Abstract
Hyperbaric oxygen therapy (HBOT) is used for a number of applications, including the treatment of diabetic foot ulcers and CO poisoning. However, we and others have shown that HBOT can mobilize cellular antioxidant defenses, suggesting that it may also be useful under circumstances in which tissue protection from oxidative damage is desired. To test the protective properties of hyperbaric oxygen (HBO) on a tissue level, we evaluated the ability of a preconditioning treatment regimen to protect cutaneous tissue from UV-A-induced oxidative damage. Three groups of hairless SKH1-E mice were exposed to UV-A 3 days per week for 22 weeks, with two of these groups receiving an HBO pretreatment either two or four times per week. UV-A exposure increased apoptosis and proliferation of the skin tissue, indicating elevated levels of epithelial damage and repair. Pretreatment with HBO significantly reduced UV-A-induced apoptosis and proliferation. A morphometric analysis of microscopic tissue folds also showed a significant increase in skin creasing following UV-A exposure, which was prevented by HBO pretreatment. Likewise, skin elasticity was found to be greatest in the group treated with HBO four times per week. The effects of HBO were also apparent systemically as reductions in caspase-3 activity and expression were observed in the liver. Our findings support a protective function of HBO pretreatment from a direct oxidative challenge of UV-A to skin tissue. Similar protection of other tissues may likewise be achievable.
Keywords: Hyperbaric oxygen, Preconditioning, UV-A, Skin, Apoptosis, Liver, SKH1-E mice
Introduction
Hyperbaric oxygen therapy (HBOT) is a method of treatment in which patients inspire 100 % oxygen at a pressure greater than 1 atm (Londahl 2012). At present, HBOT has been approved for use in a number of clinical settings, including the treatment of carbon monoxide poisoning, air/gas embolisms, and chronic wounds (Gill and Bell 2004). It has also been shown to significantly enhance the healing of diabetic foot ulcers (Abidia et al. 2003; Kessler et al. 2003; Duzgun et al. 2008), improve quality of life (Londahl et al. 2011), and decrease the need for limb amputations in these patients (Faglia et al. 1996; Thackham et al. 2008; Bishop and Mudge 2012). It is currently known that HBOT increases the partial pressure of oxygen in hypoxic tissue (Al-Waili and Butler 2006; Babchin et al. 2011) and attenuates the inflammatory response by interfering with cytokine production and activity (Alex et al. 2005; Daniel et al. 2011; Lin et al. 2012). It has also been reported that HBOT increases the production of reactive oxygen species (ROS) within the tissue (Matsunami et al. 2011; Simsek et al. 2011), thereby mobilizing cellular antioxidant responses (Matsunami et al. 2009; Godman et al. 2010b; He et al. 2011). Accordingly, hyperbaric oxygen (HBO) has been acknowledged as an effective preconditioning agent under circumstances in which protection from oxidative damage is desired, such as in rat models of myocardial infarction (Han et al. 2008; Sun et al. 2011) and cerebral ischemia–reperfusion injury (Li et al. 2008, 2009; Cheng et al. 2011).
We previously reported that HBOT efficiently induces the expression of a number of cytoprotective genes in human microvascular endothelial cells (HMEC-1), including molecular chaperones and Nrf2-regulated antioxidant genes (Godman et al. 2010a, b). These changes in gene expression conferred significant protection against cell death by exposures to both hyperthermia and the oxidizing agent t-butyl-hydroperoxide in vitro. The antioxidant genes activated by HBOT include heme oxygenase-1 and metallothionein, which have been reported to protect cells from oxidative stress-induced damage (Bell and Vallee 2009; Xue et al. 2009; Verma et al. 2010; Hou et al. 2012). The ability of HBOT to activate the expression of antioxidant genes has also been reported for a number of other cell and tissue types (Shiraishi et al. 1983; Padgaonkar et al. 1997; Dennog et al. 1999; Rothfuss et al. 2001). However, little is known about the functional consequences of these gene expression changes and whether HBOT can indeed protect tissues from oxidative damage. We were therefore interested in determining whether HBO preconditioning can protect cutaneous tissue from UV-A radiation, a known inducer of oxidative stress (Phillipson et al. 2002; Marrot et al. 2005; Hseu et al. 2012; Svobodová et al. 2012). UV-A penetrates the dermal–epidermal junction and hypodermis of the skin and imparts tissue damage via the generation of ROS. These molecules can oxidize proteins and lipids and react with DNA to cause single-strand breaks and base modifications (Svobodova et al. 2011). This process imparts a number of molecular changes to skin tissue, including the increased turnover of keratinocytes (El-Abaseri et al. 2006) and an overwhelming of cellular protective responses, ultimately leading to increased levels of cell death (Gentile et al. 2003; He et al. 2005; Pustisek and Situm 2011). If HBO does indeed have protective properties as a preconditioning agent, we reasoned that it should be clearly manifested by the suppression of these markers of UV-A-induced skin damage. These studies would have direct implications for the protection of the skin from UV-A radiation (Svobodova et al. 2011; Poljsak and Dahmane 2012) and further demonstrate the utility of HBO for tissue protection from chronic, repetitive oxidative stress.
In these studies, three groups of hairless SKH1-E mice were exposed to an escalating UV-A dose for 22 weeks (Benavides et al. 2009). Two groups of animals were additionally subjected to HBO preconditioning either two or four times per week under conditions that approximated clinical settings (Gill and Bell 2004; Londahl 2012). Consistent with a preconditioning model, mice exposed to HBO showed a reduced level of radiation-induced cell turnover and skin creasing. Interestingly, changes in gene expression consistent with cellular protection were also observed in the liver. Our findings demonstrate that HBO can protect skin tissue from UV-A-induced oxidative damage. Due to the systemic nature of HBO treatment, its protective effects may also extend to tissues that are not directly exposed to the stressor.
Materials and methods
Animal model
Cutaneous tissue is constantly and directly exposed to solar light, the primary environmental source of UV-A radiation (Svobodova et al. 2011; Poljsak and Dahmane 2012), and is easily accessible for experimental manipulation and noninvasive measurements. The hairless SKH1-E mouse has been used extensively in prior studies of skin physiology (Panteleyev et al. 1998; Benavides et al. 2009) and was therefore selected for these experiments. These immunocompetent, unpigmented animals permit ready exposure to UV-A radiation as well as direct visualization of skin response (Benavides et al. 2009).
