Abstract
We evaluated associations between the concentrations of heat shock proteins (hsp60 and hsp70) and their respective antibodies, alterations in maternal reproductive performance, and fetal malformations in pregnant rats with hyperglycemia. Mild diabetes (MD) or severe diabetes (SD) was induced in Sprague-Dawley rats prior to mating; non-treated non-diabetic rats (ND) served as controls. On day 21 of pregnancy, maternal blood was analyzed for hsp60 and hsp70 and their antibodies; and fetuses were weighed and analyzed for congenital malformations. Hsp and anti-hsp levels were correlated with blood glucose levels during gestation. There was a positive correlation between hsp60 and hsp70 levels and the total number of malformations (R = 0.5908, P = 0.0024; R = 0.4877, P = 0.0134, respectively) and the number of malformations per fetus (R = 0.6103, P = 0.0015; R = 0.4875, P = 0.0134, respectively). The anti-hsp60 IgG concentration was correlated with the number of malformations per fetus (R = 0.3887, P = 0.0451) and the anti-hsp70 IgG level correlated with the total number of malformations (R = 0.3999, P = 0.0387). Moreover, both hsp and anti-hsp antibodies showed negative correlations with fetal weight. The results suggest that there is a relationship between hsp60 and hsp70 levels and their respective antibodies and alterations in maternal reproductive performance and impaired fetal development and growth in pregnancies associated with diabetes.
Keywords: Diabetes, Pregnancy, Malformation, Heat shock protein
Introduction
Diabetes mellitus is one of the major chronic diseases and a universal health problem that affects all socioeconomic classes and populations in both developed and developing countries. It decreases the quality of life and promotes a significant burden on health systems (Toscano 2004). Diabetes mellitus comprises a group of metabolic diseases characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin action, or both (American Diabetes Association 2011). Impaired reproductive performance is a well-known result of the diabetic syndrome in many mammalian species, including humans (Chieri et al. 1969; Kirchick et al. 1978; Vomachka and Johnson 1982; Garris et al. 1986). Placental dysfunction contributes to an increased frequency of fetal complications in diabetic pregnancies (Daskalakis et al. 2008).
Heat shock proteins (hsp) play a crucial role in embryo-fetal development. They are involved in every stage of the reproductive process from formation of the male and female gametes to fertilization and post-fertilization development (Neuer et al. 2000; Anderson 1998; Dix et al. 1998; Luft and Dix 1999; Akerfelt et al. 2007; Zhong et al. 2011). In mice, the 68–70-kDa hsp appear early in development and are the primary products of the zygote (Morange et al. 1984). The 70-kDa hsp (hsp70) is present at the blastocyst stage during differentiation of the embryonic internal cellular mass (Wittig et al. 1983). The expression of hsp appears to be a vital component of the pre-implantation embryo. In mice (Neuer et al. 1998) and bovine (Matwee et al. 2001) blastocysts cultured in vitro, introduction of antibodies to the 60-kDa hsp (hsp60) and hsp70 (anti-hsp60 and anti-hsp70, respectively) significantly inhibited further embryo development.
There is increasing evidence that hsp70 is intimately involved with the development of hyperglycemia and diabetes mellitus. Elevated glucose levels are highly associated with reduced systemic hsp70 expression in both humans (Chung et al. 2008) and non-human primates (Kavanagh et al. 2009). A possible mechanism is suggested by the observation that the ingestion of glucose resulted in a decreased ability to induce hsp70 (Febbraio et al. 2004).
In the present study, we evaluated whether an association exists between the concentrations of hsp60 and hsp70 and their respective antibodies and alterations in maternal reproductive performance and fetal malformations in association with different models of diabetes (levels of hyperglycemia).
Material and methods
Induction of diabetes
All experimental procedures were approved by the Ethics Committee on Animal Experiments of the Botucatu Medical School—UNESP (Protocol number 787). Sprague-Dawley female adult rats, maintained under controlled conditions (temperature 22 ± 2 °C, humidity 55 ± 5 %, and 12 h light/dark cycle), were randomly assigned to three experimental groups: non-diabetic (ND, n = 5), mild diabetic (MD, n = 12), and severe diabetic (SD, n = 8). For induction of the MD group, newborn female rats received streptozotocin (STZ—Sigma, St. Louis, MO, USA), a beta (β)-cytotoxic agent, diluted in citrate buffer (0.1 M; pH 4.5) at a dose of 100 mg/kg on the first day of life by subcutaneous administration (Sinzato et al. 2011). For induction of the SD group, rats received streptozotocin diluted in citrate buffer, intravenously at a dose of 40 mg/kg on day 90 of age (Damasceno et al. 2011). ND rats received only the vehicle (citrate buffer).
Mating
At adult age (day 100), female rats were mated with non-diabetic males. The morning when spermatozoa were present in the vaginal smear was designated day 0 of pregnancy. The mating procedure consisted of 15 consecutive days, which comprised approximately three estral cycles. Non-mated female rats in this period were considered infertile and excluded from the study.
Pregnancy
On days 0, 7, 14, and 20 of pregnancy, in the late evening, blood glucose was measured and the mean blood glucose level calculated. On days 0 and 21 of pregnancy, rats were weighed to calculate the maternal weight gain (MWG) during pregnancy. After weighing on day 21 of pregnancy, rats were anesthetized with sodium thiopental (Thiopentax®, 50 mg/kg) and exsanguinated for collection of maternal blood for determination of heat shock proteins and antibodies. Laparotomy was then performed to remove the uterine horns for weighing of the litter. The ovaries were also removed and the corpora lutea counted and analyzed under a stereomicroscope. The numbers of implantations and live fetuses were counted to calculate the ratio of fetal viability (total number of live fetuses/total number of implantations).
Determination of hsp60 and hsp70 and respective antibodies
Serum hsp and antibodies were quantified in undiluted serum by commercial ELISA kits (hsp60—IB09693 IBL-America, anti-hsp60—IB09638 IBL-America, hsp70—EKS-715 Assay Designs and Stressgen, and anti-hsp70—EKS-750 Assay Designs and Stressgen).
