Abstract
Heat shock protein 27 (HSP27) shows attenuated expression in human coronary arteries as the extent of atherosclerosis progresses. In mice, overexpression of HSP27 reduces atherogenesis, yet the precise mechanism(s) are incompletely understood. Inflammation plays a central role in atherogenesis, and of particular interest is the balance of pro- and anti-inflammatory factors produced by macrophages. As nuclear factor-kappa B (NF-κB) is a key immune signaling modulator in atherogenesis, and macrophages are known to secrete HSP27, we sought to determine if recombinant HSP27 (rHSP27) alters NF-κB signaling in macrophages. Treatment of THP-1 macrophages with rHSP27 resulted in the degradation of an inhibitor of NF-κB, IκBα, nuclear translocation of the NF-κB p65 subunit, and increased NF-κB transcriptional activity. Treatment of THP-1 macrophages with rHSP27 yielded increased expression of a variety of genes, including the pro-inflammatory factors, IL-1β, and TNF-α. However, rHSP27 also increased the expression of the anti-inflammatory factors IL-10 and GM-CSF both at the mRNA and protein levels. Our study suggests that in macrophages, activation of NF-κB signaling by rHSP27 is associated with upregulated expression and secretion of key pro- and anti-inflammatory cytokines. Moreover, we surmise that it is the balance in expression of these mediators and antagonists of inflammation, and hence atherogenesis, that yields a favorable net effect of HSP27 on the vessel wall.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-012-0356-0) contains supplementary material, which is available to authorized users.
Keywords: Heat shock protein 27 (HSP27), Macrophage, Nuclear factor-kappa B (NF-κB) signaling, Atherosclerosis
Introduction
Macrophages are present in the atherosclerotic plaque at various stages of development and play a major role in the onset and progression of atherosclerotic lesion development, in part through participation in both the initiation and resolution of inflammation (Ley et al. 2011). In the atherosclerotic plaque, nuclear factor-kappa B (NF-κB) is a key transcription factor that mediates important macrophage functions including survival, apoptosis, proliferation, and inflammation (Gordon et al. 2011; Lawrence and Fong 2010). In the classical NF-κB pathway, the inactive heterodimers, p50/p65, are present in the cytoplasm and under basal conditions are bound to inhibitors of NF-κB (IκB). In response to inflammatory stimuli, a cascade of phosphorylation events increases the activity of the IκB kinase (IKK) complex that acts to phosphorylate IκBα, leading to its degradation by the proteasome, and consequently, dissociation from the NF-κB dimers. Release of IκBα from p50/p65 promotes translocation of these subunits to the nucleus where they interact with specific promoter regions to modulate transcription of target genes (Oeckinghaus and Ghosh 2009).
NF-κB has been associated with pro-inflammatory responses in atherosclerosis (Monaco et al. 2004; Zhang et al. 2009); however, emerging in vivo data suggests that NF-κB also regulates anti-inflammatory processes in the atherosclerotic plaque (Xanthoulea et al. 2005). For example, in a study of atherosclerosis-prone low-density lipoprotein receptor knock-out (LDLR−/−) mice with a macrophage-restricted deletion of IKK2, the mice developed more severe atherosclerosis and had markedly decreased levels of the anti-inflammatory cytokine, IL-10 (Kanters et al. 2003). In addition, macrophages deficient in the NF-κB p50 subunit have a prolonged production of the inflammatory cytokine, tumor necrosis factor (TNF)-α, in response to lipopolysaccharide (LPS). Conversely, atherosclerosis-prone LDLR−/− mice with a hematopoeitc deficiency for the p50 subunit have smaller lesions (41 %) in the aortic root compared to LDLR−/− control mice; however, the lesions are more inflammatory, as characterized by an increase in leukocyte infiltration (Kanters et al. 2004).
In humans, there is evidence that a genetic deletion in the promoter of the NFKB1 gene results in decreased levels of the p50 subunit and is associated with a higher risk for coronary heart disease (Karban et al. 2004; Park et al. 2007; Vogel et al. 2011). These studies suggest that there are protective roles for NF-κB signaling in atherosclerosis and exemplifies the importance of macrophages in balancing pro- and anti-inflammatory signals in the developing lesion. Therefore, understanding the factors that promote the dominance of the anti-inflammatory versus inflammatory effects of NF-κB signaling in macrophages may be instrumental for the development of novel therapeutics for the prevention and/or treatment of atherosclerosis.
Heat shock protein 27 (HSP27), is a highly conserved, 27-kDa stress protein. Although the chaperone activity and function of HSP27 is well documented, emerging evidence suggests that HSP27 has multiple other (pleiotropic) functions. For example, HSP27 is an anti-apoptotic factor that interacts with cytochrome c and interferes with caspase activation complexes (Arrigo 2007; Bruey et al. 2000). Moreover, HSP27 acts as a stabilizer of actin and microtubules of the cytoskeleton (Benjamin and McMillan 1998; Pockley 2002). Recently, we unraveled a novel function of HSP27 by demonstrating that HSP27 is an estrogen receptor beta (ERβ)-associated protein that regulates estrogen signaling (Al-Madhoun et al. 2007; Miller et al. 2005). Treatment of macrophages with a selective ERβ agonist, 8β-VE2, increases the secretion of HSP27 and treatment of ovariectomized ApoE−/− mice with 8β-VE2 results in smaller atherosclerotic lesions in the aortic arch (Sun et al. 2011). In addition, global overexpression of HSP27 in ApoE−/− mice fed a high fat diet for 4 weeks results in reduced atherosclerotic lesion area—a protective effect associated with elevated levels of HSP27 in the serum (Rayner et al. 2008). In previous studies that have explored how extracellular HSP27 regulates macrophages, our lab as well as others have demonstrated that exogenous HSP27 lowers the levels of the inflammatory cytokine interleukin-1β (IL-1β) in macrophages exposed to acetylated low-density lipoprotein, augments extracellular levels of the anti-inflammatory cytokine interleukin-10 (IL-10), as well as attenuates macrophage adhesion and migration (De et al. 2000; Miller-Graziano et al. 2008; Rayner et al. 2008). However, despite the substantial evidence that extracelluar HSP27 can act as an anti-inflammatory, the role of extracellular HSP27 in the regulation of NF-κB signaling is unclear. Several studies have examined the affect of intracellular HSP27 on NF-κB; however, these indeterminate results are mainly in cell types that have not been demonstrated to secrete HSP27, such as smooth muscle cells (Parcellier et al. 2003; Voegeli et al. 2008). Therefore, in the current study, we sought to examine whether extracellular HSP27 could modulate NF-κB signaling in macrophages. As will be described, we demonstrate that treatment of human macrophages with recombinant (r)HSP27 induces the degradation of IκBα, the nuclear translocation of the NF-κB p65 subunit, and activates downstream NF-κB transcriptional activity. In addition, we explored the transcriptional profile of macrophages treated with rHSP27 using NF-κB-pathway-specific qRT-PCR arrays. Among the regulated genes, are both pro-inflammatory and anti-inflammatory cytokines. Hence, we surmise that in order to exert its anti-atherosclerotic effects, HSP27 produces a favorable effect on the balance of these pro- and anti-inflammatory factors at the level of the vessel wall macrophage.
Materials and methods
Recombinant (r)HSP27 and rC1 production
Compliment (c)DNA encoding His-tagged full length HSP27 and an N-terminal truncated mutant, C1, was constructed into a pET-21a vector, and the plasmids were transformed into Rosetta™(DE3)-competent Escherichia coli (Novagen). rHSP27 and rC1 protein was purified with Ni-NTA resin (Qiagen, Valencia, CA) and refolded by dialysis. After removal of endotoxin using Detoxi-gel columns (Fisher Scientific, Pittsburgh, PA), the purity of final rHSP27 and rC1 protein was more than 95 % by SDS-PAGE and the endotoxin concentration was <5 EU/mg protein (LAL assay).
