Abstract
Introduction
To examine changes in insulin regimens and glycemic control during the 24 months after initiation of insulin in patients with type 2 diabetes mellitus.
Methods
Data were collected over a 24-month period from patients requiring insulin initiation as part of usual care, in a prospective, observational study. Changes in insulin regimens and hemoglobin A1c (HbA1c) were examined within countries (Germany, Greece, Spain) and overall.
Results
Prandial insulin only was most commonly initiated in Germany, while basal or premixed formulations were initiated in Greece and Spain. In Germany, compared with Greece or Spain, the patients were slightly younger and had a shorter diabetes duration when initiating insulin. For patients overall, 76.1% did not change their insulin regimen between initiation and 24 months. The most obvious change was a shift from prandial to basal/bolus in Germany, with almost doubling of mean daily insulin dose; in Greece and Spain, more patients stopped using insulin and the trend to more complex regimens was not seen. Overall, mean (SD) HbA1c decreased from baseline (9.4 [1.7]%) to 6 months (7.2 [1.0]%), but with little further change through 24 months (7.2 [1.1]%). HbA1c change with basal/bolus insulin (−2.6 [2.0]%, baseline 10.1%) was greater than with basal only (−2.0 [1.8]%, baseline 9.3%). Mean HbA1c less than 7% was achieved and maintained over 24 months in Germany, but was not achieved at any time in Greece or Spain.
Conclusions
Within 24 months of insulin initiation, the majority of patients with type 2 diabetes remained on the same insulin regimen initially instigated, despite the well-established progressive loss of prandial and basal endogenous insulin secretion. Adequate glycemic control was best achieved where insulin dosage adjustments and insulin intensification took place.
Keywords: Basal/bolus insulin, Glycemic control, Insulin therapy, Insulin regimen, Prandial insulin, Type 2 diabetes
Introduction
Position statements from the American Diabetes Association and the European Association for the Study of Diabetes recommend an individual target of hemoglobin A1c (HbA1c) [1–3]. The targets and the management of glycemia should be individualized and must be an integrated part of overall care for each particular patient [1, 3, 4].
For patients with type 2 diabetes mellitus, clinical guidelines generally propose a step-wise progression of pharmacotherapy, with basal insulin as the next step when lifestyle changes and initial oral therapies have failed [1, 5]. Various consecutive insulin regimens have been suggested, although with conflicting results regarding the use of basal versus prandial insulin [6, 7] and with little consensus regarding the best options for intensification [2–5, 8]. The UK Prospective Diabetes Study (UKPDS) group reported that an initial decrease in HbA1c was seen when patients started insulin, but there was a continual increase when followed long-term [9]. This is in contrast to the Outcome Reduction with Initial Glargine Intervention (ORIGIN) trial, which showed that in early diabetes basal insulin only could be a long-term option [10]. Therefore, it is unclear how alternative insulin regimens perform under real-world conditions in regard to glycemic control.
The INSulin TItration-GAining an understanding of the burden of Type 2 diabetes in Europe (INSTIGATE) observational study was designed to examine patients with type 2 diabetes who initiated insulin as part of their usual care [11]. Patients were initially recruited in France, Germany, Greece, Spain, and the UK, and results during the first 6 months have been reported [12]. Further follow-up continued in Germany, Greece, and Spain, and the present paper describes the changing clinical outcomes during the 24-month follow-up period.
Patients and Methods
Patient Population and Study Design
Patients were recruited at primary and secondary care centres that routinely treated a large number of patients with type 2 diabetes, according to normal country-specific treatment practices for insulin initiation. Investigators were diabetologists, endocrinologists, and primary care physicians who were either directly responsible for initiating insulin therapy or were actively involved in further routine management of patients who had initiated insulin therapy. The study was noninterventional and, therefore, all treatment decisions were made by the physicians in consultation with the patients as part of usual care. For each country, local requirements for ethical review, informed consent, and other regulatory approvals for an observational study were met.
Patient characteristics, therapy prior to insulin initiation, and differences between participating countries were described previously [11]. Patients had contact with their physicians within the normal course of therapy, and information was collected at baseline and approximately 3, 6, 12, 18, and 24 months after insulin initiation. For each patient throughout the study, insulin regimens and use of oral antidiabetic drugs (OADs) were recorded. HbA1c and fasting blood glucose concentrations were measured at local laboratories using standard methods. Self-reported episodes of hypoglycemia were recorded retrospectively at each visit.