Placental and fetal weights
The placentas and term fetuses were removed and individually weighed. Each fetus was classified by the mean ± standard deviation (SD) according to the mean values of fetal weights of the non-diabetic group (ND): as small for pregnancy age (SPA) when weight was smaller than ND mean − 1.7× SD, appropriate for pregnancy age (APA) when weight was included in ND mean ± 1.7× SD, and large for pregnancy age (LPA) when weight was greater than ND mean + 1.7× SD (Soulimane-Mokhtari et al. 2005).
Analysis of visceral and skeletal malformations (MF)
Half of the fetuses were fixed in Bodian’s solution and serial sections prepared as described by Wilson (1965) for visceral examination. The remaining fetuses were prepared for skeletal examination by the staining procedure of Staples and Schnell (1964). In addition to the skeletal analyses, counting of the ossification sites was performed according to the methodology of Aliverti et al. (1979), which determines the degree of fetal development.
Statistical analysis
The normality of continuous variables was tested using the Kolmogorov and Smirnov’s test. For variables normally distributed, data was analyzed using the one-way analysis of variance (ANOVA) followed by the Tukey’s multiple comparisons test. If the variables were not normally distributed, non-parametric statistical methods were used. To compare continuous non-parametric variables between multiple groups, the Kruskal–Wallis analysis of variance (ANOVA) by ranks test was performed. Fisher’s exact test was used to compare proportions, and the Spearman correlation coefficient to assess the relationship between variables. A P value < 0.05 was considered significant.
Results
The blood glucose levels during pregnancy are shown in Table 1. At days 0, 7, and 20, maternal blood glucose levels in MD rats were higher than levels of ND rats. During all stages of pregnancy, SD rats presented with higher blood glucose levels compared to both ND and MD rats.
Table 1.
Blood glucose levels of dams throughout the pregnancy
| ND | MD | SD | |
|---|---|---|---|
| Glucose mean (mg/dL) | 89.33 ± 11.57 | 107.79 ± 23.95* | 469.92 ± 68.03*,** |
| Day 0 (mg/dL) | 95.00 ± 7.97 | 112.54 ± 16.46* | 484.13 ± 82.99*,** |
| Day 7 (mg/dL) | 100.67 ± 5.50 | 130.77 ± 11.89* | 470.14 ± 39.82*,** |
| Day 14 (mg/dL) | 83.67 ± 9.52 | 94.23 ± 25.60 | 471.80 ± 35.69*,** |
| Day 20 (mg/dL) | 78.00 ± 6.81 | 93.62 ± 18.89* | 445.00 ± 104.39*,** |
Blood glucose levels were measured on days 0, 7, 14, and 21 of pregnancy from non-diabetic (ND), mild diabetic (MD), and severe diabetic (SD) rats. Data are presented as mean ± standard deviation
*P < 0.05, compared to ND; **P < 0.05—compared to MD
Data related to maternal reproductive performance are shown in Table 2. Rats from the MD group had reduced maternal weight gain (119 g) and litter weight (84 g) compared with ND rats (156 g and 107 g; P < 0.01 and P < 0.05, respectively). Rats from the SD group had reduced maternal weight gain (84 g) and litter weight (63 g) during pregnancy compared to both the ND (P < 0.001) and MD (P < 0.05) groups. Moreover, both MD and SD rats had a reduced number of implantations (11) and live fetuses (10) compared to the ND group (13; P < 0.05 and P < 0.01, respectively). The number of corpora lutea did not show differences among experimental groups. Both MD and SD groups had a reduced ratio of fetal viability (86 % and 87 %, respectively) compared to ND rats (97 %; P < 0.0001 and P < 0.001, respectively).
Table 2.
Reproductive performance and fetal and placental weights of non-diabetic (ND), mild diabetic (MD), and severe diabetic (SD) rats at the end of pregnancy
| ND | MD | SD | |
|---|---|---|---|
| Number of corpora lutea | 14.36 ± 2.44 | 14.00 ± 3.41 | 12.25 ± 2.49 |
| Number of implantation | 13.26 ± 2.82 | 11.44 ± 2.99* | 10.88 ± 2.36* |
| Number of live fetus | 12.82 ± 2.91 | 9.78 ± 3.40* | 9.50 ± 2.78* |
| Fetal viability ratio (%) | 96.67 | 85.50* | 87.36* |
| Maternal weight gain (g) | 155.80 ± 18.91 | 118.58 ± 23.83* | 83.50 ± 11.67*,** |
| Litter weight (g) | 107.08 ± 19.51 | 83.75 ± 16.85* | 62.72 ± 15.50*,** |
| Fetal weight (g) | 5.41 ± 0.43 | 5.17 ± 0.82* | 4.34 ± 0.55*,** |
| Placental weight (g) | 0.57 ± 0.13 | 0.51 ± 0.10* | 0.65 ± 0.10*,** |
Data are presented as mean ± standard deviation, except fetal viability ratio, which is presented in percentage
*P < 0.05, compared to ND; **P < 0.05, compared to MD
We obtained 77 fetuses and placentas from ND rats, 151 from MD rats, and 76 from SD rats (Table 2). Fetuses born from MD rats had reduced weight compared to fetuses from ND rats (5.2 g vs. 5.4 g, respectively; P < 0.05). Similarly, fetuses from SD rats had reduced weight (4.3 g) compared to both the ND and MD groups (P < 0.001). The placentas of MD rats had reduced weight compared to ND rats (0.5 g vs. 0.6 g; P < 0.01); and the placental weight of SD rats was higher (0.7 g) compared to both the ND and MD groups (P < 0.01).
Rats in the ND group had predominantly fetuses with appropriate weights for gestational age (APA 92.7 %). The remaining fetuses were evenly distributed between small for pregnancy age (SPA 3.9 %) and large for pregnancy age (LPA 3.4 %). In the MD group, 70.8 % of fetuses was classified as APA, 19.0 % as SPA, and 10.2 % as LPA. SD rats had only 24.8 % of fetuses with appropriate weight, 74.6 % of fetuses was classified as small and 0.6 % large for pregnancy age. There was a significant increase in SPA fetuses and reduction of APA fetuses in the MD and SD groups compared to the ND group (P < 0.0001). The proportion of LGA fetuses in the MD group was significantly higher compared to the ND and SD groups (P < 0.0001); comparing the last two groups, the SD group presented with a lower incidence (P = 0.011) of large fetuses compared to ND fetuses (Fig. 1).