Chaperone activity assay
The dithiothreitol (DTT)-induced aggregation of insulin was performed in the absence or presence of rHSP27 in phosphate buffer solution (PBS; pH 7.4), to measure the chaperone activity of previously purified and refolded rHSP27. Insulin (40 μM) and different concentrations of rHSP27 (Insulin to rHSP27 ratios of 10:1, 100:1, and no rHSP27) in PBS were incubated for 10 min at 43 °C. Aggregation was monitored by measuring the absorbance at 320 nm in a Synergy Mx Monochromator-based microplate reader (BioTek, Winooski, VT) for 30 min at 43 °C after adding DTT to a final concentration of 20 mM.
Cell culture
The THP-1 human monocytic cell line (ATCC) was maintained and subcultured in RPMI-1640 growth media supplemented with 10 % FBS, penicillin, streptomycin, and sodium pyruvate (Invitrogen-Gibco). The cells were maintained in 100-mm tissue culture plates at a density of 0.2 × 106–1 × 106 cells/mL. For experiments, cells were plated at a density of 1 × 106 cells/mL and differentiated to macrophages with 50 ng/mL of phorbol myristate acetate (PMA, Sigma, St. Louis, MO). Unless otherwise specified, THP-1 cells were differentiated with PMA for 24 h then cultured in fresh media (no PMA) for an additional 24 h.
IκBα immunoblotting
THP-1 cells were treated with LPS (10 ng/mL), rHSP27 (250 μg/mL, 9.6 μM), or rC1 (150 μg/mL, 9.6 μM). Total protein was harvested after 15, 30, and 60 min, using RIPA lysis buffer. The protein concentrations of cell lysates were quantified using the Bicinchoninic acid assay (BCA). The samples were subject to Western blotting for IκBα (US Biologicals, Swampscott, MA). β-Actin was used as a loading control (Anti-beta Actin antibody, AC-15, ab6276, Abcam, Cambridge, UK).
Immunolabeling for p65
THP-1 cells were treated with either LPS (10 ng/mL), rHSP27 (250 μg/mL), rC1 (150 μg/mL) for 30 min. At this time, the cells were fixed with 4 % paraformaldehyde and stained with NF-κB-p65 antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or Hoechst dye (Pierce Biotechnology, Rockford, IL). Photomicrographs were obtained using a fluorescent microscope (Olympus).
NF-κB reporter assay
The human monocytic cell line, THP1-XBlue™, in which cells are stably transfected with an NF-κB-inducible secreted embryonic alkaline phosphatase (SEAP) gene, was purchased from Invivogen (San Diego, CA). The cells were maintained and subcultured in complete RPMI-1640 media supplemented with Zeocin (200 μg/mL) as a selective antibiotic. Cells were subject to treatment for 24 h with rHSP27 (250 μg/mL, 9.6 μM) or rC1 (150 μg /mL, 9.6 μM). Where indicated, the the NF-kB inhibitor BAY 11-7082 (EMD Biosciences, Gibbstown, NJ) was used at the indicated concentration for 1 h prior to treatments. The conditioned media from each treatment was then analyzed for the presence of SEAP using QUANTI-Blue™ detection reagent (Invivogen) according to the manufacturer’s instructions. Briefly, QUANTI-Blue™ detection reagent was mixed with cell supernatant (10:1) and incubated at 37 °C for up to 1 h. Optical absorbance at 655 nm was then measured using the BioTek Synergy Mx microplate reader. Media only controls (no cells) were assayed to ensure that there was no endogenous SEAP activity in the treatment media. The NF-κB activity for the various treatments is represented as the fold change in absorbance compared to the control group.
NF-κB pathway-specific PCR array
THP-1 cells were plated at a density of 2 × 106 cells/well in a six-well plate or at 1 × 106 cells/well in a 12-well plate and differentiated with PMA (50 ng/mL) for 2 days. Cells were maintained in RPMI-1640 only (control), rC1 (150 μg/mL), or rHSP27 (250 μg/mL) with or without the addition of polymyxin B (PMB, EMD Chemicals; at 10 μg/mL) for 6 or 24 h. Where indicated, cells were pretreated with BAY 11-7082 (10 μM; EMD Biosciences) for 1 h. RNA was isolated and purified using Trizol (Invitrogen) and RNeasy minikit (Qiagen) with DNase treatment. Purified RNA concentration was measured on a NanoDrop 1000 (Thermo Scientific, Wilmington, DE). RNA integrity was measured on an Agilent 2100 Bioanalyzer RNA nano chip (Agilent Technologies, Santa Clara, CA). Real-time PCR arrays for human NF-κB signaling pathway (RT2Profiler PCR arrays by SABiosciences, Qiagen) were used to screen the mRNA expression of 84 genes involved in the NF-κB pathway. cDNA synthesis and real-time PCR arrays were performed according to manufacturer's instructions and ran on a LightCycler 480 instrument (Roche, Indianapolis, IN). Analysis was performed using web-based software and analysis tools provided by SABiosciences using the delta-delta Ct method (Pfaffl 2001).
qRT-PCR
cDNA was synthesized using a transcriptor first strand cDNA synthesis kit (Roche). qRT-PCR was performed using the light cycler 480 SYBR Green I Master kit (Roche). Primers for human GM-CSF and GAPDH were from Invitrogen. Primer sequences were as follows: GM-CSF-5′ACCATGATGGCCAGCCACTACAA3′ (F) and 5′GGGATGACAAGCAGAAAGTCCTTCA3′ (R); GAPDH-5′ CCACTCCTCCACCTTTGAC3′ (F) and 5′ACCCTGTTGCTGTAGCCA3′ (R). Analysis was performed using the Pfaffl method (Pfaffl 2001).
ELISA
Cell culture supernatants were collected from THP-1 cells. IL-10 and GM-CSF were measured using a commercial kit from R&D Systems, Inc. (Minneapolis, MN). Assays were performed according to the manufacturer’s instructions.
Gel filtration chromatography
The size of rHSP27 and rC1 was measured on a Gilson FPLC system with a SuperoseTM 6 10/300 GL column (GE Healthcare). Firstly, the column was balanced by 15 mL 1× PBS with a flow speed of 0.25 mL/min, then 50 μL of purified rHSP27 or rC1 (5 mg/mL) was injected to the column, and proteins were finally eluted with 0.25 mL/min 1× PBS for 100 min. The protein concentrations were determined by absorbance at O.D. 280 nm.
Immunoblot for phosphorylation of rHSP27
The phosphorylation status of rHSP27 was examined by separation on SDS-PAGE, followed by transfer to Nitrocellulose using the iBlot® 7-Minute Blotting System (Invitrogen™). Following the transfer, the membrane was blocked in 1 % bovine serum albumin in 1× PBS with 0.05 % Tween 20 (PBS-T). A 1:1,000 dilution of HSP27 (pS82) antibody (Assay Biotech) or 1:10,000 anti-HSP27 polyclonal antibody (Rabbit anti-HSP27, prepared by Cedarlane) were used as primary antibody and incubated at room temperature for 2 h. The membrane was washed with PBS-T buffer and then incubated with the respective secondary antibody, HRP conjugated anti-Rabbit IgG (dilution of 1/2,000, 111-036-045, Jackson Immunology) at room temperature for 1 h and washed again with PBS-T. The final image was obtained by incubating the membrane in AmershamTM ECL plus Western blotting detection system (GE Healthcare) and imaged by the the FluorChem® Q Imaging System (Cell Biosciences, Santa Clara, CA).
