coordinate Regulation of Stromal and Thylakoid-Bound Isoforms
The ratio of bound to soluble isoprene synthase activity was independent of two regulators of foliar isoprene emission: leaf age and light. Leaf age regulates the capacity of a leaf to emit isoprene (i.e. its basal emission rate). Because extractable isoprene synthase activity correlated with foliar isoprene emission during leaf development, our extraction and assay conditions appear to adequately represent in vivo changes in the basal emission rate. Changes in the ratio of bound to soluble isoprene synthase activity might indicate that the two enzymes were differentially expressed. In addition, one protein may be developmentally regulated with respect to the presence of a lipid modification. Cleavage of a phosphatidylinositol lipid membrane anchor has been shown to be developmentally controlled, resulting in differing soluble-to-bound ratios as a function of development (Hortsch and Goodman, 1990), and plant protein isoprenylation appears to be developmentally regulated in some cases (e.g. Morehead et al., 1995).
Light modulates instantaneous leaf isoprene emission, and light-dependent dynamic regulation of isoprene production may occur by a number of mechanisms (see Wildermuth and Fall, 1996). Although we found no difference in the ratio of stromal to thylakoid-bound isoprene synthase activities when dark- or light-exposed leaves were extracted in the dark or light, respectively, this result may only account for nondynamic changes in anchoring. In special cases, such as that of the D1 polypeptide of PSII, light-dependent dynamic anchoring is rapidly reversible (Mattoo et al., 1989). In addition, because total extractable activity did not change with light/dark extraction conditions, either we did not capture the full light activation of the isoprene synthases or, conversely, we artificially represented the light activation of the enzymes by assaying for isoprene synthase activity at pH 8.0 and 8 mm Mg2+, conditions similar to those of the plastid stroma in the light. The high Km values for DMAPP (1–8 mm) for both thylakoid-bound and stromal forms of isoprene synthase suggests that we did not assay the isoprene synthases in their light-activated states, which presumably would have lower Km values than the dark-inactive enzymes. In addition, the light-activated states of the thylakoid-bound and stromal isoprene synthases may differ as they do for stromal and thylakoid light-harvesting complex II phosphatase activities (Hammer et al., 1995). For example, a thylakoid-bound protein may exclusively activate thylakoid-bound isoprene synthase in the light.
Attempts to activate thylakoid-bound isoprene synthase (via phosphorylation or the Fd/thioredoxin system) using an in vitro willow thylakoid system with conditions similar to those described by Elich et al. (1992) and Queiroz-Claret and Meunier (1995) have not yet resulted in activation. The Km values for prenyldiphosphate-utilizing plastidic enzymes are typically 1 to 10 μm, e.g. limonene synthase has a Km for geranyl diphosphate of 1.8 μm (Rajaonarivony et al., 1992a). Unless plastidic DMAPP levels increase greater than an order of magnitude in the light, it is unlikely that the isoprene synthases truly have such high Km values in vivo. Methods for quantitating DMAPP levels in leaves and plastids need to be developed, perhaps based on the work of McCaskill and Croteau (1993), to measure light-dependent changes in plastidic DMAPP levels and to ascertain the validity of the in vitro Km values we have measured.
Implications for the Regulation and the Role of Isoprene Formation
The existence of stromal and thylakoid-bound isoforms of isoprene synthase may provide insight into the regulation and the possible function(s) of isoprene production. Recent studies report that light-activated plastidic enzymes (such as the Calvin-cycle enzymes sedoheptulose bisphosphatase, phosphoribulokinase, NADP-GAPD, and Fru bisphosphatase) are present in the stroma and are bound to thylakoids (Hermoso et al., 1989, 1992; Adler et al., 1993; Suss et al., 1993; Anderson et al., 1996). As Anderson et al. (1996) discuss, perhaps this thylakoid association promotes the efficient use of coupling-factor-1-generated ATP by phosphoribulokinase; Pi (generated by sedoheptulose bisphosphatase and Fru bisphosphatase) by coupling factor 1 to produce ATP; and NADPH (produced by Fd-NADP reductase) by NADP-GAPD. In addition, reductive light modulation of a redox-sensitive disulfide on a thylakoid-bound enzyme by thioredoxin would be facilitated. In this scenario, the thylakoid-bound enzymes are most active in vivo, with the soluble forms using excess substrate or cofactor. Isoprene biosynthesis is light dependent (e.g. Monson and Fall, 1989), and elucidation of the mechanisms responsible for the light activation of isoprene synthase may support a specific regulatory role for its thylakoid association. In addition, the conversion of DMAPP to isoprene results in the production of pyrophosphate (Silver and Fall, 1995). Hydrolysis by a thylakoid-bound pyrophosphatase (e.g. Jiang et al., 1997) may facilitate isoprene biosynthesis by removing inhibitory inorganic pyrophosphate; the Pi produced could also then be available to coupling factor 1 for the production of ATP.
