Abstract
Many brain diseases have been linked to abnormal oxygen metabolism and blood perfusion; nevertheless, there is still a lack of robust diagnostic tools for directly imaging cerebral metabolic rate of oxygen (CMRO2) and cerebral blood flow (CBF), as well as the oxygen extraction fraction (OEF) that reflects the balance between CMRO2 and CBF. This study employed the recently developed in vivo 17O MR spectroscopic imaging to simultaneously assess CMRO2, CBF and OEF in the brain using a preclinical middle cerebral arterial occlusion mouse model with a brief inhalation of 17O-labeled oxygen gas. The results demonstrated high sensitivity and reliability of the noninvasive 17O-MR approach for rapidly imaging CMRO2, CBF and OEF abnormalities in the ischemic cortex of the MCAO mouse brain. It was found that in the ischemic brain regions both CMRO2 and CBF were substantially lower than that of intact brain regions, even for the mildly damaged brain regions that were unable to be clearly identified by the conventional MRI. In contrast, OEF was higher in the MCAO affected brain regions. This study demonstrates a promising 17O MRI technique for imaging abnormal oxygen metabolism and perfusion in the diseased brain regions. This 17O MRI technique is advantageous because of its robustness, simplicity, noninvasiveness and reliability: features that are essential to potentially translate it to human patients for early diagnosis and monitoring of treatment efficacy.
Keywords: In vivo17O MRS imaging, CMRO2, CBF, OEF, Stroke
Introduction
The brain is a highly aerobic organ that consumes oxygen extensively in order to produce chemical energy in the form of the adenosine triphosphate (ATP) molecule. The brain’s oxygen metabolism relies on an adequate oxygen supply facilitated by efficient blood circulation in the capillary bed. Deficits or abnormalities in cerebral oxygen metabolism and perfusion have been linked to many brain diseases such as stroke and tumor. Direct assessment and imaging of the cerebral metabolic rate of oxygen (CMRO2) and blood flow (CBF) may provide potential biomarkers for clinical diagnosis and monitoring treatment efficacy of brain diseases. Another highly relevant physiological parameter is the oxygen extraction fraction (OEF) that reflects the interplay between the oxygen supplied through blood flow and the oxygen demand of brain tissues.
Positron emission tomography (PET) is the sole, established neuroimaging modality capable of imaging all parameters of CMRO2, CBF and OEF using three radioactive tracers of 15O-isotope-labled oxygen gas (15O2), water (H215O) and carbon monoxide (C15O) (Mintun et al., 1984; Ter-Pogossian et al., 1970). Due to the limitations of methodology complexity, invasiveness and radiation exposure, PET has not become a standard clinical diagnosis tool for imaging abnormal CMRO2, CBF or OEF in patients (see more details in Discussion).
An alternative imaging approach for potentially mapping CMRO2 and CBF is to apply in vivo 17O magnetic resonance (MR) based imaging approaches in combination with either the inhalation of non-radioactive 17O-isotope-labeled oxygen gas (17O2) for assessing CMRO2 or with a bolus injection of H217O tracer for imaging CBF (Arai et al., 1991; Atkinson and Thulborn, 2010; Fiat et al., 1992; Kwong et al., 1991; Mateescu, 2003; Mateescu et al., 1991; Mateescu et al., 1990; Mellon et al., 2010; Pekar et al., 1991; Ronen et al., 1998; Zhu et al., 2009; Zhu et al., 2002; Zhu et al., 2007). Although there is a similarity between PET and 17O MR imaging methods, only the latter specifically detects the metabolically generated H217O without confounding signals from 17O2 and this merit significantly simplifies both imaging procedure and post-imaging quantification of CMRO2 (see review articles (Zhu and Chen, 2011; Zhu et al., 2005) and the cited references therein). Several studies have also shown the ability of high-field MR scanners to greatly improve the 17O detection sensitivity (Lu et al., 2012; Thelwall et al., 2003; Zhu et al., 2001). Furthermore, a recent study has suggested that the H217O metabolically generated in the brain tissue could serve as an endogenous perfusion tracer during the post-17O2-inhalation period for indirect assessment of CBF (Zhu et al., 2010).