Statistical Analysis
Demographic characteristics, treatment status, and HbA1c concentrations were assessed using descriptive summary statistics, with mean, SD, median, minimum, maximum, and quartiles calculated for continuous variables. Depending on skewing of the data, either mean and SD or median and quartiles were used for description. For categorical variables, absolute number of patients and percentages based on total number of patients per country and overall were calculated.
Results
A total of 564 patients, out of 726 taking part in the initial 6-month part of the INSTIGATE study, were followed for up to 24 months in Germany (155/256 patients; 60.5%), Greece (237/263; 90.1%), and Spain (172/207; 83.1%). The characteristics at the time of insulin initiation of these patients, as well as of those only participating in the initial 6-month part of the study, are summarized in Table 1. Patients starting insulin were, on average, younger and with a shorter duration of diabetes in Germany compared with Greece or Spain, although HbA1c and fasting glucose concentrations were similar.
Table 1.
Baseline characteristics of patients in Germany, Greece, and Spain who continued in the INSTIGATE study for up to 24 months after initiation of insulin, and for patients in these countries overall who continued or did not continue beyond 6 months in the study
| Overall (N = 726) | Patients continuing the study for up to 24 months (N = 564) | ||||
|---|---|---|---|---|---|
| Patients not continuing | Patients continuing | Germany | Greece | Spain | |
| Number of patients | 162 | 564 | 155 | 237 | 172 |
| Completed 24-month period (n, %) | – | 498 (88.3) | 119 (76.8) | 227 (95.8) | 152 (88.4) |
| Age (years) | 63.6 (13.5) | 64.3 (11.4) | 61.0 (12.1) | 66.0 (9.9) | 64.9 (12.0) |
| Gender, male/female (%) | 56.6/43.4 | 54.4/45.6 | 56.1/43.9 | 52.3/47.7 | 55.8/44.2 |
| Time since diagnosis (years) | 7.8 (8.0) | 10.1 (7.2) | 6.5 (6.2) | 11.8 (7.0) | 10.9 (7.1) |
| BMI (kg/m2) | 29.6 (6.2) | 29.3 (5.4) | 30.6 (5.9) | 28.2 (4.7) | 29.6 (5.7) |
| HbA1c (%) | 9.3 (2.0) | 9.4 (1.7) | 9.2 (2.0) | 9.7 (1.6) | 9.2 (1.5) |
| Fasting glucose (mmol/L) | 11.9 (4.8) | 12.2 (4.0) | 11.7 (4.7) | 12.8 (3.9) | 11.9 (3.5) |
Values are mean (SD), except for patients completing 24 months and gender which are percentages of patients; mean (SD) are based on the number of patients with non-missing values and percentages are based on the total number of patients
BMI body mass index, HbA 1c hemoglobin A1c, INSTIGATE INSulin TItration-GAining an understanding of the burden of Type 2 diabetes in Europe
Insulin regimens being used at baseline, 12, and 24 months after insulin initiation are summarized in Table 2. Prandial insulin only was initially used by a large proportion of patients in Germany (46.5%) and this decreased by 12 months (30.1%), with a concomitant increase in use of a basal/bolus regimen. In Greece and Spain, basal insulin only or premixed formulations were used by most patients and there were only small changes from baseline up to 24 months. Of the 498 patients overall with data at 24 months, 379 (76.1%) did not change their regimen between initiation and 24 months. Of the patients who did change, 16 on basal only and 16 on prandial only at baseline shifted to a basal/bolus regimen. During the observation period of 24 months, 48 patients (9.6%) stopped the insulin treatment, the majority of whom (27 patients) had started on basal only.
Table 2.