Perhaps the stromal and thylakoid-bound isoprene synthases serve a protective function in chloroplasts, and the isoforms act similar to the stromal and thylakoid-bound ascorbate peroxidases. Miyake and Asada (1992) proposed that thylakoid-bound ascorbate peroxidase is the primary scavenger of hydrogen peroxide photoproduced at the thylakoids, and that stromal ascorbate peroxidase acts as a secondary scavenging system for any additional nonreduced hydrogen peroxide. If isoprene acts to stabilize plant membranes against thermal damage, as postulated by Sharkey and Singsaas (1995), it would be beneficial to produce isoprene at the thylakoids for direct partitioning of this nonpolar hydrocarbon into the thylakoid bilayer. The production of isoprene in the stroma by the stromal isoform would presumably allow isoprene to partition into other plant membranes as it exits from the plastid stroma out of the leaf. It is not yet known if exposure of leaves grown at low temperature to high temperature, a stress that is known to increase the isoprene emission rate from zero to high levels in kudzu (Sharkey and Loreto, 1993) and aspen (Monson et al., 1994) leaves, will lead to the coordinate expression of both the stromal and thylakoid-bound isoforms of isoprene synthase. Determining whether isoprene synthase isoforms are encoded by one or two genes, and characterizing these genes, will help to unravel the complexity of plastidic isoprene biosynthesis.
ACKNOWLEDGMENTS
We thank the following individuals for their assistance in this work: Michele Nemecek-Marshall for valuable advice; James Rhudy for willow isoprene synthase extractions; Felicia Tomasko for care of greenhouse plants; and Cheryl Wojciechowski for synthesis of DMAPP and maintenance and calibration of the gas chromatograph.
Abbreviations:
- Benz-HCl
benzamidine hydrochloride
- Chl
chlorophyll
- DEPC
diethylpyrocarbonate
- DMAPP
dimethylallyl diphosphate
- GAPD
glyceraldehyde-3-phosphate dehydrogenase
- GB
grinding buffer
- NEM
N-ethylmaleimide
- PEB
plant-extract buffer
- PG
phenylglyoxal
Footnotes
This research was supported by grants from the National Science Foundation (ATM-9312153 and ATM-9633285), the Southern Oxidants Study (North Carolina State University subcontract 91-0074-12), and the U.S. Environmental Protection Agency (R 825259-01-0). M.C.W. was also supported by the National Science Foundation Atmospheric Chemistry Traineeship (EAR-9256339).