Fig. 1.
Fetal weight classification. Fetal weight classification is defined as small for pregnancy age (SPA), appropriate for pregnancy age (APA) or large for pregnancy age (LPA)
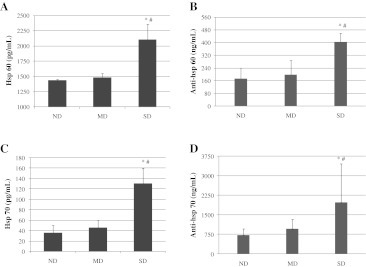
There was a significant increase in hsp60 concentration in SD rats (2,109 pg/mL) comparing to both the ND (1,437 pg/mL) and MD (1,486 pg/mL) groups (P < 0.0001 and P < 0.001, respectively). No significant difference in hsp60 protein levels was found between MD and ND rats (Fig. 2A). Similarly, the concentration of anti-hsp60 in the SD group (404 ng/mL) was significantly higher as opposed to the ND (172 ng/mL) and MD (198 ng/mL) groups (P < 0.001). No significant difference in anti-hsp60 levels was found between MD and ND rats (Fig. 2B).
Fig. 2.
Concentration of hsp60, hsp70, anti-hsp60, and anti-hsp70 in serum from non-diabetic, mild diabetic, and severe diabetic rats. Sera from rats without diabetes (ND), mild diabetes (MD), and severe diabetes (SD) were tested for hsp60 (a), anti-hsp60 (b), hsp70 (c), and anti-hsp70 (d) by ELISA. Data are presented as mean ± standard deviation. *P < 0.05, compared to ND; #P < 0.05, compared to MD
There was a significant increase in concentration of hsp70 in SD rats (1,301 pg/mL) comparing to both the ND (45.8 pg/mL) and MD (35.8 pg/mL) groups (P < 0.001). No significant difference in hsp70 protein levels was found between MD and ND rats (Fig. 2C). Similarly, the concentration of anti-hsp70 in the SD group (1,974 ng/mL) was significantly higher as opposed to the ND (718 ng/mL) and MD (961 ng/mL) groups (P < 0.05). No significant difference in anti-hsp70 levels was found between MD and ND rats (Fig. 2D).
The incidence of fetal visceral and skeletal malformations is shown in Table 3. Fetuses from MD rats presented only one type of malformation at a higher incidence compared to fetuses from ND rats: an enlarged trachea (14.3 % vs. 0.0 %, respectively; P = 0.0286). As expected, several malformations were found in the SD group and some of these showed a significant increase in comparison with the ND and/or MD groups: microphthalmia (P = 0.0370), altered crystalline (P < 0.0001), dilated renal pelvis (P = 0.0121 and P = 0.0068, respectively), enlarged ureter (P = 0.0054 and P < 0.0001, respectively), sinuous ureter (P = 0.0497), and enlarged trachea (P = 0.0055). There were no significant differences between the skeletal malformations found in fetuses from ND and MD rats (P > 0.05). However, fetuses born from SD rats presented with a higher incidence of almost all skeletal malformations in comparison with both ND and MD rats: abnormally shaped sternebrae (P = 0.0006 and P < 0.0001, respectively), incomplete ossification of sternebrae (P = 0.0024 and P = 0.0039, respectively), supranumerary ribs, bipartite ossification of vertebral centrum, and abnormal ossification of vertebral centrum (P < 0.0001).
Table 3.
Incidence of visceral and skeletal malformations in fetuses from non-diabetic (ND), mild diabetic (MD) and severe diabetic (SD) rats
| MF (%) | ND | MD | SD |
|---|---|---|---|
| Number examined fetuses (visceral) | 32 | 70 | 36 |
| Total number of malformations | 17 | 45 | 78 |
| Enlarged lateral ventricle | 0 (0.00) | 1 (1.43) | 0 (0.00) |
| Microphthalmia | 0 (0.00) | 0 (0.00) | 3 (8.33)# |
| Altered crystalline | 0 (0.00) | 0 (0.00) | 23 (63.89)*# |
| Dilated renal pelvis | 0 (0.00) | 2 (2.86) | 7 (19.44)*# |
| Ectopic kidney | 1 (3.13) | 3 (4.29) | 1 (2.78) |
| Enlarged ureter | 6 (18.75) | 9 (12.86) | 19 (52.78)*# |
| Sinuous ureter | 5 (15.63) | 7 (10.00) | 9 (25.00)# |
| Ectopic testis | 0 (0.00) | 2 (2.86) | 0 (0.00) |
| Enlarged nasal cavity | 0 (0.00) | 4 (5.71) | 3 (8.33) |
| Altered diaphragm | 0 (0.00) | 1 (1.43) | 0 (0.00) |
| Enlarged trachea | 0 (0.00) | 10 (14.29)* | 8 (22.22)* |
| Enlarged esophagus | 0 (0.00) | 3 (4.29) | 2 (5.56) |
| Enlarged bronchus | 0 (0.00) | 3 (4.29) | 3 (8.33) |
| Number examined fetuses (skeletal) | 38 | 77 | 40 |
| Total number of malformations | 11 | 39 | 103 |
| Abnormally shaped sternebrae | 5 (13.16) | 6 (7.79) | 20 (50.00)*,** |
| Incomplete ossif. sternebrae | 0 (0.00) | 3 (3.90) | 9 (22.50)*,** |
| Absent sternebrae | 0 (0.00) | 3 (3.90) | 2 (5.00) |
| Supranumerary rib | 5 (13.16) | 13 (16.88) | 35 (87.50)*,** |
| Bipartite ossif. vert. centrum | 0 (0.00) | 4 (5.20) | 14 (35.00)*,** |
| Abnormal ossif. vert. centrum | 1 (2.63) | 10 (12.99) | 23 (57.50)*,** |
Data are presented as count (percentage)
ossify ossification, vert vertebrae
*P < 0.05, compared to ND; **P < 0.05, compared to MD
The fetuses from SD rats showed a significantly reduced number of anterior (2.8) and posterior (0.3) phalanges, metatarsus (4.2), caudal vertebrae (4.0) and total ossification sites (21.2; P < 0.001) compared with fetuses from ND and MD rats. In addition, fetuses from MD rats had reduced ossification in posterior phalanges (2.0), caudal vertebrae (4.6), and total ossification sites (24.9; P < 0.01) compared with fetuses from ND rats (Table 4).