Insulin regimens used at insulin initiation and 12 and 24 month after insulin initiation
| Overall | Germany | Greece | Spain | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 months | 24 months | Baseline | 12 months | 24 months | Baseline | 12 months | 24 months | Baseline | 12 months | 24 months | |
| Evaluable patients | 564 | 562 | 498 | 155 | 153 | 119 | 237 | 237 | 227 | 172 | 172 | 152 |
| Insulin regimen (%) | ||||||||||||
| Basal only | 44.5 | 40.4 | 39.2 | 11.6 | 7.8 | 12.6 | 49.4 | 43.5 | 38.3 | 67.4 | 65.1 | 61.2 |
| Premixed only | 21.6 | 22.4 | 20.1 | 8.4 | 7.2 | 7.6 | 30.8 | 32.1 | 29.1 | 20.9 | 22.7 | 16.4 |
| Prandial only | 17.7 | 12.5 | 12.4 | 46.5 | 30.1 | 31.1 | 6.3 | 5.9 | 7.5 | 7.6 | 5.8 | 5.3 |
| Basal/bolus | 14.0 | 20.8 | 16.7 | 29.7 | 49.0 | 41.2 | 12.2 | 15.6 | 13.7 | 2.3 | 2.9 | 2.0 |
| Other | 2.1 | 3.2 | 2.0 | 3.9 | 5.2 | 0.8 | 1.3 | 2.5 | 2.2 | 1.7 | 2.3 | 2.6 |
| No insulins | 0 | 0.7 | 9.6 | 0 | 0.7 | 6.7 | 0 | 0.4 | 9.3 | 0 | 1.2 | 12.5 |
Values show percentages of evaluable patients
Mean (SD) total daily insulin dose increased in Germany from 0.28 (0.17) IU/kg at baseline to 0.53 (0.34) IU/kg at 6 months, with little further change up to 24 months (0.59 [0.41] IU/kg). Much smaller changes in mean daily insulin dose were observed in Greece (baseline: 0.41 [0.20] and 24 months: 0.54 [0.25] IU/kg) and Spain (baseline: 0.27 [0.15] and 24 months: 0.34 [0.18] IU/kg).
Concomitant OADs were not used at the time of insulin initiation by 60.0% of patients in Germany, and this percentage did not change during the 24 months of insulin use. In Greece, no OAD use was reported by a similar proportion of patients at baseline (57.0%), but this decreased to 47.6% at 24 months, with a concomitant increase in the proportion taking one OAD from 30.4% at baseline to 41.0% at 24 months. The respective percentages for no OAD use for Spain were 40.1% at baseline and 27.0% at 24 months, with increases in the proportion taking one (baseline 40.1% to 24 months 48.7%) and two OADs (baseline 19.2% to 24 months 23.7%). OAD use included a sulfonylurea for 18.6% and 18.5% of patients in Greece at baseline and 24 months, 23.8% and 27.6%, respectively, in Spain, but only 5.2% and 3.4% in Germany.