LC-MS/MS
The phosphorylation status of rHSP27 was examined by LC-MS/MS (Health Canada). rHSP27 was identified by LC MS/MS analysis on the Nano-Acquity ultra-performance liquid chromatography system (UPLC, Waters, Milford, MA) coupled to a 7-tesla hybrid linear ion-trap Fourier transform ion cyclotron resonance mass spectrometer (LTQ-FT ICR, Thermo Fisher, San Jose, CA). Specifically, proteins were separated by SDS-PAGE gels and stained with Coomassie, then reduced with 10 mM dithiothreitol (DTT), alkylated with 55 mM iodoacetamide and digested with 20 ng trypsin in 25 mM ammonium bicarbonate solution at 37 °C overnight. The peptides were extracted with 50 % acetonitrile/0.1 % trifluroacetic acid and dried by speedVac concentrator, and reconstituted in 0.2 % formic acid. 2,5-Dihydroxybenoic acid was used as the matrix for MS/MS measurements. Nano LC MS/MS analysis of the peptides was conducted using a reversed-phase Symmetry C18 trapping column (180 μm × 20 mm) and a C18 analytical column of 1.7 μm BEH130 (100 μm × 100 mm, UPLC, Waters) through a 90-min gradient from 5 to 40 % of acentonitrile containing 0.1 % FA, then 85 % at a flow rate of 0.5 μl/min. Automated data-dependent acquisition was employed to obtained MS and MS/MS measurements at a mass range of m/z 300–2,000. Dynamic exclusion was enabled for a period of 180 s.
Protein identification was performed using an in-house Mascot Server (version 2.3.0, Matrix Science, London, UK), and the data were searched against the National Center for Biotechnology Information (NCBInr) database for human protein. The parameter settings allowed trypsin digestion for maximum two missed cleavage sites and a fixed peptide modification of cysteine carbamidomethylation. Mass tolerance was set up to 0.1 Da for MS/MS measurement.
MTT assay
Mitochondrial respiration is an indicator of cell viability and can be assessed by the mitochondrial-dependent reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). MTT assays were carried out using MTT solution [Thiazolyl Blue Tetrazolium Bromide (Sigma) dissolved in PBS at a concentration of 5 mg/mL] before being diluted 20-fold in cell culture media and added in equal amounts to cell culture wells. Macrophages were then incubated at 37 °C for 4 h. The yellow MTT was metabolized by mitochondria of living cells and resulted in accumulation of membrane impermeable purple formazan crystals. At this point, the media and MTT solution were aspirated followed by solubilization of the crystals with a 7.3:1 mixture of 2-propanol to 0.2 M HCl. The wells were then scraped and mixed thoroughly in order to dissolve the formazan crystals. The absorbance, which is proportional to the number of surviving cells, was then read in a BioTek Synergy Mx microplate reader at 570 nm.
Cytotoxicity (LDH) assay
Lactate dehydrogenase (LDH) release from cells treated with rHSP27 or rC1 was measured using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega). THP-1 macrophages were treated with rHSP27 (9.6 μM) or rC1 (9.6 μM). After 24 h, 50 μL aliquots of the conditioned media from all wells were removed and placed in a new 96-well plate, to which the LDH substrate solution was added. The plate was protected from light and incubated at room temperature for 30 min, after which a stop solution was added to each well and absorbance was read at 490 nm.
Statistical analysis
Statistical analysis was performed using Sigma Stat 3.5 software. ANOVA was performed, followed by the Student Newman Keuls post-hoc test for comparing individual variables where applicable. A p < 0.05 was considered significant. All data (n ≥ 3) are expressed as mean ± standard error of the mean (SEM).
Results
rHSP27 induces IκBα degradation in THP-1 macrophages
To focus on the extracellular functions of HSP27, a recombinant form (rHSP27) was produced. In addition to the full-length protein, an N-terminal truncated mutant, referred to as rC1, was produced as a functionally null control for the recombinant protein production. In studies to examine the recombinant protein characteristics, we found that rHSP27 was unphosphorylated (“Electronic supplementary material” (ESM) Fig. S1) and could form large oligomers (1,000—5,000 kD) while rC1 appeared to be around 100 kd (ESM Fig.S2). In order to verify that the recombinant proteins are not cytotoxic, we used MTT assays to assess the viability of THP-1 macrophages treated with these proteins, and observed no differences in viability between cells that were treated with LPS, rHSP27, or rC1 (ESM Fig. S3). Since many of the downstream experiments we performed, including the NF-κB reporter assay, involved measurement of secreted factors in the conditioned media, it was important to ensure that membrane integrity was preserved in the presence of the various treatments. In order to address this, we used LDH levels as a surrogate marker for membrane integrity. LDH levels from conditioned media collected after treatment with rHSP27 or rC1 were not different from the control group (ESM Fig. S3). Hence, these data suggest that neither rHSP27 nor rC1 had measureable cytotoxic effects on THP-1 macrophages.
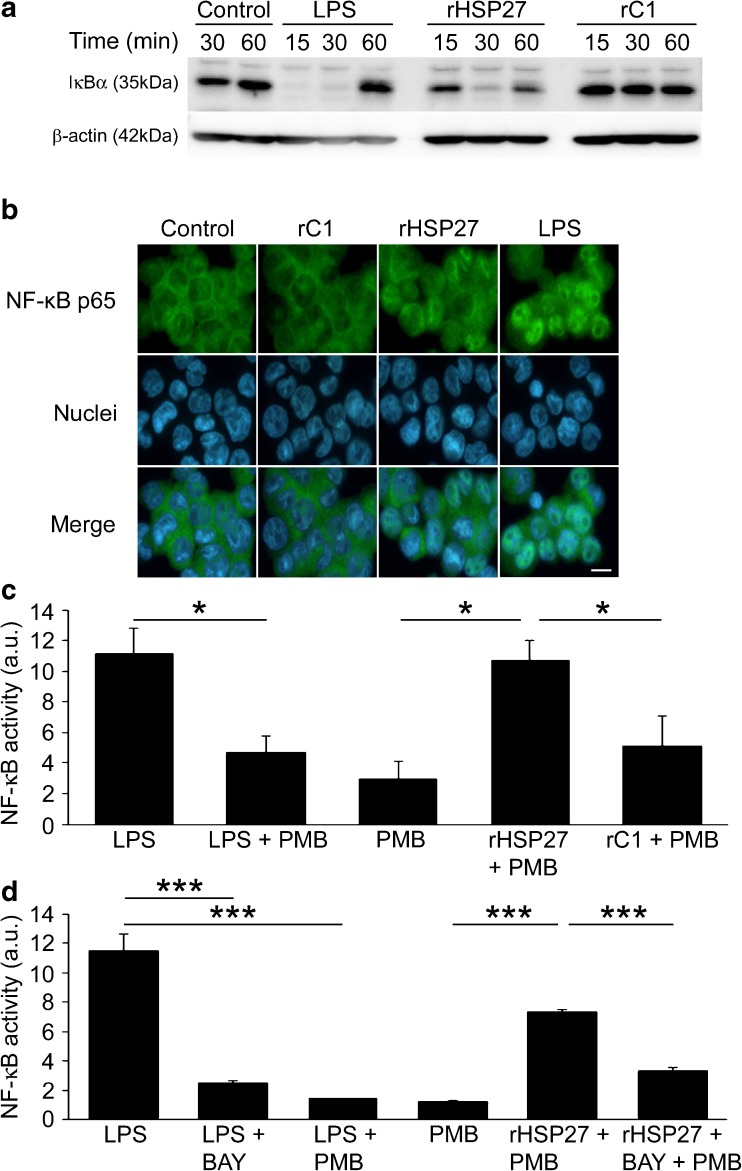
In order to explore the impact of rHSP27 on modulating NF-κB activity, we initially focused on one of the early steps in the NF-κB activation pathway, IκBα degradation (Baeuerle and Baltimore 1989). Using total cell lysate from THP-1 macrophages, we monitored the degradation of IκBα by immunoblotting (Fig. 1a). As expected, there was strong expression of IκBα in the control group. LPS, a potent activator of the NF-κB pathway, and therefore IκBα degradation, was used as a positive control (Muller et al. 1993). After 15 min of treatment with LPS, IκBα was not detectable by immunoblotting, indicating that it was rapidly degraded. This degradation was maintained up to 30 min and by 60 min, IκBα protein expression appeared to be restored to levels similar to those observed in the control. After 30 min of treatment with rHSP27, IκBα was not detectable by immunoblotting, indicating that rHSP27 modulates the degradation of IκBα. The N-terminal truncation mutant, rC1 did not induce IκBα degradation. In summary, treatment of THP-1 macrophages with rHSP27 leads to the degradation of IκBα, while equimolar concentrations of the functionally null rC1 did not.