Experimental design
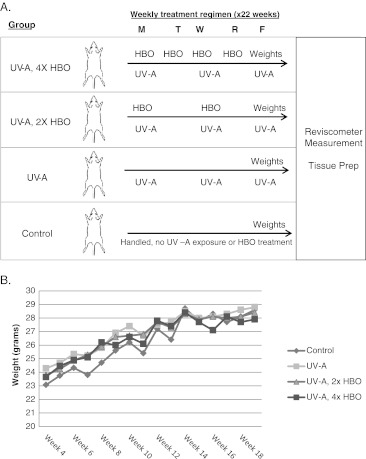
Thirty-seven SKH1-E mice obtained from Charles River Laboratories, International, Inc. (Wilmington, MA, USA) were used in these experiments. All mice were housed and treated in accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of Connecticut. Mice were randomly assigned to one of four groups (n = 8–10 per group): UV-A + 2× HBO (100 % O2, 2.4 atm, 1 h, twice per week); UV-A + 4× HBO (100 % O2, 2.4 atm, 1 h, 4 days per week); UV-A only; or control (mice were handled, but no UV-A exposure nor HBO pretreatment was conducted). Animals undergoing UV-A exposure were subjected to a whole-body irradiation three times per week using 15-W, F15T8/BLB black light bulbs (General Electric, Fairfield, CT, USA; Fig. 1a). An escalating UV-A dose over a period of 22 consecutive weeks was employed, starting at 90 mJ/cm2 and increasing 10 % per week until a dose of 175 mJ/cm2 was reached. HBO preconditioning treatments were administered using an OxyCure 3000 hyperbaric incubator (OxyHeal Health Group, National City, CA, USA). Intermittent, normobaric air breaks may be included in HBOT treatment regimens in order to reduce the risk of seizures resulting from CNS oxygen toxicity (Chavko and McCarron 2006). However, at the standard clinical dose of 2.4 atm, this response is exceedingly rare and has been shown to occur at a rate of only 0.0024 % (Yildiz et al. 2004). Because oxygen toxicity to cells and tissues was not a significant issue in our study, and the inclusion of air breaks would have substantially complicated our treatment protocol, this was not included. However, all animals were monitored during and after treatment, and no indications of distress or seizure were observed. Animals were also weighed on a weekly basis in order to assess any impact of treatment on the growth rate and overall health. Following the treatment period, mice were euthanized and their skin and liver tissues removed. Tissue samples were fixed in 4 % paraformaldehyde and then processed for sectioning and histological analysis. Others were snap-frozen and used for biochemical assays.
Fig. 1.
Experimental design. a Figure illustrating the basis of our experimental design. HBO dosage = 100 % O2, 2.4 atm absolute, 1 h. b Average animal weights. No effects of treatment on growth rate and animal health were observed
Preparation of cytosolic liver extracts
To prepare cytosolic liver extracts, sections of tissue weighing 28.5 ± 1.5 mg were obtained from each animal. Each sample was added to 400 μL of buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 2 mM MgCl2, 15 mM sucrose) containing 0.1 % Nonidet-P40 (NP40) cell lysis buffer. Tissue was lysed via homogenization and centrifuged at 10,000×g for 5 min. The supernatant (20 μL) was removed from each sample and used to create pools representative of all treatment groups. Extracts were stored at −80 °C.
Immunohistochemistry
Skin and liver tissues were dehydrated, formalin-fixed, and embedded in paraffin according to standard procedures, and 10-μm-thick sections were cut and mounted on glass slides. The slides were de-paraffinized and rehydrated according to standard protocols and then heated in 10 mM citrate buffer (pH 6.0) for 20 min for epitope retrieval. The skin tissue was blocked at room temperature for 30 min using 5 % serum (in phosphate-buffered saline) and incubated for 1 h with diluted primary antibodies (1:100 in 5 % serum) against proliferating cell nuclear antigen (PCNA; rabbit polyclonal, SC-7907, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or cleaved/active caspase-3 (rabbit polyclonal, no. 9662S, Cell Signaling Technologies, Beverly, MA, USA). The liver tissue was similarly blocked and incubated with diluted primary antibodies against cleaved caspase-3 only. CY3-conjugated secondary antibodies (goat polyclonal, no. 111-165-144, Jackson ImmunoResearch, West Grove, PA, USA) diluted 1:100 in 5 % serum were selected for a 30-min incubation. A DAPI counterstain was performed to facilitate the visualization of tissue nuclei, and coverslips were mounted on slides for imaging.
TUNEL staining
Skin and liver tissues were prepared and mounted on glass slides as described previously. Slides were de-paraffinized and rehydrated according to standard protocols and heated in 10 mM citrate buffer (pH 6.0) for 20 min. An in situ Cell Death Detection Kit (no. 11684795910, Roche Applied Science, Indianapolis, IN, USA) was employed to stain for DNA fragmentation (apoptosis) according to the manufacturer’s instructions, and coverslips were mounted on slides for imaging. The data were quantified by determining the number of terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL)-positive cells per length of epidermis. Since some normal-appearing cells were weakly labeled, only cells showing intense staining and an apoptotic morphology (e.g., “rounding-up”) were counted as positive.
Analysis of skin micro-creasing
Histological samples were utilized to quantify microscopic folds within cutaneous tissue. Tissue sections were imaged, and measurements of the depths of individual “micro-creases” were made using an equal number of images and animals per treatment group (n = 8). Individual measurements within each group were summed to generate a total micro-creasing value.
Skin elasticity
The skin elasticity of animals was assessed using a Reviscometer RVM 600 probe (C-K Electronic, Köln, Germany). This device uses two parallel sensors to measure the propagation of ultrasonic waves through the tissue and is sensitive to the density and orientation of collagen fibers and the moisture content of the tissue. Wave propagation (resonance running time, RRT) is expressed in arbitrary units (Uhoda and Piérard 2003; Ruvolo et al. 2007; Sommerfeld 2007).
Caspase-3 activity
Fifty microliters of the supernatant from individual cytosolic liver extracts was diluted to 0.5 μg/μL and added to 50 μL of 2× reaction mixture (20 mM PIPES, pH 7.4, 4 mM EDTA, 0.2 % CHAPS, 10 mM DTT) containing 0.2 mM of the fluorogenic substrate acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (DEVD-AMC; Enzo Life Sciences, Farmington, NY, USA). The reaction was carried out on a 96-well plate, and the fluorescence was measured using a microplate reader (excitation/emission, 360/460) at the start of the reaction and after 15 min. Protein concentrations were determined using the BioRad Protein Assay reagent according to the manufacturer’s instructions (BioRad, Hercules, CA, USA). The enzyme activity was quantified by dividing the change in fluorescence after 15 min by the total amount of protein in each reaction mixture (Kuratnik et al. 2012).
Results
Experimental design
To determine the effect of HBO preconditioning on the skin tissue, hairless SKH1-E mice were sorted randomly into four groups (shown schematically in Fig. 1a). Three groups of animals were exposed to UV-A three times per week, with two of these groups receiving an HBO pretreatment either two or four times per week. An unexposed and untreated control group was run in parallel. The HBO dose was similar to that used clinically: 1 h exposure at 2.4 atm in 100 % O2. The safety of HBOT at this intensity is well established, with only minor side effects such as inflammation of the middle ear and reversible myopia being the most frequently reported (Heyneman and Lawless-Liday 2002; Tiaka et al. 2012). Accordingly, no major deviation in growth curves was observed for the groups receiving HBO pretreatment (Fig. 1b).