Stroke is a leading cause of death and disability (Roger et al., 2011). The pathology, progression and treatment efficacy of stroke are extremely susceptible to the status of the cerebral oxygen metabolism (i.e., CMRO2) and blood perfusion (i.e., CBF), as well as their balance that can be quantified by OEF. New evidence has indicated that the measures of CMRO2 and OEF may provide a better assessment of viable brain tissue after a stroke attack for potential treatment compared to perfusion- or diffusion-weighted MRI, thus, the high-field 17O imaging approach might be potentially useful for the stroke patient management (Delapaz and Gupte, 2011; Heiss, 2011; Heiss and Sobesky, 2008; Zhu and Chen, 2011).
The objectives of this study were to exploit the feasibility of the 17O MR-based neuroimaging approach at high field for noninvasively and simultaneously imaging CMRO2, CBF and OEF using a preclinical mouse model of middle cerebral artery (MCA) occlusion (MCAO); and to test the applicability of the 17O imaging approach for detecting the abnormal oxygen metabolism and perfusion associated with the brain stroke.
Materials and Methods
17O Isotope Mass Balance and 17O MRI
The majority (>99.9%) of the oxygen atoms in oxygen gas or water are 16O isotopes that do not have any MR signal. The sole MR-detectable oxygen isotope is 17O, which is non-radioactive and stable, but has a very low abundance (0.037%) in nature. The principle behind the 17O-MR based CMRO2 imaging technique is to introduce the 17O-labeled oxygen gas (17O2) into the animal or human body via inhalation (for several minutes) while imaging the dynamic change of the 17O-labeled water (H217O) metabolized from the 17O2 in the brain (see review articles (Zhu and Chen, 2011; Zhu et al., 2005) and the cited references therein).
The inhaled oxygen gas will bind to hemoglobin in the blood through lung exchange, and then enter the brain via the feeding arteries and blood circulation. The labeled oxygen molecules will be metabolized in the mitochondria of brain cells to produce 17O labeled water molecules, which can be detected using a MR scanner. The net chemical reaction of this process is described as following:
Note: the 17O2 gas (reactant) is not visible to the 17O MR detection and only the final metabolic product, H217O, provides a MR signal. This makes the direct detection of H217O using 17O MRI simple, robust, and noninvasive.
After the brief 17O2 inhalation, the inhaled gas is switched back to the regular, non-17O-labeled oxygen gas. Thus, no additional H217O molecules will be generated through oxygen metabolism. The produced H217O molecules during the 17O2 inhalation period may serve as an endogenous tracer for CBF measurements.
The 17O MR images have been applied to directly determine the two important physiological quantities: CMRO2 and CBF. The quantification is based on the mass balance equation of 17O labeled water content during and after the 17O2 gas inhalation given by Eq. [1] (Zhang et al., 2004a; Zhu et al., 2002),
| [1] |
where Cb(t) is the 17O-water content of the brain tissue metabolized from the inhaled 17O2 gas (calibrated by the natural abundance 17O-water signal measured before the 17O2 gas inhalation); Ca(t) is the metabolic 17O-water content of the artery blood; α is the 17O-isotope enrichment of inhaled 17O2 gas and is a known constant; k1 is another known constant (k1=1.86) consistently across a wide range of brain physiology and non-physiology conditions (Zhu et al., 2010)).