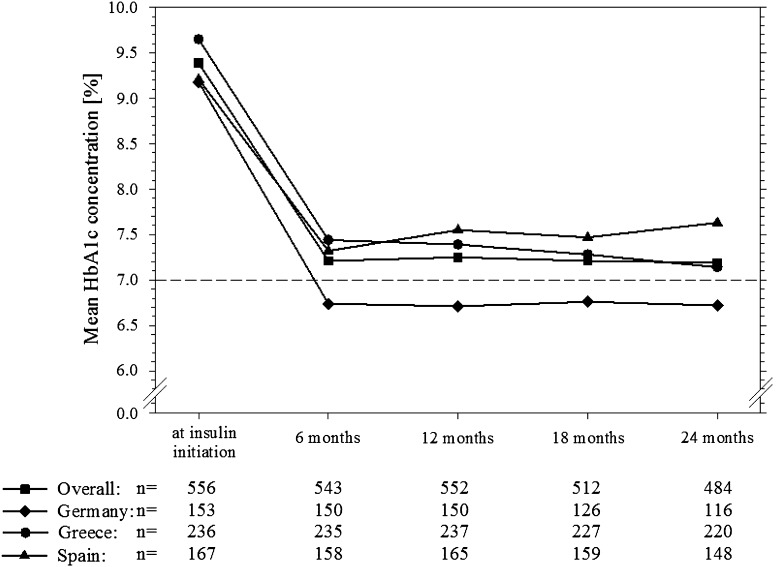
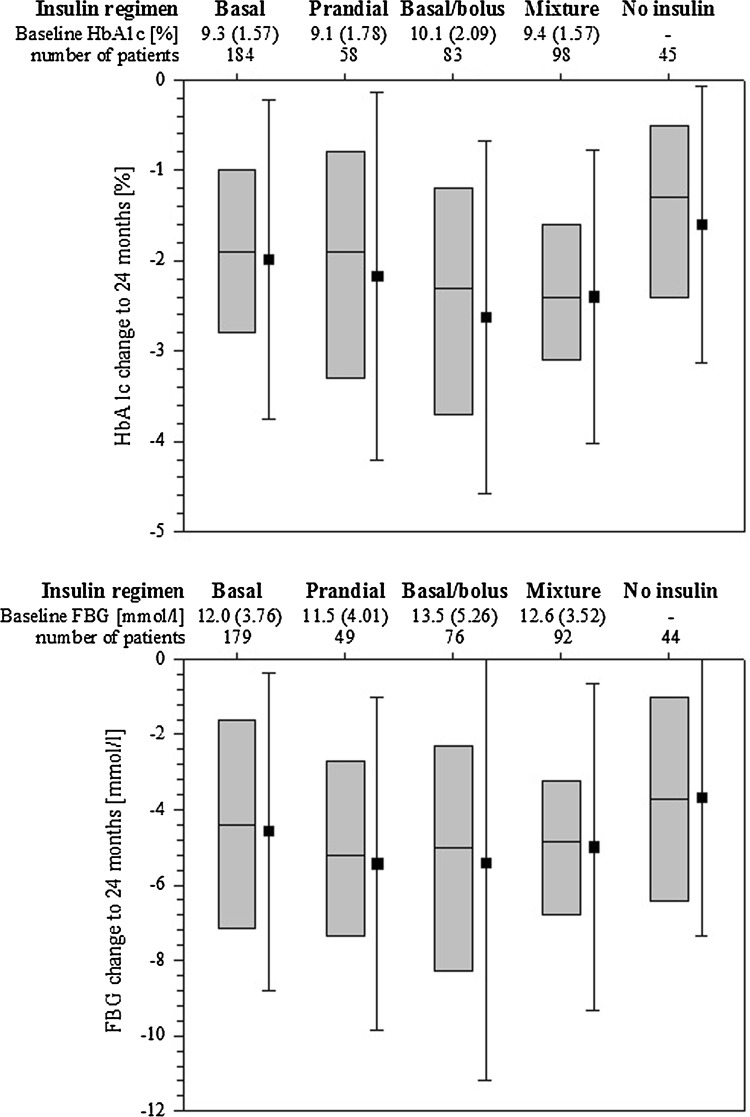
Overall mean (SD) HbA1c (Fig. 1) decreased from baseline (9.4 [1.7]%) to 6 months (7.2 [1.0]%), with little further change to 24 months (7.2 [1.1]%). A mean HbA1c below 7% was achieved and maintained only in Germany, whereas in Spain a slight increase in mean HbA1c was seen after the initial decrease at 6 months. The HbA1c and fasting glucose changes from baseline to 24 months, according to the insulin regimen being taken at the 24-month time point, are shown in Fig. 2. Mean HbA1c change observed with basal/bolus regimens (−2.6 [2.0]%) was similar to premixed regimens (−2.4 [1.6]%). Mean decreases in HbA1c were lower for patients using only one type of insulin (basal only: −2.0 [1.8]%, prandial only: −2.2 [2.0]%). Changes in fasting blood glucose concentrations according to insulin regimen largely reflected the changes in HbA1c concentration. At insulin initiation, blood glucose monitoring was performed by 87% of patients, which increased to 98% at 12 months and 99% at 24 months, with no big differences between countries.
Fig. 1.
Mean HbA1c concentration at each time-point from insulin initiation to 24 months for patients in each country and for all patients combined. HbA 1c hemoglobin A1c
Fig. 2.
Change in HbA1c and FBG from baseline to 24 months according to the insulin regimen being used at the 24-month time point. Values shown are mean with SD and simplified box plots (lower quartile, median, upper quartile); number of patients are for patients on specified insulin regimen with data at 24 months; values for an additional 10 patients with other unspecified insulin regimens are not shown. FBG fasting blood glucose, HbA 1c hemoglobin A1c
There was a small increase in mean (SD) body mass index (BMI) from 29.3 (5.4) kg/m2 at baseline to 29.9 (5.6) kg/m2 at 12 months and 30.1 (5.3) kg/m2 at 24 months, with no obvious differences between countries.