Fig. 1.
a IκBα degradation in rHSP27-treated macrophages. PMA-differentiated THP-1 cells were treated with either LPS (10 ng/mL), rHSP27 (9.6 μM), or rC1 (9.6 μM) for the times indicated. Equal amounts of whole cell lysates were subject to Western blotting with an antibody directed against IκBα. β-Actin was used as a loading control. b Nuclear translocation of the NF-κB p65 subunit in response to rHSP27. PMA-differentiated THP-1 cells were treated with media alone (control), rC1 (9.6 μM), rHSP27 (9.6 μM), or LPS (10 ng/mL) for 30 min. The cells were then stained with an antibody to p65 (green) and the nuclear stain Hoechst (blue). Scale bar = 10 μm. c NF-κB activity in rHSP27-treated macrophages. NF-κB reporter assays were performed using THP-1 Blue macrophages. PMA-differentiated cells were treated with LPS (10 ng/mL), Polymyxin B (10 μg/mL), rHSP27 (9.6 μM), or C1 (9.6 μM) for 24 h. Cell supernatants were then analyzed for presence of secreted embryonic alkaline phosphatase (SEAP) as a reporter of NF-κB activation. One-way ANOVA *p < 0.05; n = 3–4. d Reduction of rHSP27-induced NF-κB activity in the presence of BAY 11-7082, an inhibitor of IκBα phosphorylation. Inhibition of NF-κB signaling was achieved by pre-treating the cells for 1 h with BAY 11-7082 (10 μM). One-way ANOVA ***p < 0.001; n = 3
rHSP27 promotes nuclear translocation of the NF-κB-p65 subunit
In the classical pathway of NF-κB activation, active NF-κB dimers (e.g., p65) will translocate to the nucleus in order to regulate transcription of target genes (ten Hove et al. 1999). To further understand the regulation of NF-κB by HSP27, we monitored the nuclear translocation of the p65 subunit using fluorescence microscopy (Fig. 1b). Both the control and rC1 groups demonstrated p65 immunolabeling (green) mainly concentrated in the cytoplasm, showing sparse p65 staining in the nuclei. In contrast, the cells treated with rHSP27 and LPS (as a positive control) show a higher density of p65 immunolabeling in the nuclei.
rHSP27 promotes NF-κB transcriptional activity in macrophages
In order to determine if rHSP27 could activate NF-κB transcriptional activity, we used NF-κB reporter gene assays. A THP-1 cell line stably transfected with an NF-κB inducible SEAP reporter gene (THP1-XBlue™) was used to monitor NF-κB activation. rHSP27 led to a 3.6-fold increase in NF-κB reporter gene activity (p = 0.018) compared to cells that were untreated (Fig. 1c). However, rC1, used at the same molar concentration as rHSP27, did not activate the NF-κB reporter gene. LPS was used as a positive control for the activation of the NF-κB reporter gene activity. To control for the presence of possible residual endotoxin in the recombinant protein samples, PMB was added to the culture media to inhibit the biological activity of endotoxin (Nishibori et al. 2009; Wang and Quinn 2010). Indeed, PMB reduced the effects of LPS, therefore confirming its efficacy in reducing the effects of endotoxin. As well, since the SEAP reporter construct has promoter regions to which the transcription factor AP-1 can also bind, we used a chemical inhibitor of NF-κB activation, BAY 11-7082, to elucidate the specificity of the NF-κB reporter assay. BAY 11-7082 inhibits the phosphorylation of IκBα by IKKs, thus inhibiting IκBα degradation and NF-κB activation (Lappas et al. 2005). In the presence of this inhibitor, rHSP27-induced activation of NF-κB was partially reversed (2.2-fold reduction, p < 0.001; Fig. 1d).
rHSP27 alters macrophage transcriptional profile
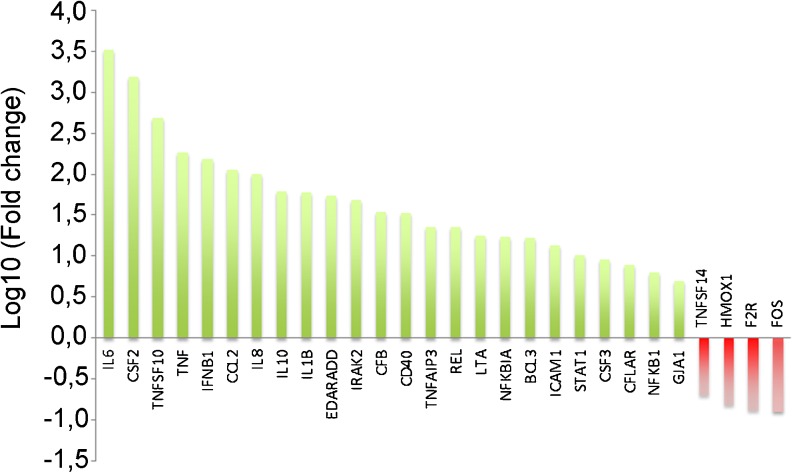
Given that rHSP27 treatment lead to an increase in NF-κB transcriptional activity, we next sought to determine the transcriptional targets that are regulated in this context. To do so, we used NF-κB pathway-focused qRT-PCR arrays. The qRT-PCR arrays were designed to profile the expression of 84 key genes that are related to NF-κB-mediated signal transduction. The array included genes that encode cytokines, receptors, membrane molecules, transcription factors, kinases, and inhibitors of NF-κB. In this experiment, the transcriptional profile of THP-1 macrophages treated with rHSP27 (in media supplemented with PMB) was compared to a control group (media with PMB). The fold change as well as the 95 % confidence interval for the up/down-regulated genes on the array were analyzed and genes with a fold change greater than 5 and a p value less than 0.05 were considered significant. The specific genes and their fold change compared to control are shown on a logarithmic scale (Fig. 2). As expected, rHSP27 resulted in the alteration of both pro- and anti-inflammatory genes. For example, two common pro-inflammatory genes, TNF-α and IL-1β, showed increased expression with rHSP27 treatment. In contrast, as described in detail below, two key anti-inflammatory factors, IL-10 and GM-CSF (CSF2), also showed increased gene expression.
Fig. 2.