LITERATURE CITED
- Adler K, Arkona C, Manteuffel R, Suss K-H. Electron-microscope localization of chloroplast proteins by immunogold labelling on cryo-embedded spinach leaves. Cell Biol Int. 1993;17:213–220. [Google Scholar]
- Anderson LE, Gibbons JT, Wang X. Distribution of ten enzymes of carbon metabolism in pea (Pisum sativum) chloroplasts. Int J Plant Sci. 1996;157:525–538. [Google Scholar]
- Bach TJ. Some new aspects of isoprenoid biosynthesis in plants: a review. Lipids. 1995;30:191–202. doi: 10.1007/BF02537822. [DOI] [PubMed] [Google Scholar]
- Bhatnagar RS, Gordon JI. Understanding covalent modifications of proteins by lipids: where cell biology and biophysics mingle. Trends Cell Biol. 1997;7:14–20. doi: 10.1016/S0962-8924(97)10044-7. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chameides W, Lindsay R, Richardson J, Kiang C. The role of biogenic hydrocarbons in urban photochemical smog: Atlanta as a case study. Science. 1988;241:1473–1475. doi: 10.1126/science.3420404. [DOI] [PubMed] [Google Scholar]
- Chappell J. Biochemistry and molecular biology of the isoprenoid biosynthetic pathway in plants. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:521–547. [Google Scholar]
- Chen A, Kroon PA, Poulter CD. Isoprenyl diphosphate synthases: protein sequence comparisons, a phylogenetic tree, and predictions of secondary structure. Protein Sci. 1994;3:600–607. doi: 10.1002/pro.5560030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon DS, Brown SA. Localization, purification, and characterization of dimethylallyl pyrophosphate: umbelliferone dimethylallyltransferase from Ruta graveolens. Arch Biochem Biophys. 1976;177:74–83. doi: 10.1016/0003-9861(76)90417-3. [DOI] [PubMed] [Google Scholar]
- Elich TD, Edelman M, Mattoo AK. Identification, characterization, and resolution of the in vivo phosphorylated form of the D1 photosystem II reaction center protein. J Biol Chem. 1992;267:3523–3529. [PubMed] [Google Scholar]
- Ferri G, Comerio G, Iadarola P, Zapponi MC, Speranza ML. Subunit structure and activity of glyceraldehyde-3-phosphate dehydrogenase from spinach chloroplasts. Biochim Biophys Acta. 1978;522:19–31. doi: 10.1016/0005-2744(78)90318-2. [DOI] [PubMed] [Google Scholar]
- Gegenheimer P. Preparation of extracts from plants. Methods Enzymol. 1990;182:174–193. doi: 10.1016/0076-6879(90)82016-u. [DOI] [PubMed] [Google Scholar]
- Gray JC, Rochford RJ, Packman LC. Proteolytic removal of the C-terminal transmembrane region of cytochrome f during extraction from turnip and charlock leaves generates a water-soluble monomeric form of the protein. Eur J Biochem. 1994;223:481–488. doi: 10.1111/j.1432-1033.1994.tb19016.x. [DOI] [PubMed] [Google Scholar]
- Greenberg JP, Zimmerman PR, Taylor BE, Silver GM, Fall R. Sub-parts per billion detection of isoprene using a reduction gas detector with a portable gas chromatograph. Atmos Environ. 1993;27A:2689–2692. [Google Scholar]
- Guenther A, Zimmerman P, Wildermuth M. Natural volatile organic compound emission rate estimates for U.S. woodland landscapes. Atmos Environ. 1994;28:1197–1210. [Google Scholar]
- Hager A, Holocher K. Localization of the xanthophyll-cycle enzyme violaxanthin de-epoxidase within the thylakoid lumen and abolition of its mobility by a (light-dependent) pH decrease. Planta. 1994;192:581–589. [Google Scholar]
- Hammer MF, Sarath G, Osterman JC, Markwell J. Assessing modulation of stromal and thylakoid light-harvesting complex-II phosphatase activities with phosphopeptide substrates. Photosynth Res. 1995;44:107–115. doi: 10.1007/BF00018301. [DOI] [PubMed] [Google Scholar]
- Hemingway RW, Karchesy JJ (1988) Chemistry and Significance of Condensed Tannins. Plenum Press, New York