Effects of HBO preconditioning on cell turnover in the skin
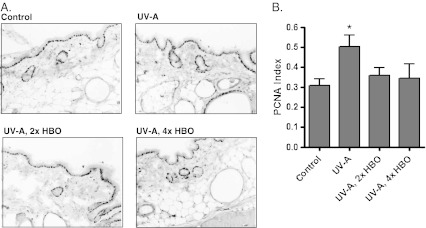
To determine the effect of UV-A and HBO preconditioning on keratinocyte turnover, cutaneous tissue was stained for the cell proliferation marker PCNA (von Neubeck et al. 2012), and a proliferation index was derived by calculating the ratio of PCNA-positive nuclei to the total nuclei within the tissue (Kuratnik et al. 2012; Rigatti et al. 2012). Figure 2a, b shows representative images and the quantified data, respectively. The PCNA staining index in the group treated only with UV-A was significantly higher than that of the control group. HBO pretreatment, either two or four times weekly, reduced PCNA staining to the control levels, consistent with a protective effect from UV-A-induced damage.
Fig. 2.
Analysis of PCNA staining in skin tissue. a Representative PCNA immunohistochemical staining of the skin tissue from the different groups. Darkly stained nuclei (mostly in the epidermis) are PCNA-positive. Magnification, ×200. b Quantified staining. The ratio of PCNA-positive nuclei to the total nuclei was obtained to calculate the staining index. The UV-A group showed a higher staining index than the control, whereas the HBOT groups were not significantly different from the control (Bartlett’s test for variance: *p < 0.05, n = 8)
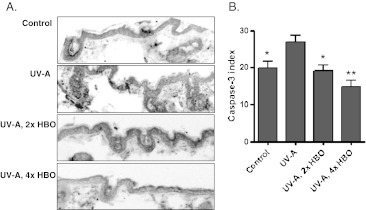
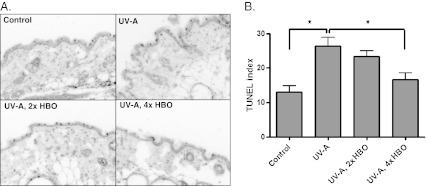
To determine whether HBO preconditioning suppressed apoptosis associated with chronic UV-A exposure, we quantified caspase-3 activation in the four groups. Figure 3a, b shows representative images and the quantified staining for active caspase-3, respectively. Animals exposed to UV-A showed a higher staining index than the control animals. Likewise, both groups pretreated with HBO had significantly lower levels of active caspase-3 staining in comparison to the UV-A-exposed animals. A TUNEL stain for DNA fragmentation (Fig. 4a) generated similar results. The labeling index (Fig. 4b) was significantly higher in the UV-A-only group relative to the control or HBO-preconditioned animals.
Fig. 3.
Analysis of active caspase-3 in skin tissue. a Representative cleaved caspase-3 immunohistochemical staining in cutaneous tissue from the different groups. An antibody recognizing both the full-length caspase-3 protein and the 17-kDa fragment resulting from enzymatic cleavage was employed. Magnification, ×200. b Quantified staining. Cells staining for cleaved/active caspase-3 were counted and controlled for field area. The UV-A group showed a higher staining index than the control and either HBO group (ANOVA with Tukey’s post hoc test relative to the UV-A group: *p < 0.05, **p < 0.001, n = 8)
Fig. 4.
TUNEL staining in the skin tissue for DNA fragmentation. a Representative TUNEL-stained images. Magnification, ×200. b Quantification of TUNEL-positive cells per length of epidermis. Cells that showed intense TUNEL staining and an apoptotic morphology (e.g., “rounding-up”) were scored positive (ANOVA with Tukey’s multiple comparison test: *p < 0.01)
HBO preconditioning and structural changes in the skin
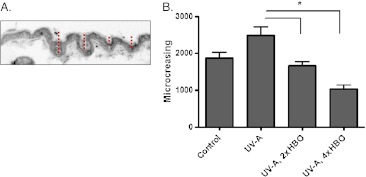
To assess the impact of experimental treatment on the skin structure, we measured the total depth of skin “micro-creases” (microscopic tissue folds) per animal using hematoxylin and eosin-stained sections. The red bars in Fig. 5a show how the micro-creasing measurements were obtained; an equal number of animals and images within each treatment group were analyzed. The degree of creasing was significantly higher in the animals treated with UV-A alone, relative to the control or those receiving HBO preconditioning (Fig. 5b). This is consistent with an HBO-induced protection of the tissue.
Fig. 5.
Skin micro-creasing. a Skin micro-creasing was quantified by measuring the depths of folds on microscopic sections. The red bars show an example of such a measurement on a tissue section. b Images obtained from all treatment groups were quantified for micro-creasing. An equal number of images and animals were analyzed for each group (n = 8). Depth measurements were summed to obtain the total micro-creasing value, which is shown in the graph (*p < 0.01 and 0.001 for the 2× HBO and 4× HBO groups, respectively, as determined by ANOVA and Tukey’s multiple comparisons test)
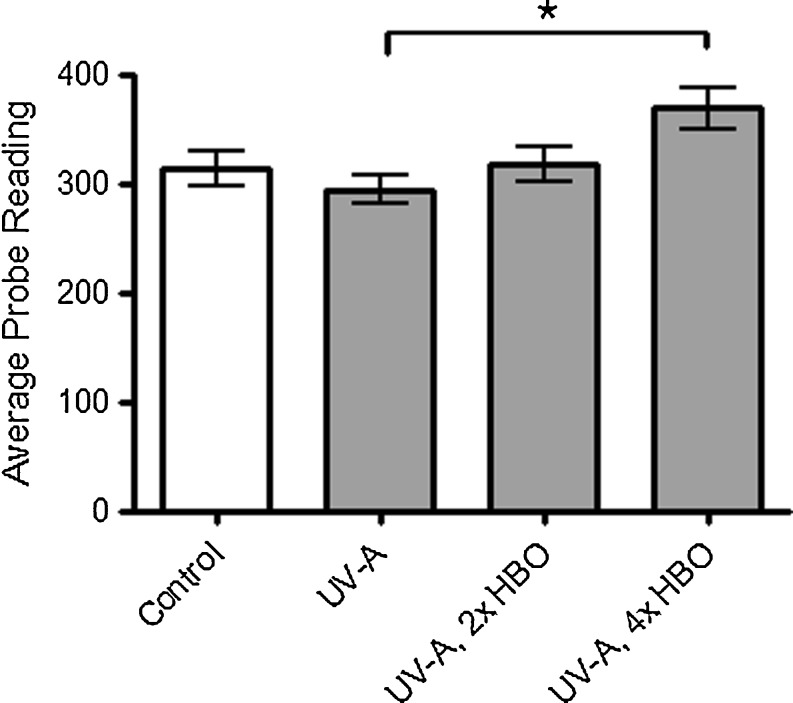
Using a Reviscometer 600, we determined the ability of HBO preconditioning to prevent the loss of skin elasticity incurred from chronic UV-A exposure. Although UV-A exposure only showed a trend to decreasing skin elasticity, animals preconditioned four times a week with HBO exhibited a statistically significant increase in elasticity relative to UV-A-treated animals (Fig. 6).
Fig. 6.