Focused gene expression profiling of PMA differentiated THP-1 cells treated with rHSP27. NF-κB pathway-focused qPCR arrays were used to screen the expression of genes involved in this pathway. THP-1 macrophages were treated with rHSP27 (9.6 μM) supplemented with polymyxin B (PMB, 10 μg/mL). The delta delta Ct method was then used to calculate the fold change of genes that are up/down-regulated in rHSP27-treated macrophages compared to control. Genes with fold change >5 and a p value < 0.05 were considered to be significant and are shown on a logarithmic scale
rHSP27 induces NF-κB-dependent GM-CSF expression and secretion in THP-1 macrophages
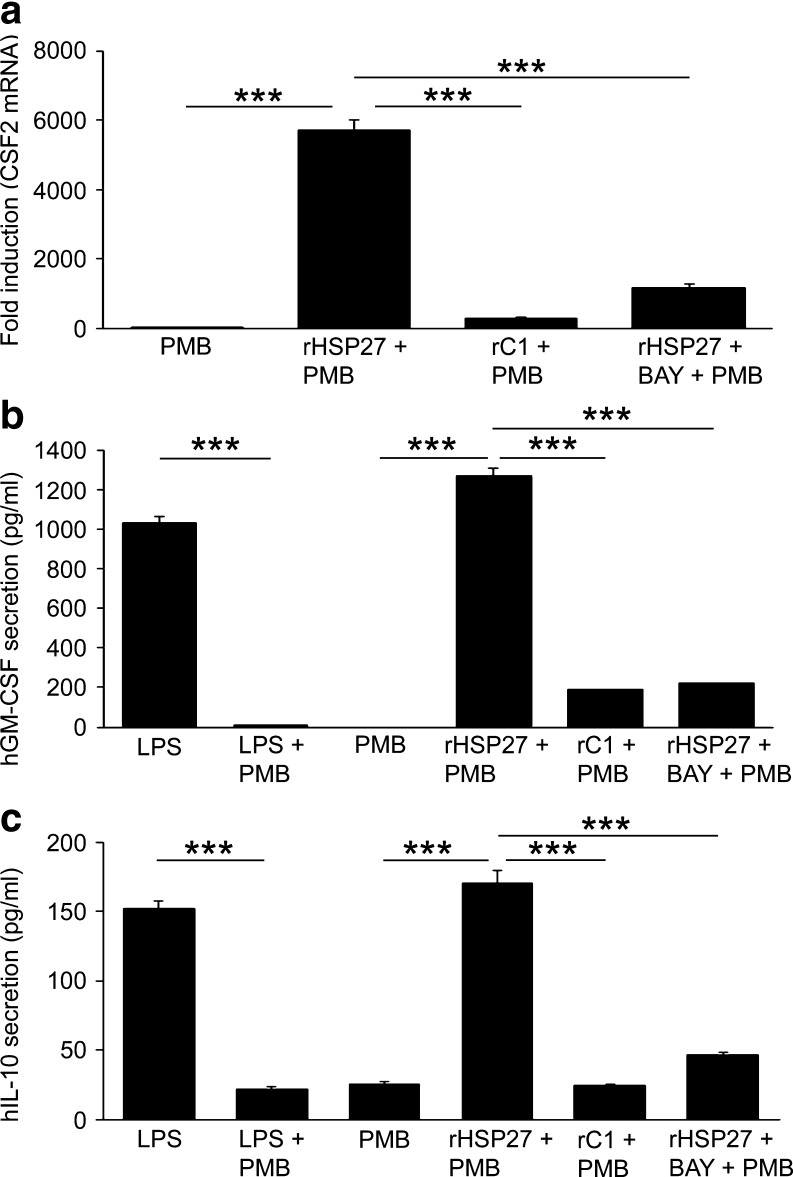
GM-CSF, encoded by the gene CSF2, showed one of the highest fold increases in response to rHSP27 treatment (5,700-fold increase; p < 0.001, n = 3; Fig. 3a)—an effect not observed when macrophages were treated with rC1. NF-κB inhibition with BAY 11-7082 blocked the effect of rHSP27 on GM-CSF gene expression, suggesting that this effect is in fact NF-κB mediated.
Fig. 3.
a GM-CSF mRNA levels rise in rHSP27-treated macrophages. PMA-differentiated THP-1 cells were treated with media (control), rC1 (9.6 μM), or rHSP27 (9.6 μM) ± BAY 11-7082 (10 μM) for 24 h. Inhibition of NF-κB signaling was achieved by pre-treating the cells for 1 h with BAY 11-7082 (10 μM). All treatments were supplemented with Polymyxin B (PMB, 10 μg/mL). One-way ANOVA *p < 0.001); n = 3. b GM-CSF is secreted by THP-1 macrophages in response to rHSP27. PMA-differentiated THP-1 cells were treated with media, LPS (10 ng/mL), rHSP27 (9.6 μM), or rC1 (9.6 μM) ± BAY 11-7082 (10 μM) for 24 h. Inhibition of NF-κB signaling was achieved by pre-treating the cells for 1 h with BAY 11-7082 (10 μM). Polymyxin B (PMB, 10 μg/mL) was used as indicated. One-way ANOVA *p < 0.001; n = 3. c IL-10 is secreted by THP-1 macrophages in response to rHSP27. THP-1 macrophages were treated with media, LPS (10 ng/mL), rHSP27 (9.6 μM), or rC1 (9.6 μM) ± BAY 11-7082 (10 μM) for 24 h. Inhibition of NF-κB signaling was achieved by pre-treating the cells for 1 h with BAY 11-7082 (10 μM). Polymyxin B (PMB, 10 μg/mL) was used as indicated. One-way ANOVA *p < 0.001; n = 3
GM-CSF, a secreted cytokine, was assayed by ELISA in the conditioned media of THP-1 macrophages to determine whether the transcriptional upregulation of this gene was reflected at the protein level. LPS was used as a positive control, as it has previously been demonstrated to induce the transcription of the CSF2 gene (Jawan et al. 2008), while rC1 was used as a negative control as it did not activate NF-κB signaling in our assays. rHSP27 was found to increase GM-CSF secretion by 1,270 fold and pretreatment of macrophages with BAY 11-7082 blocked this effect, suggesting that this effect is in fact NF-κB mediated (Fig. 3b).
rHSP27 induces NF-κB-dependent IL-10 secretion from THP-1 macrophages
IL-10 is widely described as an anti-inflammatory cytokine that is secreted from immune cells (Murray 2006). In addition, the up-regulation of IL-10 by extracellular HSP27 has been previously demonstrated in other models (De et al. 2000; Miller-Graziano et al. 2008; Rayner et al. 2008). We observed a similar affect on IL-10 gene expression in the qPCR arrays, and thus secreted IL-10 levels were measured in order to confirm that the increase in gene expression was reflected at the protein level. rHSP27 lead to a 6.8-fold increase in secreted IL-10 levels (p < 0.001; Fig. 3c). In line with our previous observations, rC1 did not have an effect on IL-10 levels while NF-κB inhibition by BAY 11-7082 blocked the rHSP27-induced increase in IL-10.
Discussion
HSP27 is protective against the development of atherosclerosis (Rayner et al. 2008, 2009). The exact mechanisms by which this protein acts to exert its anti-atherogenic effects remain unknown. In order to develop novel therapies that take advantage of these protective effects, the underlying mechanism(s) of action of HSP27 must be elucidated. Specifically, the objective of this study was to assess the involvement of NF-κB signaling pathway as a mechanism by which HSP27 exerts anti-inflammatory effects in macrophages. The two main findings of this study were that (1) rHSP27 treatment of macrophages leads to activation of the NF-κB transcriptional pathway and (2) macrophages treated with rHSP27 exhibit a differential transcriptional profile, which may account for some of the atheroprotective affects of extracellular HSP27 that were observed in previous studies (Rayner et al 2009). To elucidate the effects of rHSP27 on the activation of NF-κB in macrophages, we used several different approaches that allowed us to examine steps in the activation pathway—specifically the degradation of IκBα, translocation of the p65 subunit, transcriptional activation of an NF-κB reporter gene, as well as endogenous target genes, are all evidence that treatment of THP-1 macrophages with rHSP27 leads to activation of the NF-κB pathway.
The significance of NF-κB signaling in atherosclerosis and inflammation has been investigated and discussed in many studies (Dabek et al. 2010). However, despite the amount of research that has been done to better understand this transcriptional pathway, controversies remain with respect to the positive or negative impacts of NF-κB in atherosclerosis. Traditionally, the activation of this pathway is associated with a pro-inflammatory state, as it may lead to the upregulation of pro-inflammatory cytokines such as TNF-α and IL-1β (Ghosh et al. 2010). However, NF-κB is also involved in many protective and survival pathways, as it has been observed to induce anti-inflammatory factors such as IL-10 and Arginase I (Lawrence and Fong 2010). Based on the current state of research, it appears that a variety of factors can modulate NF-κB activity. Therefore, understanding the context and the stimuli under which the NF-κB pathway is activated is essential for our understanding and development of the therapeutic potential of HSP27 as an anti-atherogenic agent.