Table 4.
Ossification sites of fetuses from non-diabetic (ND), mild diabetic (MD), and severe diabetic (SD) rats
| ND | MD | SD | |
|---|---|---|---|
| Anterior phalanges | 3.96 ± 0.10 | 3.71 ± 0.42 | 2.77 ± 0.57*,** |
| Metacarpus | 4.00 ± 0.00 | 4.00 ± 0.00 | 3.93 ± 0.10 |
| Posterior phalanges | 3.38 ± 0.63 | 1.97 ± 0.96* | 0.31 ± 0.59*,** |
| Metatarsus | 4.93 ± 0.12 | 4.66 ± 0.37 | 4.19 ± 0.20*,** |
| Caudal vertebrae | 5.48 ± 0.33 | 4.62 ± 0.50* | 4.04 ± 0.24*,** |
| Sternebrae | 6.00 ± 0.00 | 5.94 ± 0.11 | 5.94 ± 0.11 |
| Total | 27.75 ± 0.97 | 24.90 ± 1.94* | 21.18 ± 1.53*,** |
Data are presented as mean ± standard deviation
*P < 0.05, compared to ND; **P < 0.05, compared to MD
The relationship between heat shock proteins (hsp60 and hsp70) and their respective antibodies and maternal reproductive performance and fetal malformations is summarized in Tables 5 and 6, respectively. The hsp60 concentration was positively correlated with the level of anti-hsp60 IgG (R = 0.7493, P < 0.0001). The same occurred between the levels of hsp70 and anti-hsp70 IgG (R = 0.4789, P = 0.0154). We observed a positive correlation between both hsp and anti-hsp and blood glucose levels at all measured stages of pregnancy, except for the lack of an association between anti-hsp70 and blood glucose on day 20 of pregnancy (R = 0.3245, P = 0.0987). There was a positive correlation between hsp60 and hsp70 and the total number of malformations (R = 0.5908, P = 0.0024; R = 0.4877, P = 0.0134, respectively) and the number of malformations per fetus (R = 0.6103, P = 0.0015; R = 0.4875, P = 0.0134, respectively). Antibodies to hsp were also positively correlated with malformations: anti-hsp60 and the number of malformations per fetus (R = 0.3887, P = 0.0451), and anti-hsp70 and the total number of malformations (R = 0.3999, P = 0.0387). Moreover, both hsp and anti-hsp showed very significant negative correlations with fetal weight.
Table 5.
Relationship between heat shock proteins and maternal data and fetal malformations
| Hsp60 | Hsp70 | |||
|---|---|---|---|---|
| R | P | R | P | |
| Anti-hsp60 | 0.7493 | <0.0001 | – | – |
| Anti-hsp70 | – | – | 0.4789 | 0.0154 |
| Glucose day 0 | 0.7682 | <0.0001 | 0.6991 | 0.0001 |
| Glucose day 7 | 0.7274 | <0.0001 | 0.7226 | <0.0001 |
| Glucose day 14 | 0.5919 | 0.0047 | 0.6425 | 0.0013 |
| Glucose day 20 | 0.7077 | 0.0003 | 0.6256 | 0.0018 |
| Glucose mean | 0.8509 | <0.0001 | 0.7363 | <0.0001 |
| Total MF | 0.5908 | 0.0024 | 0.4877 | 0.0134 |
| MF/fetus | 0.6103 | 0.0015 | 0.4875 | 0.0134 |
| Fetal weight | −0.6859 | 0.0002 | −0.6216 | 0.0009 |
MF malformation
Table 6.
Relationship between antibodies to heat shock proteins and maternal data and fetal malformations
| Anti-hsp60 | Anti-hsp70 | |||
|---|---|---|---|---|
| R | P | R | P | |
| Hsp60 | 0.7493 | <0.0001 | – | – |
| Hsp70 | – | – | 0.4789 | 0.0154 |
| Glucose day 0 | 0.6694 | 0.0001 | 0.5337 | 0.0041 |
| Glucose day 7 | 0.6277 | 0.0006 | 0.4904 | 0.0094 |
| Glucose day 14 | 0.6305 | 0.0010 | 0.4089 | 0.0342 |
| Glucose day 20 | 0.5345 | 0.0071 | 0.3245 | 0.0987 |
| Glucose mean | 0.6958 | <0.0001 | 0.5116 | 0.0064 |
| Total MF | 0.3465 | 0.0766 | 0.3999 | 0.0387 |
| MF/fetus | 0.3887 | 0.0451 | 0.3142 | 0.1104 |
| Fetal weight | −0.6925 | <0.0001 | −0.4525 | 0.0178 |
MF malformation
Discussion
To reproduce the hyperglycemia of human uncontrolled type-1 diabetes, experimental models have been developed to mimic the severe diabetic state (Eriksson et al. 2000, 2003; Rudge et al. 2007; Volpato et al. 2008; de Souza et al. 2009; Damasceno et al. 2011). Additional models reproduce the level of hyperglycemia typical of type-2 and gestational diabetes mellitus. In laboratory animals, these latter types of diabetes are classified as mild diabetes (Portha et al. 1974; Tsuji et al. 1988; Oh et al. 1991; Caluwaerts et al. 2003; Soulimane-Mokhtari et al. 2005; Sinzato et al. 2009). In our study, as expected, SD rats had the highest, and MD rats had intermediate circulating blood glucose levels throughout pregnancy compared to non-diabetic rats.
In the present study, the SD rats tended to have a reduced number of corpora lutea, evidence of a reduction in the number of oocytes liberated during the ovulation process. Likewise, these rats had a reduction in the number of implanted embryos and live fetuses as consequences of their hyperglycemic intrauterine environment. Diabetic rats, both MD and SD, had a reduced maternal weight gain during pregnancy due to metabolic changes caused by hyperglycemia. Moreover, the same rats showed reduced weights of their litters. The maternal weight gain and litter weight were reduced as blood glucose levels increased. In humans, inadequate maternal weight gain has been linked to an increased risk of delivery of a small-for-gestational-age infant (Cnattingius et al. 1998).