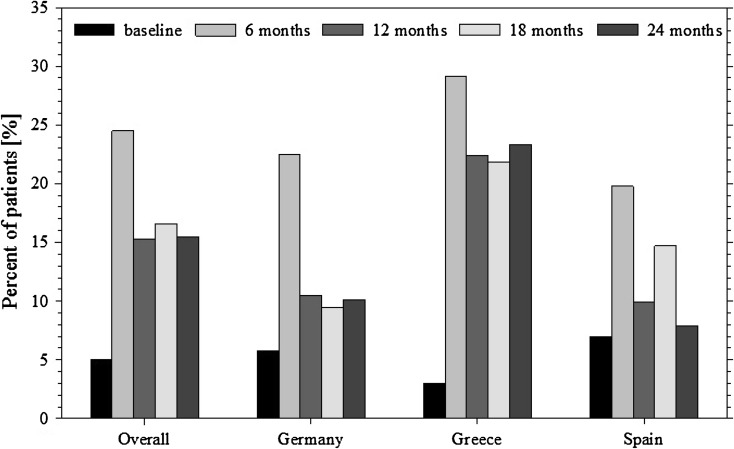
At least one episode of hypoglycemia (Fig. 3) was reported by 5.0% of patients overall at baseline for the prior 3 months; assessments at each 6-monthly interval during the study indicated an increase to 24.5% at 6 months, and 15.3%, 16.6%, and 15.5% at the following visits (12, 18, and 24 months, respectively). The hypoglycemia incidence at 24 months was higher in Greece (23.3%) than Germany (10.1%) or Spain (7.9%). Throughout the study, seven patients reported hypoglycemia requiring hospitalization and confirmed by glucose readings, which included two prior to insulin initiation.
Fig. 3.
Proportion of patients reporting at least one episode of hypoglycemia during each period throughout the study. Bars show percentages of patients in the 6 months prior to each study visit, except for baseline where the value is for the prior 3 months; percentages are based on the total number of patients per visit, by country and overall
Discussion
The present report of the INSTIGATE observational study assessed data from up to 24 months of follow-up for patients who initiated insulin in Germany, Greece, and Spain. Most guidelines/position statements recommend the initial use of basal insulin, with subsequent addition of prandial insulin when HbA1c levels remain elevated [1–5]. In the present study, a majority of patients in Germany started on prandial insulin only, whereas most patients in Greece and Spain started on basal insulin only or premixed formulations. The difference in initial treatment patterns may reflect the fact that all of the investigators in Germany were specialist diabetologists [11] and may have adopted a different approach, based on different national guidelines [13]. Overall, changes in insulin regimens over the 24-month study were small, and 76% of patients did not change their insulin regimen from that initiated.
In Germany, mean HbA1c concentration was maintained below 7% from 6 months onwards. This could be due to the predominant use of multiple injections in Germany, starting with a prandial approach and intensification to basal/bolus insulin, with increasing overall insulin dosage at 12 and 24 months. The maintenance of HbA1c below 7% was consistent with the previously reported German study using a basal/bolus regimen [14]. In Greece, a slow decline in mean HbA1c was seen, almost reaching target levels at 24 months. An increase in use of a basal/bolus regimen at 12 and 24 months and a clinically relevant increase in insulin dosage could be seen, as well as an increase in the proportion of patients using concomitant OADs. However, 21 patients in Greece stopped using insulin altogether. In Spain, the lowest mean HbA1c was achieved at 6 months with a rise to above 7.5% after 24 months. This outcome might be expected given the progressive pathophysiology of type 2 diabetes and the fact that there was little change in treatment regimens and insulin dosage in Spain, with the majority of patients remaining on basal insulin only.
The authors would suggest that the greater improvement in glycemic control seen in Germany is most likely due to the insulin intensification, with more complex regimens and higher insulin dosage. Limitations of this interpretation were that the patient cohorts were not totally comparable between countries (e.g., the German cohort was younger and had a shorter duration of diabetes), the study was only observational and, therefore, no randomization or standardization of the treatment took place. In addition, the authors observed only a limited number of patients and not all were willing to continue for 24 month, which may have caused a selection bias.