The NF-κB pathway-specific qRT-PCR arrays used in this study confirmed that there is a transcriptional outcome of rHSP27-induced activation of the NF-κB pathway in THP-1 macrophages. Overall, many genes were differentially regulated with rHSP27 treatment. This may be in part due to the highly sensitive state of activated macrophages and the role they play in inflammation and response to cytokine signaling. As rHSP27 activated the NF-κB reporter gene, it was expected that genes involved in the activation cascade would be differentially regulated. As a result of rHSP27 treatment, 24 genes were found to be significantly upregulated and 4 were found to be significantly downregulated. The rHSP27-regulated genes have varying functions and can be broadly categorized as either pro- or anti-atherogenic. Although several pro-atherogenic genes were induced by rHSP27 (e.g., IL-1β and TNF-α), there were also important anti-atherogenic factors that were significantly up-regulated. Of note, IL-10 and GM-CSF were induced at both the mRNA and protein level by the rHSP27 treatment of THP-1 macrophages and pre-treatment with BAY 11-7082 blocked this effect, suggesting that this effect is in fact NF-κB mediated. The up-regulation of IL-10 is consistent with previous literature and is supportive of the notion that HSP27 is an anti-inflammatory factor (De et al. 2000; Miller-Graziano et al. 2008). In addition, the up-regulation of IL-10 by rHSP27 is consistent with previous studies demonstrating the protective actions of IL-10 in various models of atherosclerosis (Caligiuri et al. 2003; Han et al. 2010; Mallat et al. 1999; Namiki et al. 2004; Potteaux et al. 2004; Yoshioka et al. 2004). For example, the deletion of IL-10 in atherosclerosis-prone ApoE−/− mice results in increased lesion size, a more robust inflammatory Th1 response, as well as increased systemic coagulation and vascular thrombosis, thereby suggesting that the abrogation of IL-10 may be detrimental for preventing or halting the development of atherosclerosis (Caligiuri et al. 2003). Additionally, intramuscular gene transfer of IL-10 cDNA results in elevated IL-10 serum levels as well as reduced expression of Th1-inflammatory cytokines and atherosclerotic plaque area in ApoE−/− mice (Namiki et al. 2004). Finally, in humans, George et al. (2012) found that serum IL-10 levels were lower in patients with clinically overt coronary artery disease (e.g., a previous myocardial infarction) as compared to “stable” patients with disease burden but without clinical events (George et al. 2012).
GM-CSF is another cytokine that is markedly upregulated by rHSP27. Several studies link GM-CSF to vascular remodeling and atherosclerosis (Ditiatkovski et al. 2006; Haghighat et al. 2007; Plenz et al. 2003; Shaposhnik et al. 2007; Weissen-Plenz et al. 2008). For example, GM-CSF deficiency in ApoE−/− mice worsens atherosclerotic lesion size and increases inflammatory cytokine expression (Ditiatkovski et al. 2006). GM-CSF deficiency also affects the vascular collagenous matrix and may play a role in maintaining vessel wall integrity (Plenz et al. 2003; Weissen-Plenz et al. 2008).
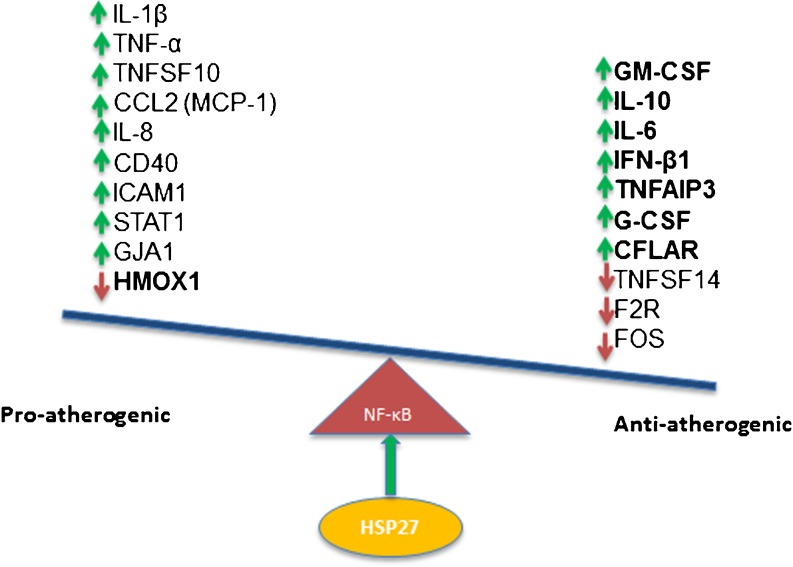
As initial studies suggest that serum HSP27 levels are lower in patients with vascular disease, this protein may indeed be a biomarker of atherosclerosis (Martin-Ventura et al. 2004, 2006; Miller et al. 2005). Therefore, it is attractive to consider the possible strategy of administering rHSP27 to patients at risk of or laden with CAD. Although data from the current study suggest that rHSP27 treatment of macrophages in vitro leads to upregulation of both pro- and anti-inflammatory genes, we know that elevated extracellular HSP27 in vivo is anti-atherogenic (Rayner et al. 2008, 2009). Moreover, in preliminary experiments, we show that administration of rHSP27 to ApoE−/− mice attenuates atherogenesis and surrogate markers of plaque inflammation (Chen et al. 2009). Hence, we have summarized the transcriptional effects of rHSP27 in a schematic diagram (Fig. 4), highlighting the concept that the consequence of rHSP27-mediated NF-κB activation is the net effect of a variety of seemingly competing anti-atherogenic and potentially pro-atherogenic effects. In summary, our data demonstrate the activation of NF-κB signaling in macrophages by rHSP27 results in an altered transcriptional profile. While it remains unclear how HSP27 is able to activate NF-κB signaling, especially at the cell surface, future studies examining this signaling paradigm will provide further insight into the mechanisms of HSP27 atheroprotection and will be helpful in developing novel therapeutic approaches for atherosclerosis.
Fig. 4.
Schematic diagram representing the transcriptional outcome of HSP27-mediated activation of NF-κB in THP-1 macrophages. Given the previously described anti-atherogenic effects of HSP27, it is speculated that the net effect of these transcriptional regulations is likely tipping the balance towards anti-inflammatory effects and suppression of atherosclerosis
Electronic supplementary material
rHSP27 is not phosphorylated. a Western blot demonstrating rHSP27 is not phosphorylated b LC-MS/MS confirmed rHSP27 is not phosphorylated (PPT 184 kb)
Gel filtration of rHSP27 and rC1 to demonstrate molecular size in 1× PBS buffer (pH = 7.4). The size of rHSP27 and rC1 was measured by gel filtration. The size of most of rHSP27 (>90 %) is between 1,000—5,000 kD with <10 % in the range of 150 kD. The size of recombinant C1 appears to be around 100 kD (PPT 40 kb)
a Assays of cell viability and cytotoxicity. THP1 cells that were treated with rHSP27 (9.6 μM) or rC1 (9.6 μM) show no changes in viability or membrane integrity as demonstrated by MTT (a) and LDH (b) assays, respectively (PSD 2915 kb)
Acknowledgments
This work was supported by operating grants MOP 80204 from the Canadian Institute for Health Research (CIHR) and T6335 from the Heart and Stroke Foundation of Ontario. CIHR and Medtronic collectively provide EOB with a peer-reviewed Research Chair (URC #57093; IGO 94418). SS was supported by an Ontario Graduate Scholarship and a CIHR IGH Women’s Health Council Masters Award. TS was supported by a studentship from the Heart and Stroke Foundation of Ontario and by a CIHR Frederick Banting and Charles Best Canada Graduate Doctoral Award. JR was supported by a CIHR Frederick Banting and Charles Best Canada Graduate Doctoral Award. CMC was supported by a postdoctoral fellowship from le Fonds de Recherche en Santé du Québec (FRSQ) and the Ernest and Margaret Ford cardiology endowed research fellowship from the University of Ottawa Heart Institute.
Disclosures
None.