Regulation of fetal growth varies with the stage of gestation and is characterized by a major role for nutrient availability to the fetus and by the fact that the fetus and the placenta form a functional unit (Alsat et al. 1995). Placental structure and function change as a result of maternal diabetes. In the present study, both MD and SD rats had altered placental and fetal weights. In the MD group, the placental weight was reduced and this can be associated to alterations in diabetes-derived placental cyto-architecture (Sinzato et al. 2011). The placental weight in the SD group was increased, interpreted as a compensatory mechanism to maximize maternal–fetal exchanges and nutrients supply to the developing fetus. Despite this alteration, hyperglycemia of the maternal environment can lead to pancreatic functional exhaustion in fetuses that contribute to impaired growth and development (Calderon et al. 1992). The fetal weight classification showed increased rates of SPA fetuses, reduced rates of APA fetuses, and decreased number of ossification sites of fetuses in both the MD and SD groups, providing evidence of delayed somatic development. The nature and extent of these changes depend on the type of diabetes and on the gestational period (Vambergue and Fajardy 2011). In humans, during the first trimester of pregnancy, embryonic growth might be controlled by nutrient supply and by locally active growth factors. Later, fetal growth depends essentially upon maternal–placental cooperation in delivering nutrients to the fetus. Fetal growth seems to be regulated by fetal insulin and insulin-like growth factors (IGF-1 and IGF-2), with growth hormone (GH) playing only a secondary role (Alsat et al. 1995).
Our findings of an increase in circulating hsp concentrations in SD rats with high levels of hyperglycemia corroborate previous studies. There are two reports of elevated serum hsp70 levels in type-1 diabetic patients (Oglesbee et al. 2005; Gruden et al. 2009). In another study, Hunter-Lavin et al. (2004) showed that serum hsp70 levels were higher in non-insulin treated type-2 diabetes subjects in comparison to insulin-treated subjects. They concluded that hsp70 may therefore be a suitable marker of the severity of the clinical condition and may be useful in the monitoring of type-2 diabetes as well as other diseases associated with oxidative stress. Yabunaka et al. (1995) showed that levels of hsp70 in mononuclear lymphoid cells were significantly higher in diabetic patients compared with normal controls. Similarly, hsp60 and hsp70 were found to be induced in lymphocytes of patients suffering from type-2 diabetic nephropathy compared to controls (Calabrese et al. 2007). In contrast, other studies have reported reduced systemic hsp70 expression in association with elevated glucose levels in both humans (Bruce et al. 2003; Chung et al. 2008) and non-human primates (Kavanagh et al. 2009). This apparent discrepancy remains to be resolved.
Increased concentrations of hsp70 in mononuclear cells of peripheral blood obtained from women in early pregnancy were associated with subsequent miscarriages, stillbirths, and preterm births (Tan et al. 2007). Child et al. (2006) reported that serum anti-hsp70 antibody levels are significantly elevated at 16 weeks of gestation in women who later gave birth to babies with birth defects (cleft lip or palate or neural tube defects). A recent study identified altered production of heat shock proteins in development of chemical-induced cleft palate formation in mice (Zhu et al. 2012). As evidenced in our results, hsp70 levels and its antibody were higher in severe diabetic rats, which also presented with high rates of embryonic death and congenital malformations. Further studies are required to clarify the association between circulating hsp70 and intrauterine growth restriction.
The higher incidence of fetal malformations, found in fetuses from SD rats in our study, is also consistent with previous investigations. As in human diabetic pregnancies, malformations in streptozotocin-induced experimental models of diabetes occur mainly in the neural system, heart, and skeleton (Eriksson 2009; Schaefer-Graf et al. 2000; Simán et al. 2000). Both increased oxidative stress and nitrosative stress are crucial features in diabetes-induced embryopathy and have been characterized in chemical-induced and genetic models of diabetes and even in mild diabetic experimental models (Eriksson et al. 2003; Jawerbaum and González 2006; Ornoy 2007). Impairment of the oxidative and nitrosative stress balance can deregulate multiple signaling pathways and cause massive cell damage, apoptotic events, and defective embryonic and fetal development (Sivan et al. 1997; Reece et al. 2005; Morgan et al. 2008; Sugimura et al. 2009). In addition, the malformation rate is clearly correlated with increased glucose concentrations (Jawerbaum and White 2010). Experimental results support the notion of hyperglycemia as a teratogen, since high glucose levels (Dienelt and Zur Nieden 2011) or maternal diabetes in vivo as well as exposure to high glucose concentration cause embryonic maldevelopment. Several studies have shown that fetuses from mild diabetic rats have a compromised intrauterine development (Saito et al. 2010; Iessi et al. 2010) and elevated oxidative stress, contributing to an increased incidence of skeletal and visceral malformations at birth (Damasceno et al. 2011).
Concentrations of hsp/anti-hsp in the present study were elevated in association with abnormal fetal weight and the occurrence of malformation. Belhia et al. (2010) showed that unexplained small-for-gestational-age fetuses were positive for IgM and IgG antibody to human hsp60 and that the specific IgM antibody level was predictive of fetal mortality. The authors concluded that detection of these antibodies indicated a placental perturbation and that a fetal autoimmune reaction to hsp60 was associated with this developmental delay.
In summary, our results suggest that there is a relationship between levels of hsp60 and hsp70 and their respective antibodies and alterations in maternal reproductive performance and impaired fetal growth and development, evidenced by intrauterine growth restriction and fetal malformations in the context of different models of diabetes (levels of hyperglycemia). Further studies are required to determine the mechanisms that result in the increased levels of hsp and antibodies, and whether hsp have a direct role in the pathogenesis of congenital malformations and/or serve as biomarkers of oxidative stress and/or altered reproductive outcome. As suggested recently (Henderson and Pockley 2012), to gain a more complete understanding of the role(s) of extracellular heat shock proteins in pathology, it may be necessary to identify and view all elements of the stress response, as well as the occurrence of antibodies to these proteins, as an interacting network rather than focusing on individual components of this system.