Calculation of CMRO2
It has been shown that during a short 17O2 inhalation period (as used in this study) the temporal changes of the Ca(t) and Cb(t)/k1 terms in Eq. [1] are similar for small animals (Zhang et al., 2004a; Zhu et al., 2002), thus, Eq. [1] becomes
| [2] |
The anti-derivative of Eq. [2] gives a linear equation with a slope determined by linear regression of the following equation:
| [3] |
Thus, CMRO2 can be calculated as:
| [4] |
Calculation of CBF
After the 17O2 inhalation, the newly formed H2O molecules will no longer be labeled with 17O thus no more replenishing H217O molecules will be generated in the brain. The CMRO2 term in Eq. [1] diminishes and Ca(t) reaches a plateau, thus:
| [5] |
where k2 is a constant. Thus the anti-derivative of Eq. [5] gives an exponential equation with an exponential decay rate (D) that is proportional to CBF/k1
| [6] |
where k3 and k4 are constants, thus,
| [7] |
Therefore, one can determine CMRO2 from the Cb(t) time course during the period of 17O2 inhalation, and CBF from the Cb(t) time course in the period after the 17O2 inhalation is halted.
Calculation of OEF
| [8] |
where Ca,O2 is the artery oxygen concentration (or input function) and is a constant for the brain. The common unit of the Ca,O2 is ml-O2/dl-blood and it can be converted to μmol/ml-blood for matching the unit of CMRO2 to CBF ratio according to Avogadro’s law, i.e., a mol of any gas occupies 22.4 liters at standard temperature and pressure. The conversion factor between them is 2.24, therefore
| [9] |
The Ca,O2 value approximately ranges from 17 ml-O2/dl-blood measured in the mouse brain (Frietsch et al., 2007) to 18.8 ml-O2/dl-blood in the rat brain (unpublished data from our lab), and Ca,O2 ≈18 ml-O2/dl-blood was applied in the present study for OEF calculation.
OEF ratio between ischemic and contralateral hemispheres
In this study, we determined the OEF values from 3D chemical shift imaging (CSI) voxels covering the left and right mouse hemispheres, then calculated the OEF ratio (ROEF) between the paired voxels, located in the ischemic hemisphere (OEFI) and the “mirror” voxel in the contralateral, control hemisphere (OEFC).
| [10] |
Animal Stroke Model
Four male C57BL/6 mice (18–30g body weight) underwent a 60-minute right middle cerebral artery (MCA) occlusion 5–7 days prior to MR scanning were used in this study. The mice were anesthetized with ketamine (87 mg/kg)/xylazine (13 mg/kg) cocktail solution via intraperitoneally injection followed by constant subcutaneous infusion at 2.6 ml/kg/hr of the same cocktail solution during the MR study. The mouse head was immobilized with tooth and ear bars. Spontaneous respiration and body temperature of the animal were monitored throughout the MR experiment; warm water and warm air were used to maintain the body temperatures of mice at ~37°C. Two to three minutes of 17O2 (60% 17O enrichment, Sigma) gas was supplied to each mouse for 17O MR imaging experiments.
All experimental procedures and protocol were conducted under the guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the Washington University.
MRI/MRS Experiments
The 1H anatomic brain images and 17O MRS imaging data were acquired using a 11.7T/26-cm clear bore horizontal animal magnet (Magnex Scientific, UK) interfaced with a Varian INOVA console (Varian Inc., Palo Alto, CA). A radiofrequency (RF) probe consisting of a single loop 17O surface coil (~1.2 cm diameter) and a larger quadrature 1H coil was built for the 17O and 1H imaging of the mouse brain. T2-weighted fast spin echo image (repetition and echo times of TR/TE=4 s/15 ms; image matrix size of 256×256; field of view (FOV) of 2.5×2.5 cm2; 1 mm slice thickness, echo chain length of 8, 4 signal averages) and/or proton density gradient echo image sequences were used for acquiring the 1H anatomic brain images.
Three-dimensional (3D) 17O MRS imaging data was acquired using the Fourier Series Window (FSW) CSI technique, in which the k-space sampling is weighted according to the Fourier coefficients of a predetermined voxel shape (Hendrich et al., 1994). The following acquisition parameters were used: TR/TE=10/0.4 ms, 50-μs hard RF pulse for a nominal 90° excitation, spectral width=30 kHz; 8.5 ms acquisition time; 2×2×2 cm3 FOV; 9×9×5 phase encodes; 40 μl (or 15 μl nominal) voxel size with a cylindrical voxel shape; and 11 s temporal resolution per 3D CSI dataset. A 17×17×9 matrix of free induced decay (FID) signals was generated from the original phase encode data for each 3D 17O image.