Reviscometer 600 measurements. This probe quantifies the ability of HBO preconditioning to suppress macroscopic changes in the skin arising from UV-A exposure. There is a statistically significant increase in the probe readings (RRT, expressed in arbitrary units) of mice that received HBO preconditioning four times per week relative to mice exposed to UV-A only. A higher number indicates less damaged (i.e., more elastic) skin (ANOVA and Tukey’s post hoc test: *p < 0.01)
HBO preconditioning and liver tissue
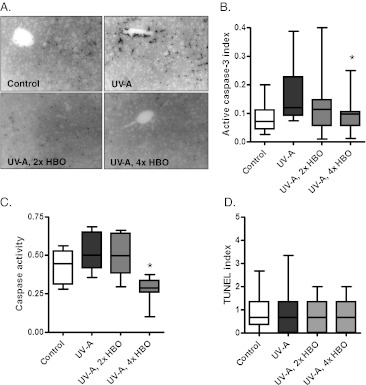
Immunohistochemical staining for cleaved/active caspase-3 was performed on hepatic tissue sections to determine whether the livers of HBO-preconditioned animals showed signs of protection following chronic UV-A exposure (Fig. 7a, b). UV-A-treated animals showed a trend of increasing caspase-3 staining relative to the control animals, with a significant reduction observed in animals receiving HBO pretreatment four times per week. We also assessed the activity of caspase-3 within the liver extracts using a DEVD-AMC substrate. Although UV-A-treated animals only showed a trend to increased caspase-3 activity relative to the controls, enzymatic activity was significantly decreased in animals receiving four HBO treatments per week (Fig. 7c). No significant changes in TUNEL staining were observed (Fig. 7d). These findings support the possibility that in addition to the skin, HBO may also be capable of exerting a protective effect on internal organs.
Fig. 7.
Response of the hepatic tissue to experimental treatment. a Representative cleaved caspase-3 immunohistochemical staining in liver from the different groups. b Quantification of cleaved caspase-3 staining. The ratio of caspase-3-positive cells to the total cells was obtained. The staining index is reduced significantly in the group treated four times per week with HBO (ANOVA and Tukey’s post hoc test: *p < 0.05) c Cleaved caspase-3 activity in cytosolic liver extracts. Enzymatic activity is significantly reduced in animals treated four times per week with HBO relative to the group treated with UV-A alone (Bartlett’s test for equal variance: *p < 0.001). d Quantification of TUNEL staining in liver tissue. TUNEL-positive cells were counted and controlled for field area. No significant differences were found among the groups (ANOVA)
Discussion
Although the types of approved medical applications vary, the benefit of HBOT stems from its ability to increase tissue levels of O2. The increased concentration of O2 can serve to stimulate a range of O2-dependent biochemical reactions. For example, the ability of NADPH oxidase to generate superoxide is strictly limited by the local concentration of O2. Increasing tissue levels of O2 can therefore enhance wound healing by enhancing the bactericidal actions of leukocytes within hypoxic, non-healing wounds. Evidence has also been obtained that the tissue levels of NO can be enhanced by hyperbaric treatment; oxygen tension at atmospheric pressures above 2.0 can increase NO production by pulmonary endothelial cells (Buras et al. 2000). In some instances, increased NO production has also been associated with enhanced cardioprotection in an ischemia–reperfusion model (Cabigas et al. 2006).
In addition to increasing the activity of O2-utilizing enzymes, HBOT can stimulate O2-dependent signaling pathways, which range from MAP kinase pathways to NF-κB (Bonomo et al. 1998; Lin et al. 2002; Shyu et al. 2009; Rinaldi et al. 2011; Wang et al. 2011). One pathway of particular interest is the Keap1/Nrf2 pathway which regulates the expression of a range of antioxidant genes that can potentially protect cells from severe oxidative stress. Employing a gene array approach, we reported that the activation of Nrf2-regulated genes, along with a number of molecular chaperones, is a dominant response of cells to HBOT (Godman et al. 2010a, b). However, since HBOT can induce such a broad range of cell- and tissue-level effects, an important question is whether, on balance, these responses are protective. As a step toward addressing this issue, we used a hairless mouse model with UV-A as a source of oxidative stress. We find that HBO preconditioning treatments provide a protective effect to UV-A-irradiated skin, as determined by reduced epidermal turnover, cutaneous creasing, and loss of skin elasticity. Effects were also observed in the liver, which can incur some damage from UV-A exposure due to the circulation of reactive molecules (Svobodova et al. 2011). When UV-A is used as the primary stressor, our mouse model indicates that HBO preconditioning provides a significant degree of protection from oxidative stress at the tissue level. Although this has implications regarding the protection of skin for various cosmetic purposes and pathological conditions, we propose that our data could also prove useful for understanding the mechanism of HBO-mediated tissue protection.
The phenomenon known as hormesis involves a favorable biological response to sub-toxic levels of stress. Hormetic agents induce an adaptive cellular response which confers resistance to harmful doses of the same stressor (Martins et al. 2011). We envision HBOT to be acting largely as a hormetic agent, stimulating the generation and sensing of reactive oxygen intermediates within the tissue (i.e., oxidative signaling), leading to the activation of protective responses including Nrf2 mobilization and antioxidant gene expression (Cypser and Johnson 2002; Rothfuss and Speit 2002; Godman et al. 2010a, b; Matsunami et al. 2011; Simsek et al. 2011). The HBO-preconditioned tissue is therefore prepared to withstand the stresses of UV-A and potentially other oxidative stressors. Even though HBOT has been reported to induce a level of oxidative stress within cells and tissues, it is only rarely associated with adverse effects. HBOT may therefore generate a spectrum of ROS that efficiently induce protective pathways without inducing extensive oxidative damage to macromolecules. In support of this conclusion, we found previously (Godman et al. 2010a) only small inductions of the cytosolic chaperones involved in protein damage responses, and no mobilization of HSPA6, which is only activated in human cells that incur substantial damage. Nonetheless, as with any hormetic agent, HBO dosing schedules need to be carefully studied to allow the development of maximal tissue protection.
In addition to upregulating antioxidant gene expression, the protective effects of HBO may also derive from a number of other reported effects of high oxygen tensions in tissues. One physiological response that may contribute to tissue protection is enhanced circulation. Saglam et al. (2008) described a significant increase in the diameter of the right brachial artery in healthy subjects following 10 HBO treatments, suggestive of long-term effects on vascular physiology. Accordingly, the HBO-mediated upregulation of nitric oxide synthase has been observed in a number of cell types (Buras et al. 2000; Liu et al. 2008; Xu et al. 2009; Lin et al. 2011; Kendall et al. 2012). HBOT also induces the expression of angiogenin, another promoter of NO synthesis, in a chronic wound model (Kendall et al. 2012). By stimulating NO production, HBOT triggers a vasodilatory response which increases blood flow and oxygen delivery to target tissues (Buerk 2007; Giles et al. 2012). Although future studies are required to test the contribution of enhanced circulation to HBO preconditioning-mediated protection, this well-documented response could facilitate the efficient flow of nutrients to the tissue and the removal of reactive molecules (such as aldehydes and other breakdown products) during times of stress.