Acknowledgments
We are grateful to Dr. Maria Terezinha Serrão Peraçoli for assistance and helpful discussions. We would also like to thank to Talísia Moreto, technician in the Laboratory of Experimental Research on Gynecology and Obstetrics. The financial support of CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) is gratefully acknowledged.
References
- Akerfelt M, Trouillet D, Mezger V, Sistonen L. Heat shock factors at a crossroad between stress and development. Ann N Y Acad Sci. 2007;1113:15–27. doi: 10.1196/annals.1391.005. [DOI] [PubMed] [Google Scholar]
- Aliverti V, Bonanomi L, Giavini E. The extent of fetal ossification as an index of delayed development in teratogenic studies on the rat. Teratology. 1979;20:237–242. doi: 10.1002/tera.1420200208. [DOI] [PubMed] [Google Scholar]
- Alsat E, Marcotty C, Gabriel R, Igout A, Frankenne F, Hennen G, Evain-Brion D. Molecular approach to intrauterine growth retardation: an overview of recent data. Reprod Fertil Dev. 1995;7:1457–1464. doi: 10.1071/RD9951457. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34:S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RL. Stress proteins and apoptosis in prenatal development, cancer and medicine. Cell Stress Chaperones. 1998;3:209–212. doi: 10.1379/1466-1268(1998)003<0209:SPAAIP>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhia F, Gremlich S, Muller-Brochut AC, Damnon F, Hohlfeld P, Witkin SS, Gerber S. Anti-60-kDa heat shock protein antibodies in fetal serum: a biomarker for unexplained small for gestational age fetuses. Gynecol Obstet Investig. 2010;70:299–305. doi: 10.1159/000314021. [DOI] [PubMed] [Google Scholar]
- Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes. 2003;52:2338–2345. doi: 10.2337/diabetes.52.9.2338. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Mancuso C, Sapienza M, Puleo E, Calafato S, Cornelius C, Finocchiaro M, Mangiameli A, Mauro M, Stella AM, Castellino P. Oxidative stress and cellular stress response in diabetic nephropathy. Cell Stress Chaperones. 2007;12:299–306. doi: 10.1379/CSC-270.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon IMP, Rudge MVC, Brasil MAM, Henry MACA. Diabete e gravidez experimental em ratas I. Indução do diabete, obtenção e evolução da prenhez. Acta Cirúrgica Brasileira. 1992;7:142–146. [Google Scholar]
- Caluwaerts S, Holemans K, Bree R, Verhaeghe J, Assche A. Is low-dose Streptozotocin in rats adequate model for gestational Diabetes mellitus? J Soc Gynecol Investig. 2003;10:216–221. doi: 10.1016/S1071-5576(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Chieri RA, Pivetta OH, Folgia VG. Altered ovulation pattern in experimental diabetes. Fertil Steril. 1969;20:661–666. doi: 10.1016/s0015-0282(16)37094-7. [DOI] [PubMed] [Google Scholar]
- Child DF, Hudson PR, Hunter-Lavin C, Mukhergee S, China S, Williams CP, Williams JH. Birth defects and anti-heat shock protein 70 antibodies in early pregnancy. Cell Stress Chaperones. 2006;11:101–105. doi: 10.1379/CSC-130R1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MD, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2008;105:1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnattingius S, Bergström R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338:147–152. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- Damasceno DC, Sinzato YK, Lima PH, Souza MS, Campos KE, Dallaqua B, Calderon IM, Rudge MV, Volpato GT. Effects of exposure to cigarette smoke prior to pregnancy in diabetic rats. Diabetol Metab Syndr. 2011;3:20. doi: 10.1186/1758-5996-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis G, Marinopoulos S, Krielesi V, Papapanagiotou A, Papantoniou N, Mesogitis S, Antsaklis A. Placental pathology in women with gestational diabetes. Acta Obstet Gynecol Scand. 2008;87(4):403–407. doi: 10.1080/00016340801908783. [DOI] [PubMed] [Google Scholar]
- Souza MSS, Lima PHO, Sinzato YK, Rudge MVC, Pereira OCM, Damasceno DC. Effects of cigarette smoke exposure on pregnancy outcome of diabetic rats and on their offspring. Reprod BioMed Online. 2009;18:562–567. doi: 10.1016/S1472-6483(10)60135-6. [DOI] [PubMed] [Google Scholar]
- Dienelt A, Zur Nieden NI. Hyperglycemia impairs skeletogenesis from embryonic stem cells by affecting osteoblast and osteoclast differentiation. Stem Cells Dev. 2011;20:465–474. doi: 10.1089/scd.2010.0205. [DOI] [PubMed] [Google Scholar]
- Dix DJ, Garges JB, Hong RL. Inhibition of HSP70-1 and HSP70-3 expression disrupts preimplantation embryogenesis and heightens embryo sensitivity to arsenic. Mol Reprod Dev. 1998;51:373–380. doi: 10.1002/(SICI)1098-2795(199812)51:4<373::AID-MRD3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Eriksson UJ. Congenital anomalies in diabetic pregnancy. Semin Fetal Neonatal Med. 2009;14:85–93. doi: 10.1016/j.siny.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Eriksson UJ, Borg LA, Cederberg J, Nordstrand H, Simán CM, Wentzel C, Wentzel P. Pathogenesis of diabetes-induced congenital malformations. Ups J Med Sci. 2000;105:53–84. doi: 10.1517/03009734000000055. [DOI] [PubMed] [Google Scholar]
- Eriksson UJ, Cederberg J, Wentzel P. Congenital malformations in offspring of diabetic mothers—animal and human studies. Rev Endocr Metab Disord. 2003;4:79–93. doi: 10.1023/A:1021879504372. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Mesa JL, Chung J, Steensberg A, Keller C, Nielsen HB, Krustrup P, Ott P, Secher NH, Pedersen BK. Glucose ingestion attenuates the exercise induced increase in circulating heat shock protein 72 and heat shock protein 60 in human cells. Cell Stress Chaperones. 2004;9:390–396. doi: 10.1379/CSC-24R1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris DR, West RL, Pekala PH. Ultrastructural and metabolic changes associated with reproductive tract atrophy and adiposity in diabetic female mice. Anat Rec. 1986;216:359–366. doi: 10.1002/ar.1092160304. [DOI] [PubMed] [Google Scholar]