For 17O imaging data acquisition, a total of 100–120 3D CSI datasets were collected during pre-inhalation (Phase I), inhalation (Phase II) and post-inhalation (Phase III) periods. Two to three minutes of 17O2 gas inhalation was applied for the noninvasive and simultaneous imaging of CMRO2, CBF and OEF.
The imaged brain H217O signal (resonance peak height) was converted to the absolute brain concentration in mM (i.e., Cb(t) in Eq. [1]) based on its signal ratio to the mean natural abundance brain H217O signal acquired during pre-inhalation (Phase I) period and the known values of the 17O natural abundance and the brain water concentration (Zhang et al., 2004a; Zhu et al., 2002). The Cb(t) time course of the 3D CSI voxel measured during Phase II was used to perform linear regression and calculate the CMRO2 value according to Eqs. [3] and [4]. The Cb(t) time course measured during Phase III was used to perform exponential regression and calculate the CBF value according to Eqs. [6] and [7]. For the voxels located in the ischemic brain regions, the initial few points of Cb(t) in Phase III, which not yet reached the peak H217O signal, were excluded from the exponential regression.
The CMRO2 and CBF values of the same voxel were used to calculate OEF according to Eq. [9]. The OEF ratio, ROEF, was determined using Eq. [10].
Statistical Analysis
All measurement results are presented as mean±standard deviation (SD). A paired student’s t test was applied for statistical analysis of the experimental data and for comparison of the results between the MCAO affected and intact brain regions. A p value of < 0.05 is considered to be statistically significant.
Results
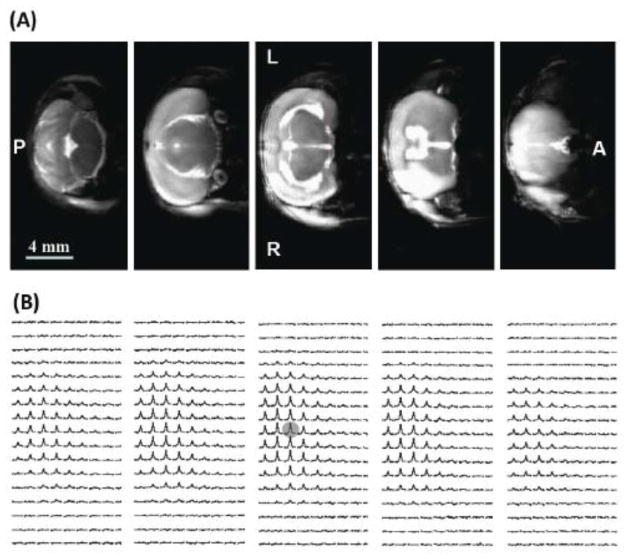
Figure 1 illustrates multi-slice T2-weighted 1H MRI (Fig. 1A) and the corresponding 3D 17O CSI of the natural abundance brain H217O signal (Fig. 1B) from a representative MCAO mouse brain. The total acquisition time was 8 min for the 1H MRI and 11 s for the 3D 17O CSI. The infarction in the right hemisphere caused by the MCA occlusion was detected as the hyper-intense lesions in the anatomic 1H images (Fig. 1A). The spatial distribution of the natural abundance H217O signal in the mouse brain was consistent with the 1H anatomic images and the 17O coil size; and the signal intensity reflected the RF field distribution of the 17O surface coil. The improved 17O detection sensitivity at 11.7 T was evident by the excellent spatial and temporal resolution of the 3D CMRO2 and/or CBF imaging in this study.
Figure 1.
(A) Multi-slice T2-weighted 1H MRI (8 minutes of total acquisition time and 0.01 μl pixel size), and (B) the corresponding 3D-CSI images of the natural abundance H217O (11 seconds of total acquisition time and 15 μl nominal voxel size) from a representative MCAO mouse brain. The lesions with hyper-intensity in the right brain hemisphere caused by the right MCA occlusion are evident from the anatomic 1H images. L: left side; R: right side; A: anterior image slice; and P: posterior image slice.