The changes in cell turnover within the skin tissue we observed following UV-A exposure likely derive in part from enhanced inflammatory signaling (Gentile et al. 2003; He et al. 2005; Rock 2009; Svobodová and Vostálová 2010; Pustisek and Situm 2011). It is well established that UV radiation induces the production and secretion of a number of pro-inflammatory cytokines (Morita et al. 1997; Krutmann 2000; Halliday 2005). Interestingly, HBOT has been reported to possess anti-inflammatory effects in other scenarios (Al-Waili and Butler 2006; Thom 2009; Daniel et al. 2011), and it can reduce the circulating levels of TNF-α, IL-1, and IL-6 (Al-Waili and Butler 2006) implicated in the cutaneous response to UV exposure (Al-Waili and Butler 2006; Thom 2009; Daniel et al. 2011). These data suggest that HBOT may be beneficial in the management of dermatological conditions arising from pathological inflammation, such as psoriasis (Schafer 2012) and atopic dermatitis (Rebane et al. 2012).
Although decreases in caspase-3 expression and activity were observed in the livers of animals pretreated with HBO four times per week, animals exposed solely to UV-A showed a trend to increasing liver caspase-3 compared to the controls. Exposure of the skin to UV-A has been shown to increase apoptosis in that tissue (Wu et al. 2011; Boyer et al. 2012; Hseu et al. 2012; Lee et al. 2012), but few studies have been conducted which examined its effects on other tissues. Svobodova et al. (2011) described a number of changes in oxidative stress-related parameters in hepatic tissue following exposure to UV radiation, which were attributed in part to the circulation of reactive signaling molecules generated in the skin and associated blood vessels. This finding may provide a partial explanation for the non-significant increases in liver caspase-3 we report here. Similarly, the significant skin protection afforded to mice receiving HBO four times per week may ultimately reduce the indications of liver stress observed in the irradiated animals. We expect that if the trend to increasing liver caspase-3 is indeed real, the results would obtain statistical significance if more animals were analyzed. If this is not the case, the reduced liver caspase-3 in the 4× HBOT group may instead result from a protraction of the natural caspase-3 increases that occur throughout an animal’s life span (Zhang et al. 2002; Kujoth et al. 2005).
In summary, the data obtained from our mouse model support a protective function of HBO preconditioning from oxidative stress when UV-A is used as the primary stressor. We report that a preconditioning treatment regimen reduced apoptosis and proliferation in the skin and prevented detrimental structural changes such as creasing and a reduction in elasticity. The protective effects of HBO were also observed systemically because reductions in the expression and the activity of apoptotic markers were also evident in the liver. These responses may be valuable for understanding the molecular mechanisms by which HBOT confers tissue protection. They may also aid in the development of novel clinical applications for HBOT.
Acknowledgments
We are indebted to Dr. Rajeev Verma, Saryn Kunajukr, and Kousanee Chheda for their help in treating animals and preparing tissues for analysis. We also thank Aashay Vyas for his assistance in developing and performing the skin micro-creasing analysis. The OxyHeal Health Group provided the hyperbaric chamber for these studies as well as some of the funding.
References
- Abidia A, Laden G, Kuhan G, Johnson BF, Wilkinson AR, Renwick PM, Masson EA, McCollum PT. The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers: a double-blind randomised-controlled trial. Eur J Vasc Endovasc Surg : Off J Eur Soc Vasc Surg. 2003;25(6):513–518. doi: 10.1053/ejvs.2002.1911. [DOI] [PubMed] [Google Scholar]
- Alex J, Laden G, Cale ARJ, Bennett S, Flowers K, Madden L, Gardiner E, McCollum PT, Griffin SC. Pretreatment with hyperbaric oxygen and its effect on neuropsychometric dysfunction and systemic inflammatory response after cardiopulmonary bypass: a prospective randomized double-blind trial. J Thorac Cardiovasc Surg. 2005;130(6):1623–1630. doi: 10.1016/j.jtcvs.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Al-Waili NS, Butler GJ. Effects of hyperbaric oxygen on inflammatory response to wound and trauma: possible mechanism of action. The Scientific World Journal. 2006;6:425–441. doi: 10.1100/tsw.2006.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babchin A, Levich E, Melamed MDY, Sivashinsky G. Osmotic phenomena in application for hyperbaric oxygen treatment. Colloids Surf B: Biointerfaces. 2011;83(1):128–132. doi: 10.1016/j.colsurfb.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Bell SG, Vallee BL. The metallothionein/thionein system: an oxidoreductive metabolic zinc link. ChemBioChem. 2009;10(1):55–62. doi: 10.1002/cbic.200800511. [DOI] [PubMed] [Google Scholar]
- Benavides F, Oberyszyn TM, VanBuskirk AM, Reeve VE, Kusewitt DF. The hairless mouse in skin research. J Dermatol Sci. 2009;53(1):10–18. doi: 10.1016/j.jdermsci.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AJ, Mudge E (2012) A retrospective study of diabetic foot ulcers treated with hyperbaric oxygen therapy. Int Wound J. doi:10.1111/j.1742-481X.2011.00936.x [DOI] [PMC free article] [PubMed]
- Bonomo SR, Davidson JD, Yu Y, Xia Y, Lin X, Mustoe TA. Hyperbaric oxygen as a signal transducer: upregulation of platelet derived growth factor-beta receptor in the presence of HBO2 and PDGF. Undersea Hyperb Med : J Undersea Hyperb Med Soc Inc. 1998;25(4):211–216. [PubMed] [Google Scholar]
- Boyer JZ, Jandova J, Janda J, Vleugels FR, Elliott DA, Sligh JE. Resveratrol-sensitized UVA induced apoptosis in human keratinocytes through mitochondrial oxidative stress and pore opening. J Photochem Photobiol B: Biol. 2012;113:42–50. doi: 10.1016/j.jphotobiol.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerk DG. Nitric oxide regulation of microvascular oxygen. Antioxid Redox Signal. 2007;9(7):829–843. doi: 10.1089/ars.2007.1551. [DOI] [PubMed] [Google Scholar]
- Buras JA, Stahl GL, Svoboda KK, Reenstra WR. Hyperbaric oxygen downregulates ICAM-1 expression induced by hypoxia and hypoglycemia: the role of NOS. Am J Physiol Cell Physiol. 2000;278(2):C292–C302. doi: 10.1152/ajpcell.2000.278.2.C292. [DOI] [PubMed] [Google Scholar]