The present results were consistent with those from the Treating To Target in Type 2 diabetes (4-T) study, where mean HbA1c was significantly lower in patients treated with premixed and/or prandial insulin, compared with basal insulin, at 1 year [6]; after 3 years, treatment intensification took place in about 70% of the patients, regardless of the initial therapy regimen, highlighting the need for further adjustment of therapy [15]. Furthermore, in a meta-analysis of data from 4,379 patients identified in 22 randomized controlled trials up to October 2008, HbA1c reductions were significantly greater with biphasic and prandial insulin than with basal insulin alone [16].
Consistent with the 6-month INSTIGATE results [12], the rate of hypoglycemia was lower in Germany at 12, 18, and 24 months, despite the more intensive regimens and lower HbA1c, compared with Greece where basal and premixed regimens were more widely used. This was in contrast to studies of patients treated with prandial insulin who had improved post-prandial glucose and HbA1c, but higher rates of hypoglycemia compared with patients treated with basal insulin alone [6, 17]. In a study by Bretzel et al. (A Parallel design comparing an OAD combination therapy with either Lantus once daily or Lispro at mealtime in Type 2 Diabetic patients failing oral treatment [APOLLO]), an overall incidence of hypoglycemic events that was four times higher with rapid-acting prandial insulin than with basal insulin was shown [7, 18]. However, it has been suggested that the use of sulfonylureas in the prandial insulin group of APOLLO might have contributed to the higher rate of hypoglycemia [19]. While only 5% of patients in Germany reported concomitant sulfonylureas in the present study, the proportion in Greece was over 18%, which may relate to the higher incidence of hypoglycemia in Greece. However, in Spain approximately 25% reported concomitant sulfonylurea use, but the percentage reporting hypoglycemic episodes were generally lowest. This might reflect the smaller HbA1c reduction, the lowest insulin dosages, and the highest percentage of patients who stopped insulin use.
Conclusion
The majority of patients did not change insulin regimen from that initiated, indicating that guidelines of adaptation to individual patient needs are not sufficiently adhered to. Therapeutic algorithms suggest that basal insulin should be followed by a combination of basal with preprandial insulin to control postprandial glucose fluctuations [20]. The present INSTIGATE results suggest that good glycemic control was attained by the highest percentage of patients when insulin was initiated earlier after diabetes diagnosis and with earlier intensification. This underlines the importance of timely escalation of insulin therapies as the pathophysiology of type 2 diabetes progresses. Insulin regimens substituting both basal and prandial insulin insufficiency in type 2 diabetes were shown to be feasible and effective in routine clinical practice.
Acknowledgments
The INSTIGATE study was funded by Eli Lilly and Company. The authors are very grateful to all of the clinical investigators involved in the INSTIGATE observational study for providing data. The authors would also like to thank Dr. Peter C. Bates, Cambridge Medical Writing Services, UK, for help in preparation and editing of the manuscript; support for this assistance was funded by Eli Lilly and Company. Dr. Liebl is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Conflict of interest
A.L., S.J., A.G. M.B., and C.C. are members of the INSTIGATE Advisory Board, and have received honoraria and travel expenses from Eli Lilly and Company. A.H. and C.N. are employees of, and H.T.S is a former employee of, Eli Lilly and Company. A.L., S.J., A.G., M.B., C.C., and H.T.S. were involved in the design and interpretation of the study, and drafting and revision of the manuscript. C.N. was responsible for statistical analysis, and A.H. was involved in interpretation of results and revision of the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(Suppl. 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathan DM, Buse JB, Davidson MB, American Diabetes Association. European Association for Study of Diabetes et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inzucchi SE, Bergenstal RM, Buse JB, American Diabetes Association. European Association for Study of Diabetes et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blonde L. Current antihyperglycaemic treatment guidelines and algorithms for patients with type 2 diabetes mellitus. Am J Med. 2010;123(Suppl. 3):S12–S18. doi: 10.1016/j.amjmed.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 5.IDF Clinical Guideline Task Force. Global guideline for type 2 diabetes. Brussels: International Diabetes Federation; 2005. p. 26–8.