Footnotes
Samira Salari and Tara Seibert contributed equally to this work
References
- Al-Madhoun AS, Chen YX, Haidari L, Rayner K, Gerthoffer W, McBride H, O’Brien ER. The interaction and cellular localization of HSP27 and ERbeta are modulated by 17beta-estradiol and HSP27 phosphorylation. Mol Cell Endocrinol. 2007;270:33–42. doi: 10.1016/j.mce.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. The cellular “networking” of mammalian Hsp27 and its functions in the control of protein folding, redox state and apoptosis. Adv Exp Med Biol. 2007;594:14–26. doi: 10.1007/978-0-387-39975-1_2. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Baltimore D. A 65-kappaD subunit of active NF-kappaB is required for inhibition of NF-kappaB by I kappaB. Genes Dev. 1989;3:1689–1698. doi: 10.1101/gad.3.11.1689. [DOI] [PubMed] [Google Scholar]
- Benjamin IJ, McMillan DR. Stress (heat shock) proteins molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. doi: 10.1161/01.RES.83.2.117. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, az-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nature Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- Caligiuri G, Rudling M, Ollivier V, Jacob MP, Michel JB, Hansson GK, Nicoletti A. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med. 2003;9:10–17. [PMC free article] [PubMed] [Google Scholar]
- Chen Y-X, Zhao X, McNulty M, O’Brien ER. Recombinant HSP27 therapy reduces serum cholesterol levels and experimental atherogenesis. Circulation. 2009;120:S1153. doi: 10.1161/CIRCULATIONAHA.107.751412. [DOI] [Google Scholar]
- Dabek J, Kulach A, Gasior Z. Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB): a new potential therapeutic target in atherosclerosis? Pharmacol Rep. 2010;62:778–783. doi: 10.1016/s1734-1140(10)70338-8. [DOI] [PubMed] [Google Scholar]
- De AK, Kodys KM, Yeh BS, Miller-Graziano C. Exaggerated human monocyte IL-10 concomitant to minimal TNF-alpha induction by heat-shock protein 27 (Hsp27) suggests Hsp27 is primarily an antiinflammatory stimulus. J Immunol. 2000;165:3951–3958. doi: 10.4049/jimmunol.165.7.3951. [DOI] [PubMed] [Google Scholar]
- Ditiatkovski M, Toh BH, Bobik A. GM-CSF deficiency reduces macrophage PPAR-gamma expression and aggravates atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2337–2344. doi: 10.1161/01.ATV.0000238357.60338.90. [DOI] [PubMed] [Google Scholar]
- George J, Schwartzenberg S, Medvedovsky D, Jonas M, Charach G, Afek A, Shamiss A. Regulatory T cells and IL-10 levels are reduced in patients with vulnerable coronary plaques. Atherosclerosis. 2012;222:519–523. doi: 10.1016/j.atherosclerosis.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Ghosh CC, Ramaswami S, Juvekar A, Vu HY, Galdieri L, Davidson D, Vancurova I. Gene-specific repression of proinflammatory cytokines in stimulated human macrophages by nuclear IkappaBalpha. J Immunol. 2010;185:3685–3693. doi: 10.4049/jimmunol.0902230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-kappaB in the heart: to be or not to NF-kappaB. Circ Res. 2011;108:1122–1132. doi: 10.1161/CIRCRESAHA.110.226928. [DOI] [PubMed] [Google Scholar]
- Haghighat A, Weiss D, Whalin MK, Cowan DP, Taylor WR. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor exacerbate atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2007;115:2049–2054. doi: 10.1161/CIRCULATIONAHA.106.665570. [DOI] [PubMed] [Google Scholar]
- Han X, Kitamoto S, Wang H, Boisvert WA. Interleukin-10 overexpression in macrophages suppresses atherosclerosis in hyperlipidemic mice. FASEB J. 2010;24:2869–2880. doi: 10.1096/fj.09-148155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawan B, Kao YH, Goto S, Pan MC, Lin YC, Hsu LW, Nakano T, Lai CY, Sun CK, Cheng YF, Tai MH, Eng HL, Wang CS, Huang CJ, Lin CR, Chen CL. Propofol pretreatment attenuates LPS-induced granulocyte-macrophage colony-stimulating factor production in cultured hepatocytes by suppressing MAPK/ERK activity and NF-kappaB translocation. Toxicol Appl Pharmacol. 2008;229:362–373. doi: 10.1016/j.taap.2008.01.044. [DOI] [PubMed] [Google Scholar]
- Kanters E, Pasparakis M, Gijbels MJJ, Vergouwe MN, Partouns-Hendriks I, Fijneman RJA, Clausen BE, Förster I, Kockx MM, Rajewsky K, Kraal G, Hofker MH, Winther MPJ. Inhibition of NF-kappaB activation in macrophages increases atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;112:1176–1185. doi: 10.1172/JCI18580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanters E, Gijbels MJJ, Made I, Vergouwe MN, Heeringa P, Kraal G, Hofker MH, Winther MPJ. Hematopoietic NF-kappaB1 deficiency results in small atherosclerotic lesions with an inflammatory phenotype. Blood. 2004;103:934–940. doi: 10.1182/blood-2003-05-1450. [DOI] [PubMed] [Google Scholar]
- Karban AS, Okazaki T, Panhuysen CI, Gallegos T, Potter JJ, Bailey-Wilson JE, Silverberg MS, Duerr RH, Cho JH, Gregersen PK, Wu Y, Achkar JP, Dassopoulos T, Mezey E, Bayless TM, Nouvet FJ, Brant SR. Functional annotation of a novel NFKB1 promoter polymorphism that increases risk for ulcerative colitis. Hum Mol Genet. 2004;13:35–45. doi: 10.1093/hmg/ddh008. [DOI] [PubMed] [Google Scholar]
- Lappas M, Yee K, Permezel M, Rice GE. Sulfasalazine and BAY 11-7082 interfere with the nuclear factor-kappa B and I kappa B kinase pathway to regulate the release of proinflammatory cytokines from human adipose tissue and skeletal muscle in vitro. Endocrinology. 2005;146:1491–1497. doi: 10.1210/en.2004-0809. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Fong C. The resolution of inflammation: anti-inflammatory roles for NF-kappaB. Int J Biochem Cell Biol. 2010;42:519–523. doi: 10.1016/j.biocel.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arterioscler Thromb Vasc Biol. 2011;31:1506–1516. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat Z, Besnard S, Duriez M, Deleuze V, Emmanuel F, Bureau MF, Soubrier F, Esposito B, Duez H, Fievet C, Staels B, Duverger N, Scherman D, Tedgui A. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–e24. doi: 10.1161/01.RES.85.8.e17. [DOI] [PubMed] [Google Scholar]
- Martin-Ventura JL, Duran MC, Blanco-Colio LM, Meilhac O, Leclercq A, Michel JB, Jensen ON, Hernandez-Merida S, Tuñón J, Vivanco F, Egido J. Identification by a differential proteomic approach of heat shock protein 27 as a potential marker of atherosclerosis. Circulation. 2004;110:2216–2219. doi: 10.1161/01.CIR.0000136814.87170.B1. [DOI] [PubMed] [Google Scholar]
- Martin-Ventura JL, Nicolas V, Houard X, Blanco-Colio LM, Leclercq A, Egido J, Vranckx R, Michel JB, Meilhac O. Biological significance of decreased HSP27 in human atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:1337–1343. doi: 10.1161/01.ATV.0000220108.97208.67. [DOI] [PubMed] [Google Scholar]
- Miller H, Poon S, Hibbert B, Rayner K, Chen YX, O’Brien ER. Modulation of estrogen signaling by the novel interaction of heat shock protein 27, a biomarker for atherosclerosis, and estrogen receptor beta: mechanistic insight into the vascular effects of estrogens. Arterioscler Thromb Vasc Biol. 2005;25:e10–e14. doi: 10.1161/01.ATV.0000156536.89752.8e. [DOI] [PubMed] [Google Scholar]
- Miller-Graziano CL, De A, Laudanski K, Herrmann T, Bandyopadhyay S (2008) HSP27: an anti-inflammatory and immunomodulatory stress protein acting to dampen immune function. 291:196–208. Ref Type: Serial (Book,Monograph) [DOI] [PubMed]