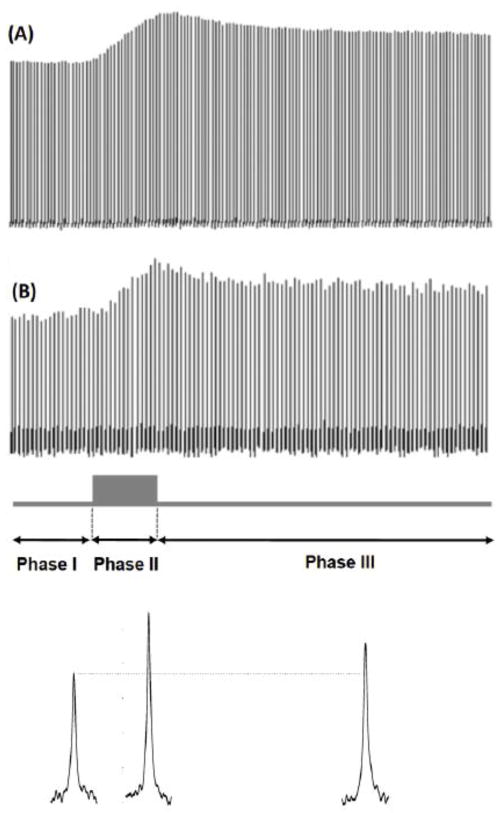
Figure 2A displays the stacked plots of the global brain H217O spectra acquired before, during and after a 2.5-min 17O2 inhalation in a representative MCAO mouse at 11.7T. The time course of the H217O signal can be divided into three distinct phases: Phase I is the pre-inhalation period with a constant natural abundance brain H217O signal; Phase II is the inhalation period with an approximately linear increase of brain H217O signal; Phase III is the post-inhalation period with an exponential decay of brain H217O signal. Similar temporal pattern was also observed in voxel-based time courses, as shown in the example of a representative voxel (see Fig. 2B) taken from the 3D 17O CSI dataset (marked in Fig. 1B) with a temporal resolution of 11 seconds per CSI volume. The quality of the 17O MR spectra is displayed at bottom of the Fig. 2B for each of the three phases. The H217O signal measured in Phase I was used to quantify the absolute brain H217O concentrations measured during Phase II and Phase III. The time courses of the H217O concentrations in Phase II and III were used to calculate the CMRO2 and CBF values, respectively.
Figure 2.
Stacked plots of 17O spectra of the H217O signals acquired before, during and after a 2.5-minute 17O2 inhalations from a representative MCAO mouse brain at 11.7T. The 17O spectra of (A) the global brain signal observed with the 17O surface coil, and (B) a representative voxel (as shadowed in Fig. 1B) taken from the 3D 17O CSI data acquired with 11 seconds per 3D CSI volume are displayed. The H217O signal time course can be divided into three phases: pre-inhalation period (Phase I: constant phase), inhalation period (Phase II: linearly increasing phase) and post-inhalation period (Phase III: exponentially decay phase). The typical single-voxel spectra for each of three phases are shown at bottom of this figure.