- 6.Holman RR, Thorne KI, Farmer AJ, 4-T Study Group et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007;357:1716–1730. doi: 10.1056/NEJMoa075392. [DOI] [PubMed] [Google Scholar]
- 7.Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet. 2008;371:1073–1084. doi: 10.1016/S0140-6736(08)60485-7. [DOI] [PubMed] [Google Scholar]
- 8.Hermansen K, Mortensen LS, Hermansen ML. Combining insulins with oral antidiabetic agents: effect on hyperglycemic control, markers of cardiovascular risk and disease. Vasc Health Risk Manag. 2008;4:561–574. doi: 10.2147/vhrm.s1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UK Prospective Diabetes Study (UKPDS) Group Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 10.The ORIGIN Trial Investigators Basal insulin and cardiovascular and other outcomes in dysglycemia. N Eng J Med. 2012;367:319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 11.Jones S, Benroubi M, Castell C, et al. Characteristics of patients with type 2 diabetes mellitus initiating insulin therapy: baseline data from the INSTIGATE study. Curr Med Res Opin. 2009;25:691–700. doi: 10.1185/03007990902739669. [DOI] [PubMed] [Google Scholar]
- 12.Liebl A, Jones S, Benroubi M, et al. Clinical outcomes after insulin initiation in patients with type 2 diabetes: 6-month data from the INSTIGATE observational study in five European countries. Curr Med Res Opin. 2011;27:887–895. doi: 10.1185/03007995.2011.555755. [DOI] [PubMed] [Google Scholar]
- 13.Matthaei S, Bierwirth R, Fritsche A, et al. Medical antihyperglycaemic treatment of type 2 diabetes mellitus: update of the evidence-based guideline of the German Diabetes Association. Exp Clin Endocrinol Diabetes. 2009;117:522–557. doi: 10.1055/s-0029-1239559. [DOI] [PubMed] [Google Scholar]
- 14.Liebl A, Prager R, Binz K, Kaiser M, Bergenstal R, Gallwitz B, PREFER Study Group Comparison of insulin analogue regimens in people with type 2 diabetes mellitus in the PREFER study: a randomized controlled trial. Diab Obes Metab. 2009;11:45–52. doi: 10.1111/j.1463-1326.2008.00915.x. [DOI] [PubMed] [Google Scholar]
- 15.Holman RR, Farmer AJ, Davies MJ, 4-T Study Group et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361:1736–1747. doi: 10.1056/NEJMoa0905479. [DOI] [PubMed] [Google Scholar]
- 16.Lasserson DS, Glasziou P, Perera R, Holman RR, Farmer AJ. Optimal insulin regimens in type 2 diabetes mellitus: systematic review and meta-analyses. Diabetologia. 2009;52:1990–2000. doi: 10.1007/s00125-009-1468-7. [DOI] [PubMed] [Google Scholar]
- 17.Heise T, Mathieu C, Hey-Hadavi J, Strack T, Lawrence D. Glycemic control with preprandial versus basal insulin in patients with type 2 diabetes mellitus poorly controlled by oral antidiabetes agents. Diabetes Technol Ther. 2010;12:135–141. doi: 10.1089/dia.2009.0105. [DOI] [PubMed] [Google Scholar]
- 18.Bretzel RG, Eckhard M, Landgraf W, Owens D, Linn T. Initiating insulin therapy in type 2 diabetic patients failing on oral hypoglycemic agents basal or prandial insulin? The APOLLO trial and beyond. Diabetes Care. 2009;32(Suppl. 2):S260–S265. doi: 10.2337/dc09-S319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnolds S, Rave K. Basal insulin glargine vs prandial insulin lispro in type 2 diabetes. Lancet. 2008;372:370–371. doi: 10.1016/S0140-6736(08)61151-4. [DOI] [PubMed] [Google Scholar]
- 20.Raccah D, Bretzel RG, Owens D, Riddle M. When basal insulin therapy in type 2 diabetes mellitus is not enough: what next? Diabetes Metab Res Rev. 2007;23:257–264. doi: 10.1002/dmrr.733. [DOI] [PubMed] [Google Scholar]