- Gruden G, Bruno G, Chaturvedi N, Burt D, Pinach S, Schalkwijk C, Stehouwer CD, Witte DR, Fuller JH, Cavallo-Perin P. ANTI-HSP60 and ANTI-HSP70 antibody levels and micro/macrovascular complications in type 1 diabetes: the EURODIAB Study. J Intern Med. 2009;266:527–536. doi: 10.1111/j.1365-2796.2009.02129.x. [DOI] [PubMed] [Google Scholar]
- Henderson B, Pockley AG. Proteotoxic stress and circulating cell stress proteins in the cardiovascular diseases. Cell Stress Chaperones. 2012;17:303–311. doi: 10.1007/s12192-011-0318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter-Lavin C, Hudson PR, Mukherjee S, Davies GK, Williams CP, Harvey JN, Child DF, Williams JH. Folate supplementation reduces serum HSP70 levels in patients with type 2 diabetes. Cell Stress Chaperones. 2004;9:344–349. doi: 10.1379/CSC-28R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iessi IL, Bueno A, Sinzato YK, Taylor KN, Rudge MV, Damasceno DC. Evaluation of neonatally-induced mild diabetes in rats: maternal and fetal repercussions. Diabetol Metab Syndr. 2010;8:37. doi: 10.1186/1758-5996-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawerbaum A, González E. Diabetic pregnancies: the challenge of developing in a pro-inflammatory environment. Curr Med Chem. 2006;13:2127–2138. doi: 10.2174/092986706777935302. [DOI] [PubMed] [Google Scholar]
- Jawerbaum A, White V. Animal models in diabetes and pregnancy. Endocr Rev. 2010;31:680–701. doi: 10.1210/er.2009-0038. [DOI] [PubMed] [Google Scholar]
- Kavanagh K, Zhang L, Wagner JD. Tissue-specific regulation and expression of heat shock proteins in type 2 diabetic monkeys. Cell Stress Chaperones. 2009;14:291–300. doi: 10.1007/s12192-008-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchick HJ, Keyes PL, Frye BE. Etiology of anovulation in the immature alloxan-diabetic rat treated with PMSG: absence of the preovulatory luteinizing hormone surge. Endocrinology. 1978;109:316–318. doi: 10.1210/endo-102-6-1867. [DOI] [PubMed] [Google Scholar]
- Luft JC, Dix DJ. HSP70 expression and function during embryogenesis. Cell Stress Chaperones. 1999;4:162–170. doi: 10.1379/1466-1268(1999)004<0162:HEAFDE>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matwee C, Kamaruddin M, Betts DH, Basrur PK, King WA. The effects of antibodies to heat shock protein 70 in fertilization and embryo development. Mol Hum Reprod. 2001;7:829–837. doi: 10.1093/molehr/7.9.829. [DOI] [PubMed] [Google Scholar]
- Morange M, Diu A, Bensaude O, Babinet C. Altered expression of heat shock proteins in mouse embryonal carcinoma cells and mouse early embryonic cells. Mol Cell Biol. 1984;4:730–735. doi: 10.1128/mcb.4.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan SC, Relaix F, Sandell LL, Loeken MR. Oxidative stress during diabetic pregnancy disrupts cardiac neural crest migration and causes outflow tract defects. Birth Defects Res A Clin Mol Teratol. 2008;82:453–463. doi: 10.1002/bdra.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuer A, Mele C, Rosenwaks Z, Witkin SS. Monoclonal antibodies to mammalian heat shock proteins impair mouse embryo development in vitro. Hum Reprod. 1998;14:987–990. doi: 10.1093/humrep/13.4.987. [DOI] [PubMed] [Google Scholar]
- Neuer A, Spandorfer SD, Giraldo P, Dieterle S, Rosenwalks Z, Witkin SS. The role of heat shock proteins in reproduction. Hum Reprod Updat. 2000;6:149–159. doi: 10.1093/humupd/6.2.149. [DOI] [PubMed] [Google Scholar]
- Oglesbee MJ, Herdman AV, Passmore GG, Hoffman WH. Diabetic ketoacidosis increases extracellular levels of the major inducible 70-kDa heat shock protein. J Biomed Biotechnol. 2005;38:900–904. doi: 10.1016/j.clinbiochem.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Oh W, Gelardi NL, Cha CJM. The cross-generation effect of neonatal macrosomia in rat pups of streptozotocin-induced diabetes. Pediatr Res. 1991;29:606–610. doi: 10.1203/00006450-199106010-00016. [DOI] [PubMed] [Google Scholar]
- Ornoy A. Embryonic oxidative stress as a mechanism of teratogenesis with special emphasis on diabetic embryopathy. Reprod Toxicol. 2007;24:31–41. doi: 10.1016/j.reprotox.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Portha B, Levacher C, Picon L, Rosselin G. Diabetogenic effect of streptozotocin on the rat during the perinatal period. Diabetes. 1974;23:888–895. doi: 10.2337/diab.23.11.889. [DOI] [PubMed] [Google Scholar]
- Reece EA, Ma XD, Zhao Z, Wu YK, Dhanasekaran D. Aberrant patterns of cellular communication in diabetes-induced embryopathy in rats: II, apoptotic pathways. Am J Obstet Gynecol. 2005;192:967–972. doi: 10.1016/j.ajog.2004.10.592. [DOI] [PubMed] [Google Scholar]
- Rudge MVC, Damasceno DC, Volpato GT, Almeida FCG, Calderon IMP, Lemonica IP. Effect of Ginkgo biloba on the reproductive outcome and oxidative stress biomarkers of streptozotocin-induced diabetic rats. Braz J Med Biol Res. 2007;40:1095–1099. doi: 10.1590/S0100-879X2006005000132. [DOI] [PubMed] [Google Scholar]