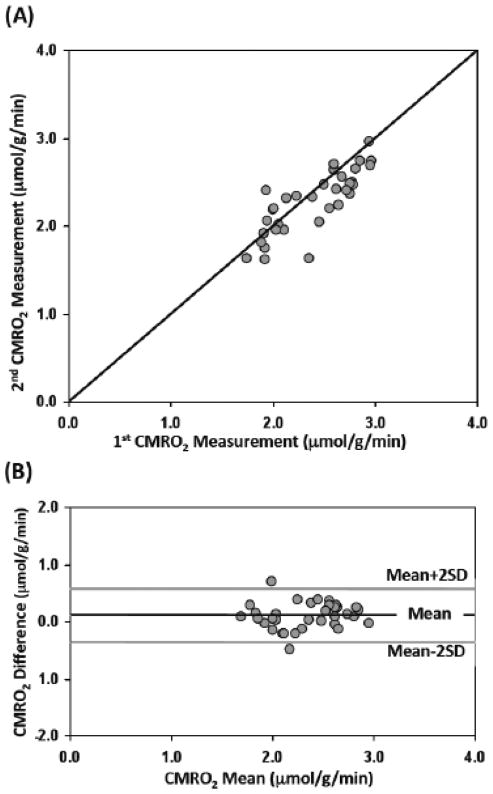
Figure 3A shows the voxel-based comparison of two repeated CMRO2 imaging measurements in the same mouse. All the data points across multiple CSI voxels (n=34) covering both ischemic and intact brain regions are located along the line of equality. The reproducibility of the CMRO2 measurements was quantitatively assessed and the Bland-Altman plot between the differences and the mean CMRO2 values of the two repeated measurements (Bland and Altman, 1986, 1999) is shown in Figure 3B. The mean (black line) and standard deviation (SD) of the difference between the 1st and 2nd CMRO2 measurements were 0.11 μmol/g/min and 0.23 μmol/g/min, respectively. The gray lines in Fig. 3B define the limits of agreement where 95% of the difference values are expected to be less than two SD. Clearly, the majority of the data points were within the range except two outliers, which, interestingly, were from two voxels located in the periphery regions of the 17O surface coil with relatively poor detection sensitivity.
Figure 3.
Comparison of two repeated CMRO2 imaging measurements in the same MCAO mouse. (A) The relationship between the 1st and 2nd CMRO2 measurements across multiple CSI voxels (n=34) with a line of equality. (B) The Bland-Altman plot of the differences verse the mean values between the two repeated CMRO2 measurements. The black line depicts the mean (0.11 μmol/g/min) of the difference between the two CMRO2 measurements and the gray lines defines the limits of agreement where 95% of the difference values are expected to be less than two standard deviation (SD=0.23 μmol/g/min).
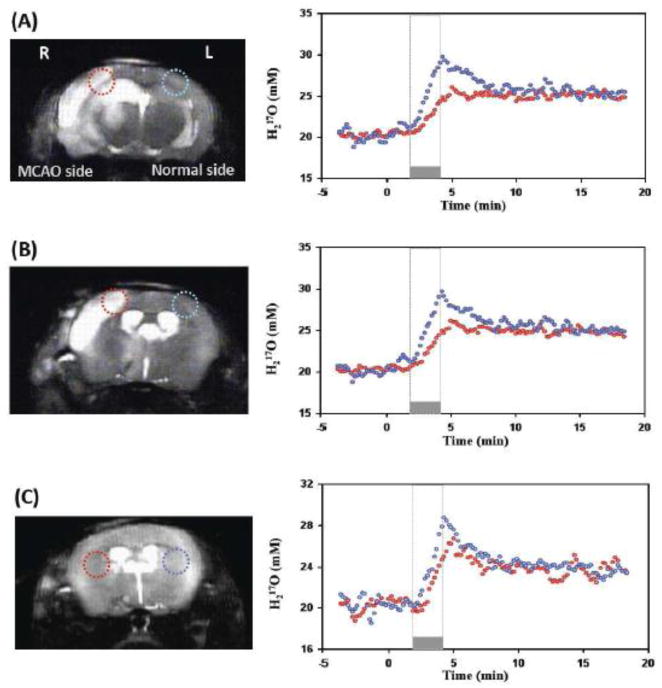
Figure 4 shows an example for comparing the time courses of the H217O water during the CMRO2 and CBF measurements between the voxels located in the MCA occluded hemisphere (red circles) and the voxels located in the contralateral, intact hemisphere (blue circles). Two anatomic image slices from a representative MCAO mouse are shown in Figs. 4A and 4B. For each image slice, two voxels symmetrically located in the lesion area and in the contralateral hemisphere were identified. The time courses of the brain H217O concentration in such paired-voxels measured before, during and after a 2.5-min inhalation of 17O2 were plotted together and analyzed carefully. It is clear that the H217O concentration increased during 17O inhalation (Phase II) followed by the H217O concentration decay during the post-inhalation period (Phase III). Both rates of H217O accumulation and decay were substantially reduced in the MCAO affected voxels compared to those of intact voxels, which revealed large reductions of CBF and CMRO2 in ischemic brain (see Fig. 4A and Fig. 4B). Figure 4C shows the results from another MCAO mouse, in which an obvious different H217O dynamic was also observed in the MCAO affected brain region but without a discernible lesion in the anatomic image.