- Hermoso R, de Felipe MR, Vivo A, Chueca A, Lazaro JJ, Gorge JL. Immunogold localization of photosynthetic fructose-1,6-bisphosphatase in pea leaf tissue. Plant Physiol. 1989;89:381–385. doi: 10.1104/pp.89.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermoso R, Fonolla J, de Felipe MR, Vivo A, Chueca A, Lazaro JJ, Lopez-Gorge JL. Double immunogold localization of thioredoxin f and photosynthetic fructose-1,6-bisphosphatase in spinach leaves. Plant Physiol Biochem. 1992;30:39–46. [Google Scholar]
- Hortsch M, Goodman CS. Drosophila fasciclin I, a neural cell adhesion molecule, has a phosphatidylinositol lipid membrane anchor that is developmentally regulated. J Biol Chem. 1990;265:15104–15109. [PubMed] [Google Scholar]
- Jiang SS, Fan LL, Yang SJ, Kuo SY, Pan RL. Purification and characterization of thylakoid membrane-bound inorganic pyrophosphatase from Spinacia oleracea L. Arch Biochem Biophys. 1997;346:105–112. doi: 10.1006/abbi.1997.0279. [DOI] [PubMed] [Google Scholar]
- Julkunen-Tiitto R, Tahvanainen J, Silvola J. Increased CO2 and nutrient status changes affect phytomass and the production of plant defensive secondary chemicals in Salix myrsinifolia (Salisb.) Oecologia. 1993;95:495–498. doi: 10.1007/BF00317433. [DOI] [PubMed] [Google Scholar]
- Kuzma J (1995) Regulation of isoprene production from plants and microorganisms. PhD thesis. University of Colorado, Boulder
- Kuzma J, Fall R. Leaf isoprene emission rate is dependent on leaf development and the level of isoprene synthase. Plant Physiol. 1993;101:435–440. doi: 10.1104/pp.101.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. [Google Scholar]
- Linder ME, Middleton P, Hepler JR, Taussig R, Gilman AG, Mumby SM. Lipid modifications of G proteins: α subunits are palmitoylated. Proc Natl Acad Sci USA. 1993;90:3675–3679. doi: 10.1073/pnas.90.8.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad RL. Chemical Reagents for Protein Modification, Ed 2. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- Makino A, Osmond B. Solubilization of ribulose-1,5-bisphosphate carboxylase from membrane fraction of pea leaves. Photosynth Res. 1991;29:79–85. doi: 10.1007/BF00035378. [DOI] [PubMed] [Google Scholar]
- Mattoo AK, Callahan FE, Mehta RA, Ohlrogge JB. Rapid in vivo acylation of acyl carrier protein with exogenous fatty acids in Spirodela oligorrhiza. Plant Physiol. 1989;89:707–711. doi: 10.1104/pp.89.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo AK, Elich TD, Ghirardi ML, Callahan FE, Edelman M. Post-translational modification of chloroplast proteins and the regulation of protein turnover. In: Battey NH, Dickinson HG, Hetherington AM, editors. Post-Translational Modifications in Plants. Cambridge, UK: Cambridge University Press; 1993. pp. 65–78. [Google Scholar]
- McCaskill D, Croteau R. Procedures for the isolation and quantification of the intermediates of the mevalonic acid pathway. Anal Biochem. 1993;215:142–149. doi: 10.1006/abio.1993.1566. [DOI] [PubMed] [Google Scholar]
- McGarvey DJ, Croteau R. Terpenoid metabolism. Plant Cell. 1995;7:1015–1026. doi: 10.1105/tpc.7.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake C, Asada K. Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radicals in thylakoids. Plant Cell Physiol. 1992;33:541–553. [Google Scholar]
- Monson RK, Fall R. Isoprene emission from aspen leaves, influence of environment and relation to photosynthesis and photorespiration. Plant Physiol. 1989;90:267–274. doi: 10.1104/pp.90.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson RK, Harley PC, Litvak ME, Wildermuth M, Guenther AB, Zimmerman PR, Fall R. Environmental and developmental controls over the seasonal pattern of isoprene emission from aspen leaves. Oecologia. 1994;99:260–270. doi: 10.1007/BF00627738. [DOI] [PubMed] [Google Scholar]
- Morehead TA, Biermann BJ, Crowell DN, Randall SK. Changes in protein isoprenylation during the growth of suspension-cultured tobacco cells. Plant Physiol. 1995;109:277–284. doi: 10.1104/pp.109.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperberg DR, Morrison DF, O'Brien PJ. Depalmitoylation of rhodopsin with hydroxylamine. Methods Enzymol. 1995;250:348–360. doi: 10.1016/0076-6879(95)50084-7. [DOI] [PubMed] [Google Scholar]