- Monaco C, Andreakos E, Kiriakidis S, Mauri C, Bicknell C, Foxwell B, Cheshire N, Paleolog E, Feldmann M. Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proc Natl Acad Sci U S A. 2004;101:5634–5639. doi: 10.1073/pnas.0401060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JM, Ziegler-Heitbrock HWL, Baeuerle PA. Nuclear factor kappa B, a mediator of lipopolysaccharide effects. Immunobiology. 1993;187:233–256. doi: 10.1016/S0171-2985(11)80342-6. [DOI] [PubMed] [Google Scholar]
- Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr Opin Pharmacol. 2006;6:379–386. doi: 10.1016/j.coph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Namiki M, Kawashima S, Yamashita T, Ozaki M, Sakoda T, Inoue N, Hirata KI, Morishita R, Kaneda Y, Yokoyama M. Intramuscular gene transfer of interleukin-10 cDNA reduces atherosclerosis in apolipoprotein E-knockout mice. Atherosclerosis. 2004;172:21–29. doi: 10.1016/j.atherosclerosis.2003.08.032. [DOI] [PubMed] [Google Scholar]
- Nishibori M, Takahashi HK, Katayama H, Mori S, Saito S, Iwagaki H, Tanakac N, Morita K, Ohtsuka A. Specific removal of monocytes from peripheral blood of septic patients by polymyxin B-immobilized filter column. Acta Med Okayama. 2009;63:65–69. doi: 10.18926/AMO/31855. [DOI] [PubMed] [Google Scholar]
- Oeckinghaus A, Ghosh S (2009) The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 1 [DOI] [PMC free article] [PubMed]
- Parcellier A, Schmitt E, Gurbuxani S, Seigneurin-Berny D, Pance A, Chantôme A, Plenchette S, Khochbin S, Solary E, Garrido C. HSP27 is a ubiquitin-binding protein involved in I-kappaBalpha proteasomal degradation. Mol Cell Biol. 2003;23:5790–5802. doi: 10.1128/MCB.23.16.5790-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Farrance IK, Fenty NM, Hagberg JM, Roth SM, Mosser DM, Wang MQ, Jo H, Okazaki T, Brant SR, Brown MD. NFKB1 promoter variation implicates shear-induced NOS3 gene expression and endothelial function in prehypertensives and stage I hypertensives. Am J Physiol Heart Circ Physiol. 2007;293:H2320–H2327. doi: 10.1152/ajpheart.00186.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenz G, Eschert H, Beissert S, Arps V, Sindermann JR, Robenek H, Völker W. Alterations in the vascular extracellular matrix of granulocyte macrophage colony-stimulating factor (GM-CSF)-deficient mice. FASEB J. 2003;17:1451–1457. doi: 10.1096/fj.02-1035com. [DOI] [PubMed] [Google Scholar]
- Pockley AG. Heat shock proteins, inflammation, and cardiovascular disease. Circulation. 2002;105:1012–1017. doi: 10.1161/hc0802.103729. [DOI] [PubMed] [Google Scholar]
- Potteaux S, Esposito B, Oostrom O, Brun V, Ardouin P, Groux H, Tedgui A, Mallat Z. Leukocyte-derived interleukin 10 is required for protection against atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:1474–1478. doi: 10.1161/01.ATV.0000134378.86443.cd. [DOI] [PubMed] [Google Scholar]
- Rayner K, Chen YX, Mcnulty M, Simard T, Zhao X, Wells DJ, Belleroche J, O’Brien ER. Extracellular release of the atheroprotective heat shock protein 27 is mediated by estrogen and competitively inhibits acLDL binding to scavenger receptor-a. Circ Res. 2008;103:133–141. doi: 10.1161/CIRCRESAHA.108.172155. [DOI] [PubMed] [Google Scholar]
- Rayner K, Sun J, Chen YX, Mcnulty M, Simard T, Zhao X, Wells DJ, Belleroche J, O’Brien ER. Heat shock protein 27 protects against atherogenesis via an estrogen-dependent mechanism: role of selective estrogen receptor beta modulation. Arterioscler Thromb Vasc Biol. 2009;29:1751–1756. doi: 10.1161/ATVBAHA.109.193656. [DOI] [PubMed] [Google Scholar]
- Shaposhnik Z, Wang X, Weinstein M, Bennett BJ, Lusis AJ. Granulocyte macrophage colony-stimulating factor regulates dendritic cell content of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2007;27:621–627. doi: 10.1161/01.ATV.0000254673.55431.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Ma X, Chen YX, Rayner K, Hibbert B, Mcnulty M, Dhaliwal B, Simard T, Ramirez D, O’Brien E. Attenuation of atherogenesis via the anti-inflammatory effects of the selective estrogen receptor beta modulator 8beta-VE2. J Cardiovasc Pharmacol. 2011;58:399–405. doi: 10.1097/FJC.0b013e318226bd16. [DOI] [PubMed] [Google Scholar]
- Hove T, Vervoordeldonk MJBM, Dekkers PEP, Reitsma PH, Deventer SJH. Lps induced translocation of NF-kappaB occurs only in a subpopulation of CD14-positive mononuclear cells. Innate Immun. 1999;5:15–21. [Google Scholar]
- Voegeli TS, Wintink AJ, Chen Y, Currie RW. Heat shock proteins 27 and 70 regulating angiotensin II-induced NF-kappaB: a possible connection to blood pressure control? Appl Physiol Nutr Metab. 2008;33:1042–1049. doi: 10.1139/H08-068. [DOI] [PubMed] [Google Scholar]
- Vogel U, Jensen MK, Due KM, Rimm EB, Wallin H, Nielsen MRS, Pedersen APT, Tiønneland A, Overvad K. The NFKB1 ATTG ins/del polymorphism and risk of coronary heart disease in three independent populations. Atherosclerosis. 2011;219:200–204. doi: 10.1016/j.atherosclerosis.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Quinn PJ. Lipopolysaccharide: biosynthetic pathway and structure modification. Prog. Lipid Res. 2010;49:97–107. doi: 10.1016/j.plipres.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Weissen-Plenz G, Eschert H, Volker W, Sindermann JR, Beissert S, Robenek H, Scheld HH, Breithardt G. Granulocyte macrophage colony-stimulating factor deficiency affects vascular elastin production and integrity of elastic lamellae. J Vasc Res. 2008;45:103–110. doi: 10.1159/000109819. [DOI] [PubMed] [Google Scholar]
- Xanthoulea S, Curfs DMJ, Hofker MH, Winther MPJ. Nuclear factor kappaB signaling in macrophage function and atherogenesis. Curr Opin Lipidology. 2005;16:536–542. doi: 10.1097/01.mol.0000180167.15820.ae. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Okada T, Maeda Y, Ikeda U, Shimpo M, Nomoto T, Takeuchi K, Nonaka-Sarukawa M, Ito T, Takahashi M, Matsushita T, Mizukami H, Hanazono Y, Kume A, Ookawara S, Kawano M, Ishibashi S, Shimada K, Ozawa K. Adeno-associated virus vector-mediated interleukin-10 gene transfer inhibits atherosclerosis in apolipoprotein E-deficient mice. Gene Ther. 2004;11:1772–1779. doi: 10.1038/sj.gt.3302348. [DOI] [PubMed] [Google Scholar]
- Zhang W, Xing SS, Sun XL, Xing QC. Overexpression of activated nuclear factor-kappaB in aorta of patients with coronary atherosclerosis. Clin Cardiol. 2009;32:E42–E47. doi: 10.1002/clc.20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rHSP27 is not phosphorylated. a Western blot demonstrating rHSP27 is not phosphorylated b LC-MS/MS confirmed rHSP27 is not phosphorylated (PPT 184 kb)
Gel filtration of rHSP27 and rC1 to demonstrate molecular size in 1× PBS buffer (pH = 7.4). The size of rHSP27 and rC1 was measured by gel filtration. The size of most of rHSP27 (>90 %) is between 1,000—5,000 kD with <10 % in the range of 150 kD. The size of recombinant C1 appears to be around 100 kD (PPT 40 kb)
a Assays of cell viability and cytotoxicity. THP1 cells that were treated with rHSP27 (9.6 μM) or rC1 (9.6 μM) show no changes in viability or membrane integrity as demonstrated by MTT (a) and LDH (b) assays, respectively (PSD 2915 kb)