- Cabigas BP, Su J, Hutchins W, Shi Y, Schaefer RB, Recinos RF, Nilakantan V, Kindwall E, Niezgoda JA, Baker JE. Hyperoxic and hyperbaric-induced cardioprotection: role of nitric oxide synthase 3. Cardiovasc Res. 2006;72(1):143–151. doi: 10.1016/j.cardiores.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Chavko M, McCarron RM. Extension of brain tolerance to hyperbaric O2 by intermittent air breaks is related to the time of CBF increase. Brain Res. 2006;1084(1):196–201. doi: 10.1016/j.brainres.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Cheng O, Ostrowski RP, Wu B, Liu W, Chen C, Zhang JH. Cyclooxygenase-2 mediates hyperbaric oxygen preconditioning in the rat model of transient global cerebral ischemia. Stroke. 2011;42(2):484–490. doi: 10.1161/STROKEAHA.110.604421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypser JR, Johnson TE. Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J Gerontol Ser A: Biol Sci Med Sci. 2002;57(3):B109–B114. doi: 10.1093/gerona/57.3.B109. [DOI] [PubMed] [Google Scholar]
- Daniel RAF, Cardoso VK, Góis E, Jr, Parra RS, Garcia SB, Rocha JJ, Féres O. Effect of hyperbaric oxygen therapy on the intestinal ischemia reperfusion injury. Acta Cir Bras. 2011;26:463–469. doi: 10.1590/S0102-86502011000600010. [DOI] [PubMed] [Google Scholar]
- Dennog C, Radermacher P, Barnett YA, Speit G. Antioxidant status in humans after exposure to hyperbaric oxygen. Mutat Res. 1999;428(1–2):83–89. doi: 10.1016/s1383-5742(99)00034-4. [DOI] [PubMed] [Google Scholar]
- Duzgun AP, Satır HZ, Ozozan O, Saylam B, Kulah B, Coskun F. Effect of hyperbaric oxygen therapy on healing of diabetic foot ulcers. J Foot Ankle Surg. 2008;47(6):515–519. doi: 10.1053/j.jfas.2008.08.002. [DOI] [PubMed] [Google Scholar]
- El-Abaseri TB, Putta S, Hansen LA. Ultraviolet irradiation induces keratinocyte proliferation and epidermal hyperplasia through the activation of the epidermal growth factor receptor. Carcinogenesis. 2006;27(2):225–231. doi: 10.1093/carcin/bgi220. [DOI] [PubMed] [Google Scholar]
- Faglia E, Favales F, Aldeghi A, Calia P, Quarantiello A, Oriani G, Michael M, Campagnoli P, Morabito A. Adjunctive systemic hyperbaric oxygen therapy in treatment of severe prevalently ischemic diabetic foot ulcer. A randomized study. Diabetes Care. 1996;19(12):1338–1343. doi: 10.2337/diacare.19.12.1338. [DOI] [PubMed] [Google Scholar]
- Gentile M, Latonen L, Laiho M. Cell cycle arrest and apoptosis provoked by UV radiation–induced DNA damage are transcriptionally highly divergent responses. Nucleic Acids Res. 2003;31(16):4779–4790. doi: 10.1093/nar/gkg675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles TD, Sander GE, Nossaman BD, Kadowitz PJ. Impaired vasodilation in the pathogenesis of hypertension: focus on nitric oxide, endothelial-derived hyperpolarizing factors, and prostaglandins. J Clin Hypertens. 2012;14(4):198–205. doi: 10.1111/j.1751-7176.2012.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill AL, Bell CNA. Hyperbaric oxygen: its uses, mechanisms of action and outcomes. QJM. 2004;97(7):385–395. doi: 10.1093/qjmed/hch074. [DOI] [PubMed] [Google Scholar]
- Godman C, Chheda K, Hightower L, Perdrizet G, Shin D-G, Giardina C. Hyperbaric oxygen induces a cytoprotective and angiogenic response in human microvascular endothelial cells. Cell Stress Chaperones. 2010;15(4):431–442. doi: 10.1007/s12192-009-0159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godman CA, Joshi R, Giardina C, Perdrizet G, Hightower LE. Hyperbaric oxygen treatment induces antioxidant gene expression. Ann N Y Acad Sci. 2010;1197(1):178–183. doi: 10.1111/j.1749-6632.2009.05393.x. [DOI] [PubMed] [Google Scholar]
- Halliday GM. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat Res Fundam Mol Mech Mutagen. 2005;571(1–2):107–120. doi: 10.1016/j.mrfmmm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Han C, Lin L, Zhang W, Zhang L, Lv S, Sun Q, Tao H, Zhang JH, Sun X. Hyperbaric oxygen preconditioning alleviates myocardial ischemic injury in rats. Exp Biol Med. 2008;233(11):1448–1453. doi: 10.3181/0801-RM-8. [DOI] [PubMed] [Google Scholar]
- He YY, Huang JL, Block ML, Hong JS, Chignell CF. Role of phagocyte oxidase in UVA-induced oxidative stress and apoptosis in keratinocytes. J Investig Dermatol. 2005;125(3):560–566. doi: 10.1111/j.0022-202X.2005.23851.x. [DOI] [PubMed] [Google Scholar]
- He X, Xu X, Fan M, Chen X, Sun X, Luo G, Chen L, Mu Q, Feng Y, Mao Q, Chao Z. Preconditioning with hyperbaric oxygen induces tolerance against renal ischemia–reperfusion injury via increased expression of heme oxygenase-1. J Surg Res. 2011;170(2):e271–e277. doi: 10.1016/j.jss.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Heyneman CA, Lawless-Liday C. Using hyperbaric oxygen to treat diabetic foot ulcers: safety and effectiveness. Crit Care Nurse. 2002;22(6):52–60. [PubMed] [Google Scholar]
- Hou W, Tian Q, Steuerwald NM, Schrum LW, Bonkovsky HL (2012) The let-7 microRNA enhances heme oxygenase-1 by suppressing Bach1 and attenuates oxidant injury in human hepatocytes. Biochim Biophys Acta (BBA)—Gene Regul Mech (0). doi:10.1016/j.bbagrm.2012.06.001 [DOI] [PMC free article] [PubMed]
- Hseu Y-C, Chou C-W, Senthil Kumar KJ, Fu K-T, Wang H-M, Hsu L-S, Kuo Y-H, Wu C-R, Chen S-C, Yang H-L. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem Toxicol. 2012;50(5):1245–1255. doi: 10.1016/j.fct.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Kendall AC, Whatmore JL, Harries LW, Winyard PG, Smerdon GR, Eggleton P. Changes in inflammatory gene expression induced by hyperbaric oxygen treatment in human endothelial cells under chronic wound conditions. Exp Cell Res. 2012;318(3):207–216. doi: 10.1016/j.yexcr.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Kessler L, Bilbault P, Ortéga F, Grasso C, Passemard R, Stephan D, Pinget M, Schneider F. Hyperbaric oxygenation accelerates the healing rate of nonischemic chronic diabetic foot ulcers. Diabetes Care. 2003;26(8):2378–2382. doi: 10.2337/diacare.26.8.2378. [DOI] [PubMed] [Google Scholar]