- Queiroz-Claret C, Meunier JC. Light activation of sedoheptulose-1,7-bisphosphatase by a reconstituted thylakoid system compared with DTT activation: evidence for protein-enzyme interactions. J Plant Physiol. 1995;145:45–49. [Google Scholar]
- Rajaonarivony JIM, Gershenzon J, Croteau R. Characterization and mechanism of (4S)-limonene synthase, a monoterpene cyclase from the glandular trichomes of peppermint (Mentha × piperita) Arch Biochem Biophys. 1992a;296:49–57. doi: 10.1016/0003-9861(92)90543-6. [DOI] [PubMed] [Google Scholar]
- Rajaonarivony JIM, Gershenzon J, Miyazaki J, Croteau R. Evidence for an essential histidine residue in 4S-limonene synthase and other terpene cyclases. Arch Biochem Biophys. 1992b;299:77–82. doi: 10.1016/0003-9861(92)90246-s. [DOI] [PubMed] [Google Scholar]
- Savage TJ, Ichii H, Hume SD, Little DB, Croteau R. Mono-terpene synthases from gymnosperms and angiosperms: stereospecificity and inactivation by cysteinyl- and arginyl-directed modifying reagents. Arch Biochem Biophys. 1995;320:257–265. doi: 10.1016/0003-9861(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Schnitzler J-P, Arenz R, Steinbrecher R, Lehning A. Characterization of an isoprene synthase from leaves of Quercus petrae (Mattuschka) Liebl. Bot Acta. 1996;109:216–221. [Google Scholar]
- Sharkey TD, Loreto F. Water stress, temperature, and light effects on the capacity for isoprene emission and photosynthesis of kudzu leaves. Oecologia. 1993;95:328–333. doi: 10.1007/BF00320984. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Singsaas EL. Why plants emit isoprene. Science. 1995;374:769. [Google Scholar]
- Shechter I, Klinger E, Rucker ML, Engstrom RG, Spirito JA, Islam MA, Boettcher BR, Weinstein DB. Solubilization, purification, and characterization of a truncated form of rat hepatic squalene synthetase. J Biol Chem. 1992;267:8628–8635. [PubMed] [Google Scholar]
- Silver G (1994) Discovery, purification, and characterization of isoprene synthase from aspen leaf extracts. PhD thesis. University of Colorado, Boulder
- Silver GM, Fall R. Enzymatic synthesis of isoprene from dimethylallyl diphosphate in aspen leaf extracts. Plant Physiol. 1991;97:1588–1591. doi: 10.1104/pp.97.4.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver GM, Fall R. Characterization of aspen isoprene synthase, an enzyme responsible for leaf isoprene emission to the atmosphere. J Biol Chem. 1995;270:13010–13016. doi: 10.1074/jbc.270.22.13010. [DOI] [PubMed] [Google Scholar]
- Suss K-H, Arkona C, Manteuffel R, Adler K. Calvin cycle multienzyme complexes are bound to chloroplast thylakoid membranes of higher plants in situ. Proc Natl Acad Sci USA. 1993;90:5514–5518. doi: 10.1073/pnas.90.12.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarshis LC, Yan M, Poulter DC, Sacchettini JC. Crystal structure of recombinant farnesyl diphosphate synthase at 2.6-Å resolution. Biochemistry. 1994;33:10871–10877. doi: 10.1021/bi00202a004. [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Fall R. Light-dependent isoprene emission. Characterization of a thylakoid-bound isoprene synthase in Salix discolor chloroplasts. Plant Physiol. 1996;112:171–182. doi: 10.1104/pp.112.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler JG, Lichtenthaler HK, May HU, Lichtenthaler FW. Is isoprene emitted by plants synthesized via the novel isopentenyl pyrophosphate pathway? Z Naturforsch. 1997;52c:15–23. [Google Scholar]
- Zeng F-Y, Weigel PH. Fatty acylation of the rat and human asialoglycoprotein receptors. J Biol Chem. 1996;271:32454–32460. doi: 10.1074/jbc.271.50.32454. [DOI] [PubMed] [Google Scholar]
- Zimmerman PR (1979) Tampa Bay photochemical oxidant study. Determination of emission rates of hydrocarbon species from indigenous species of vegetation in the Tampa/St. Petersburg, Florida area. Report EPA 904/977-028. Environmental Protection Agency, Research Triangle Park, NC