- Saito FH, Damasceno DC, Kempinas WG, Morceli G, Sinzato YK, Taylor KN, Rudge MV. Repercussions of mild diabetes on pregnancy in Wistar rats and on the fetal development. Diabetol Metab Syndr. 2010;2:26. doi: 10.1186/1758-5996-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer-Graf UM, Buchanan TA, Xiang A, Songster G, Montoro M, Kjos SL. Patterns of congenital anomalies and relationship to initial maternal fasting glucose levels in pregnancies complicated by type 2 and gestational diabetes. Am J Obstet Gynecol. 2000;182:313–320. doi: 10.1016/S0002-9378(00)70217-1. [DOI] [PubMed] [Google Scholar]
- Simán CM, Gittenberger-De Groot AC, Wisse B, Eriksson UJ. Malformations in offspring of diabetic rats: morphometric analysis of neural crest-derived organs and effects of maternal vitamin E treatment. Teratology. 2000;61:355–367. doi: 10.1002/(SICI)1096-9926(200005)61:5<355::AID-TERA7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Sinzato YK, Lima PH, Campos KE, Kiss AC, Rudge MV, Damasceno DC. Neonatally-induced diabetes: lipid profile outcomes and oxidative stress status in adult rats. Rev Assoc Med Bras. 2009;55:384–388. doi: 10.1590/S0104-42302009000400010. [DOI] [PubMed] [Google Scholar]
- Sinzato YK, Damasceno DC, Laufer-Amorim R, Rodrigues MM, Oshiiwa M, Taylor KN, Rudge MV. Plasma concentrations and placental immunostaining of interleukin-10 and tumor necrosis factor-α as predictors of alterations in the embryo-fetal organism and the placental development of diabetic rats. Braz J Med Biol Res. 2011;44:206–211. doi: 10.1590/S0100-879X2011007500015. [DOI] [PubMed] [Google Scholar]
- Sivan E, Lee YC, Wu YK, Reece EA. Free radical scavenging enzymes in fetal dysmorphogenesis among off- spring of diabetic rats. Teratology. 1997;56:343–349. doi: 10.1002/(SICI)1096-9926(199712)56:6<343::AID-TERA1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Soulimane-Mokhtari NA, Guermouche B, Yessoufou A, Saker M, Moutairou K, Hichami A, Merzouk H, Khan NA. Modulation of lipid metabolism by n-3 polyunsaturated fatty acids in gestational diabetic rats and their macrosomic offspring. Clinical Science (London) 2005;109:287–295. doi: 10.1042/CS20050028. [DOI] [PubMed] [Google Scholar]
- Staples RE, Schnell VL. Refinements in rapid clearing technic in the KOH-alizarin red S method for fetal bone. Stain Technol. 1964;39:61–63. [PubMed] [Google Scholar]
- Sugimura Y, Murase T, Oyama K, Uchida A, Sato N, Hayasaka S, Kano Y, Takagishi Y, Hayashi Y, Oiso Y, Murata Y. Prevention of neural tube defects by loss of function of inducible nitric oxide synthase in fetuses of a mouse model of streptozotocin-induced diabetes. Diabetologia. 2009;52:962–971. doi: 10.1007/s00125-009-1312-0. [DOI] [PubMed] [Google Scholar]
- Tan H, Xu Y, Xu J, Wang F, Nie S, Yang M, Yuan J, Tanguay RM, Wu T. Association of increased heat shock protein 70 levels in the lymphocyte with high risk of adverse pregnancy outcomes in early pregnancy: a nested case-control study. Cell Stress Chaperones. 2007;12:230–236. doi: 10.1379/CSC-266.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano CM. As campanhas nacionais para detecção das doenças crônicas não-transmissíveis: diabetes e hipertensão arterial. Ciências e Saúde Coletiva. 2004;9:885–895. doi: 10.1590/S1413-81232004000400010. [DOI] [Google Scholar]
- Tsuji K, Taminato T, Usami M, Ishida H, Kitano N, Fukumoto H, Koh G, Kurose T, Yamada Y, Yano H, Seino Y, Imura H. Characteristic features of insulin secretion in the streptozotocin- induced NIDDM rat model. Metabolism. 1988;37:1040–1044. doi: 10.1016/0026-0495(88)90064-9. [DOI] [PubMed] [Google Scholar]
- Vambergue A, Fajardy I. Consequences of gestational and pregestational diabetes on placental function and birth weight. World J Diabetes. 2011;2:196–203. doi: 10.4239/wjd.v2.i11.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpato GT, Damasceno DC, Rudge MVC, Padovani CR, Calderon IMP. Effect of Bauhinia forficata aqueous extract on the maternal-fetal outcome and oxidative stress biomarkers of streptozotocin-induced diabetic rats. J Ethnopharmacol. 2008;116:131–137. doi: 10.1016/j.jep.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Vomachka MS, Johnson DC. Ovulation, ovarian 17 alpha-hydroxylase activity, and serum concentrations of luteinizing hormone, estradiol, and progesterone in immature rats with diabetes mellitus induced by streptozotocin. Proc Soc Exp Biol Med. 1982;171:207–213. doi: 10.3181/00379727-171-41500. [DOI] [PubMed] [Google Scholar]
- Wilson JC. Methods for administering agents and detecting malformations in experimental animal. In: Wilson JC, Warkany J, editors. Teratology: principles and techniques. Chicago: Univ. of Chicago Press; 1965. [Google Scholar]
- Wittig S, Hensse S, Keitel C. Heat shock gene expression is regulated during teratocarinoma cell differentiation and early embryo development. Dev Biol. 1983;96:507–514. doi: 10.1016/0012-1606(83)90187-2. [DOI] [PubMed] [Google Scholar]
- Yabunaka N, Ohtsuka Y, Watanabe I, Noro H, Fujisawa H, Agishi Y. Elevated levels of heat-shock protein 70 (HSP70) in the mononuclear cells of patients with non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1995;30:143–147. doi: 10.1016/0168-8227(95)01151-X. [DOI] [PubMed] [Google Scholar]
- Zhong X, Wang T, Zhang X, Li W. Heat shock protein 70 is upregulated in the intestine of intrauterine growth retardation piglets. Cell Stress Chaperones. 2011;15:335–342. doi: 10.1007/s12192-009-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Ren C, Wan X, Zhu Y, Zhu J, Zhou H, Zhang T (2012) Gene expression of Hsp70, Hsp90 and Hsp110 families in normal palate and cleft palate during mouse embryogenesis. Toxicol Ind Health. doi:10.1177/0748233712446720 [DOI] [PubMed]