- Krutmann J. Ultraviolet A radiation-induced biological effects in human skin: relevance for photoaging and photodermatosis. J Dermatol Sci. 2000;23(Supplement 1 (0)):S22–S26. doi: 10.1016/S0923-1811(99)00077-8. [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309(5733):481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- Kuratnik A, Senapati VE, Verma R, Mellone BG, Vella AT, Giardina C. Acute sensitization of colon cancer cells to inflammatory cytokines by prophase arrest. Biochem Pharmacol. 2012;83(9):1217–1228. doi: 10.1016/j.bcp.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Cha H, Hong M, Yoon Y, Lee H, An S. Role of NF-κB–p53 crosstalk in ultraviolet A-induced cell death and G1 arrest in human dermal fibroblasts. Arch Dermatol Res. 2012;304(1):73–79. doi: 10.1007/s00403-011-1176-2. [DOI] [PubMed] [Google Scholar]
- Li J, Liu W, Ding S, Xu W, Guan Y, Zhang JH, Sun X. Hyperbaric oxygen preconditioning induces tolerance against brain ischemia–reperfusion injury by upregulation of antioxidant enzymes in rats. Brain Res. 2008;1210:223–229. doi: 10.1016/j.brainres.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Li JS, Zhang W, Kang ZM, Ding SJ, Liu WW, Zhang JH, Guan YT, Sun XJ. Hyperbaric oxygen preconditioning reduces ischemia–reperfusion injury by inhibition of apoptosis via mitochondrial pathway in rat brain. Neuroscience. 2009;159(4):1309–1315. doi: 10.1016/j.neuroscience.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Lin S, Shyu K-G, Lee C-C, Wang B-W, Chang C-C, Liu Y-C, Huang F-Y, Chang H. Hyperbaric oxygen selectively induces angiopoietin-2 in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2002;296(3):710–715. doi: 10.1016/S0006-291X(02)00924-5. [DOI] [PubMed] [Google Scholar]
- Lin CD, Wei IH, Lai CH, Hsia TC, Kao MC, Tsai MH, Wu CH. Hyperbaric oxygen upregulates cochlear constitutive nitric oxide synthase. BMC Neurosci. 2011;12:21. doi: 10.1186/1471-2202-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KC, Niu KC, Tsai KJ, Kuo JR, Wang LC, Chio CC, Chang CP. Attenuating inflammation but stimulating both angiogenesis and neurogenesis using hyperbaric oxygen in rats with traumatic brain injury. J Trauma Acute Care Surg. 2012;72(3):650–659. doi: 10.1097/TA.0b013e31823c575f. [DOI] [PubMed] [Google Scholar]
- Liu W, Li J, Sun X, Liu K, Zhang JH, Xu W, Tao H. Repetitive hyperbaric oxygen exposures enhance sensitivity to convulsion by upregulation of eNOS and nNOS. Brain Res. 2008;1201:128–134. doi: 10.1016/j.brainres.2008.01.068. [DOI] [PubMed] [Google Scholar]
- Londahl M. Hyperbaric oxygen therapy as treatment of diabetic foot ulcers. Diabetes Metab Res Rev. 2012;28(Suppl 1):78–84. doi: 10.1002/dmrr.2256. [DOI] [PubMed] [Google Scholar]
- Londahl M, Landin-Olsson M, Katzman P. Hyperbaric oxygen therapy improves health-related quality of life in patients with diabetes and chronic foot ulcer. Diabet Med: J Br Diabet Assoc. 2011;28(2):186–190. doi: 10.1111/j.1464-5491.2010.03185.x. [DOI] [PubMed] [Google Scholar]
- Marrot L, Belaïdi J-P, Jones C, Perez P, Meunler J-R. Molecular responses to stress induced in normal human Caucasian melanocytes in culture by exposure to simulated solar UV. Photochem Photobiol. 2005;81(2):367–375. doi: 10.1562/2004-10-13-RA-343.1. [DOI] [PubMed] [Google Scholar]
- Martins I, Galluzzi L, Kroemer G. Hormesis, cell death and aging. Aging. 2011;3(9):821–828. doi: 10.18632/aging.100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami T, Sato Y, Sato T, Ariga S, Shimomura T, Yukawa M. Oxidative stress and gene expression of antioxidant enzymes in the streptozotocin-induced diabetic rats under hyperbaric oxygen exposure. Int J Clin Exp Pathol. 2009;3(2):177–188. [PMC free article] [PubMed] [Google Scholar]
- Matsunami T, Sato Y, Hasegawa Y, Ariga S, Kashimura H, Sato T, Yukawa M. Enhancement of reactive oxygen species and induction of apoptosis in streptozotocin-induced diabetic rats under hyperbaric oxygen exposure. Int J Clin Exp Pathol. 2011;4(3):255–266. [PMC free article] [PubMed] [Google Scholar]
- Morita A, Grewe M, Grether-Beck S, Olaizola-Horn S, Krutmann J. Induction of proinflammatory cytokines in human epidermoid carcinoma cells by in vitro ultraviolet A1 irradiation. Photochem Photobiol. 1997;65(4):630–635. doi: 10.1111/j.1751-1097.1997.tb01904.x. [DOI] [PubMed] [Google Scholar]
- Padgaonkar VA, Giblin FJ, Fowler K, Leverenz VR, Reddan JR, Dziedzic DC. Heme oxygenase synthesis is induced in cultured lens epithelium by hyperbaric oxygen or puromycin. Exp Eye Res. 1997;65(3):435–443. doi: 10.1006/exer.1997.0356. [DOI] [PubMed] [Google Scholar]
- Panteleyev AA, Paus R, Ahmad W, Sundberg JP, Christiano AM. Molecular and functional aspects of the hairless (hr) gene in laboratory rodents and humans. Exp Dermatol. 1998;7(5):249–267. doi: 10.1111/j.1600-0625.1998.tb00295.x-i1. [DOI] [PubMed] [Google Scholar]
- Phillipson RP, Tobi SE, Morris JA, McMillan TJ. UV-A induces persistent genomic instability in human keratinocytes through an oxidative stress mechanism. Free Radic Biol Med. 2002;32(5):474–480. doi: 10.1016/S0891-5849(01)00829-2. [DOI] [PubMed] [Google Scholar]
- Poljsak B, Dahmane R. Free radicals and extrinsic skin aging. Dermatol Res Pract. 2012;2012:135206. doi: 10.1155/2012/135206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustisek N, Situm M. UV-radiation, apoptosis and skin. Coll Antropologicum. 2011;35(Suppl 2):339–341. [PubMed] [Google Scholar]
- Rebane A, Zimmermann M, Aab A, Baurecht H, Koreck A, Karelson M, Abram K, Metsalu T, Pihlap M, Meyer N, Fölster-Holst R, Nagy N, Kemeny L, Kingo K, Vilo J, Illig T, Akdis M, Franke A, Novak N, Weidinger S, Akdis CA. Mechanisms of IFN-γ-induced apoptosis of human skin keratinocytes in patients with atopic dermatitis. J Allergy Clin Immunol. 2012;129(5):1297–1306. doi: 10.1016/j.jaci.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Rigatti MJ, Verma R, Belinsky GS, Rosenberg DW, Giardina C. Pharmacological inhibition of Mdm2 triggers growth arrest and promotes DNA breakage in mouse colon tumors and human colon cancer cells. Mol Carcinog. 2012;51(5):363–378. doi: 10.1002/mc.20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi B, Cuzzocrea S, Donniacuo M, Capuano A, Palma D, Imperatore F, Mazzon E, Paola R, Sodano L, Rossi F. Hyperbaric oxygen therapy reduces the Toll-like receptor signaling pathway in multiple organ failures. Intensive Care Med. 2011;37(7):1110–1119. doi: 10.1007/s00134-011-2241-1. [DOI] [PubMed] [Google Scholar]
- Rock KL. Pathobiology of inflammation to cell death. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant. 2009;15(1 Suppl):137–138. doi: 10.1016/j.bbmt.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfuss A, Speit G. Investigations on the mechanism of hyperbaric oxygen (HBO)-induced adaptive protection against oxidative stress. Mutat Res Fundam Mol Mech Mutagen. 2002;508(1–2):157–165. doi: 10.1016/S0027-5107(02)00213-0. [DOI] [PubMed] [Google Scholar]
- Rothfuss A, Radermacher P, Speit G. Involvement of heme oxygenase-1 (HO-1) in the adaptive protection of human lymphocytes after hyperbaric oxygen (HBO) treatment. Carcinogenesis. 2001;22(12):1979–1985. doi: 10.1093/carcin/22.12.1979. [DOI] [PubMed] [Google Scholar]
- Ruvolo EC, Jr, Stamatas GN, Kollias N. Skin viscoelasticity displays site- and age-dependent angular anisotropy. Ski Pharmacol Physiol. 2007;20(6):313–321. doi: 10.1159/000108147. [DOI] [PubMed] [Google Scholar]
- Saglam M, Bozlar U, Kantarci F, Ay H, Battal B, Coskun U. Effect of hyperbaric oxygen on flow-mediated vasodilation. J Ultrasound Med. 2008;27(2):209–214. doi: 10.7863/jum.2008.27.2.209. [DOI] [PubMed] [Google Scholar]
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83(12):1583–1590. doi: 10.1016/j.bcp.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Shiraishi N, Aono K, Utsumi K. Increased metallothionein content in rat liver induced by X irradiation and exposure to high oxygen tension. Radiat Res. 1983;95(2):298–302. doi: 10.2307/3576256. [DOI] [PubMed] [Google Scholar]
- Shyu KG, Wang BW, Chang H. Hyperbaric oxygen activates discoidin domain receptor 2 via tumour necrosis factor-alpha and the p38 MAPK pathway to increase vascular smooth muscle cell migration through matrix metalloproteinase 2. Clin Sci (Lond) 2009;116(7):575–583. doi: 10.1042/CS20080215. [DOI] [PubMed] [Google Scholar]
- Simsek K, Ay H, Topal T, Ozler M, Uysal B, Ucar E, Acikel CH, Yesilyurt O, Korkmaz A, Oter S, Yildiz S. Long-term exposure to repetitive hyperbaric oxygen results in cumulative oxidative stress in rat lung tissue. Inhal Toxicol. 2011;23(3):166–172. doi: 10.3109/08958378.2011.558528. [DOI] [PubMed] [Google Scholar]
- Sommerfeld B. Randomised, placebo-controlled, double-blind, split-face study on the clinical efficacy of Tricutan® on skin firmness. Phytomedicine. 2007;14(11):711–715. doi: 10.1016/j.phymed.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Sun Q, Sun Q, Liu Y, Sun X, Tao H. Anti-apoptotic effect of hyperbaric oxygen preconditioning on a rat model of myocardial infarction. J Surg Res. 2011;171(1):41–46. doi: 10.1016/j.jss.2010.01.036. [DOI] [PubMed] [Google Scholar]
- Svobodová A, Vostálová J. Solar radiation induced skin damage: review of protective and preventive options. Int J Radiat Biol. 2010;86(12):999–1030. doi: 10.3109/09553002.2010.501842. [DOI] [PubMed] [Google Scholar]
- Svobodova AR, Galandakova A, Sianska J, Dolezal D, Ulrichova J, Vostalova J. Acute exposure to solar simulated ultraviolet radiation affects oxidative stress-related biomarkers in skin, liver and blood of hairless mice. Biol Pharm Bull. 2011;34(4):471–479. doi: 10.1248/bpb.34.471. [DOI] [PubMed] [Google Scholar]
- Svobodová A, Galandáková A, Šianská J, Doležal D, Lichnovská R, Ulrichová J, Vostálová J (2012) DNA damage after acute exposure of mice skin to physiological doses of UVB and UVA light. Arch Dermatol Res 304:407–412. doi:10.1007/s00403-012-1212-x [DOI] [PubMed]
- Thackham JA, McElwain DLS, Long RJ. The use of hyperbaric oxygen therapy to treat chronic wounds: a review. Wound Repair Regen. 2008;16(3):321–330. doi: 10.1111/j.1524-475X.2008.00372.x. [DOI] [PubMed] [Google Scholar]
- Thom SR. Oxidative stress is fundamental to hyperbaric oxygen therapy. J Appl Physiol. 2009;106(3):988–995. doi: 10.1152/japplphysiol.91004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiaka EK, Papanas N, Manolakis AC, Maltezos E. The role of hyperbaric oxygen in the treatment of diabetic foot ulcers. Angiology. 2012;63(4):302–314. doi: 10.1177/0003319711416804. [DOI] [PubMed] [Google Scholar]
- Uhoda E, Piérard GE. Irritation cutanée et vitesse de propagation d'ondes ultrasonores. Int J Cosmet Sci. 2003;25(1/2):31–35. doi: 10.1046/j.1467-2494.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- Verma R, Rigatti MJ, Belinsky GS, Godman CA, Giardina C. DNA damage response to the Mdm2 inhibitor nutlin-3. Biochem Pharmacol. 2010;79(4):565–574. doi: 10.1016/j.bcp.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubeck C, Shankaran H, Karin NJ, Kauer PM, Chrisler WB, Wang X, Robinson RJ, Waters KM, Tilton SC, Sowa MB. Cell type-dependent gene transcription profile in a three-dimensional human skin tissue model exposed to low doses of ionizing radiation: implications for medical exposures. Environ Mol Mutagen. 2012;53(4):247–259. doi: 10.1002/em.21682. [DOI] [PubMed] [Google Scholar]
- Wang BW, Lin CM, Wu GJ, Shyu KG. Tumor necrosis factor-alpha enhances hyperbaric oxygen-induced visfatin expression via JNK pathway in human coronary arterial endothelial cells. J Biomed Sci. 2011;18:27. doi: 10.1186/1423-0127-18-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N-L, Fang J-Y, Chen M, Wu C-J, Huang C-C, Hung C-F. Chrysin protects epidermal keratinocytes from UVA- and UVB-induced damage. J Agric Food Chem. 2011;59(15):8391–8400. doi: 10.1021/jf200931t. [DOI] [PubMed] [Google Scholar]
- Xu X, Wang Z, Li Q, Xiao X, Lian Q, Xu W, Sun X, Tao H, Li R. Endothelial nitric oxide synthase expression is progressively increased in primary cerebral microvascular endothelial cells during hyperbaric oxygen exposure. Oxidative Med Cell Longev. 2009;2(1):7–13. doi: 10.4161/oxim.2.1.7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Liu Q, Cai L, Wang Z, Feng W. Stable overexpression of human metallothionein-IIA in a heart-derived cell line confers oxidative protection. Toxicol Lett. 2009;188(1):70–76. doi: 10.1016/j.toxlet.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Yildiz S, Aktas S, Cimsit M, Ay H, Togrol E. Seizure incidence in 80,000 patient treatments with hyperbaric oxygen. Aviat, Space, Environ Med. 2004;75(11):992–994. [PubMed] [Google Scholar]
- Zhang Y, Chong E, Herman B. Age-associated increases in the activity of multiple caspases in Fisher 344 rat organs. Exp Gerontol. 2002;37(6):777–789. doi: 10.1016/S0531-5565(02)00013-X. [DOI] [PubMed] [Google Scholar]