Figure 4.
Comparison of CMRO2 and CBF measurements between the 17O CSI voxels located in the MAC occluded right hemisphere (red circles) and the voxels located in the contralateral left hemisphere of the same mouse brain (blue circles). (A) and (B) show the anatomic images, selected voxels and their corresponding dynamic brain H217O signal changes before, during and after a 2.5-minute 17O2 inhalation from two representative image slices in the same MCAO mouse. Both the slope of 17O signal increase during the inhalation and the exponential decay rate during the post-inhalation phase were substantially smaller in the MCAO affected voxels compared to that corresponding voxels in intact hemisphere, indicating large reductions in both CMRO2 and CBF. (C) Shows the similar results from a different MCAO mouse brain.
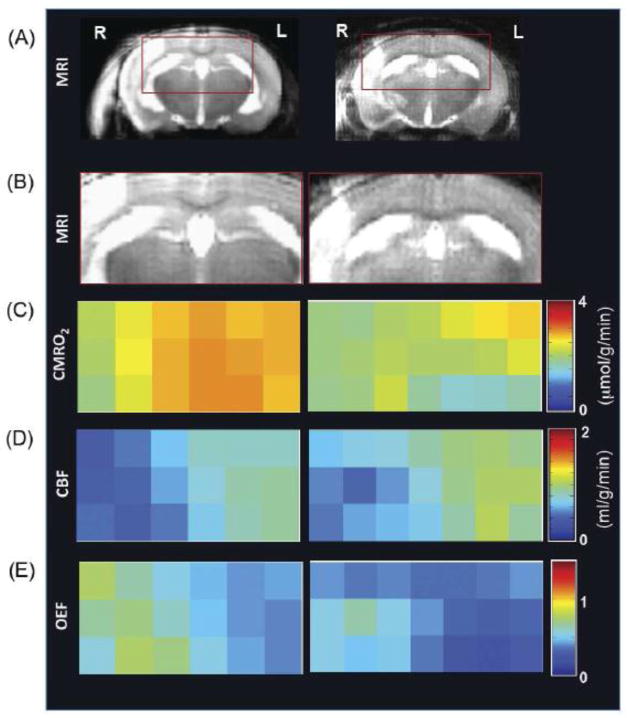
Figure 5 displays the measurement results obtained from two representative MCAO mice in imaging format. The 1H images shown in Figs. 5A and 5B define the brain region of interest with high 17O detection sensitivity including both normal and MCAO affected brain regions. Figures 5C, 5D and 5E represent the CMRO2, CBF and OEF maps of the same brain regions, respectively. Substantial reductions of CMRO2 and CBF were observed in the MCA occluded right hemisphere. In contrast, OEF increased in the MCAO affected brain regions comparing with that of the contralateral control.
Figure 5.
Imaging results obtained from two representative MCAO mice (left versus right column). Anatomic brain images selected from a representative image slice (A), expanded anatomic image region of interest with high 17O detection sensitivity covering both MCA occluded and normal brain regions (B), and the corresponding CMRO2 images (C), CBF images (D) and OEF images (E) are displayed. These images clearly demonstrate significant reductions of CMRO2 and CBF in the right hemisphere affected by MCAO compared to the intact left hemisphere. In contrast, the values of OEF were increased in the MCAO affected brain regions.
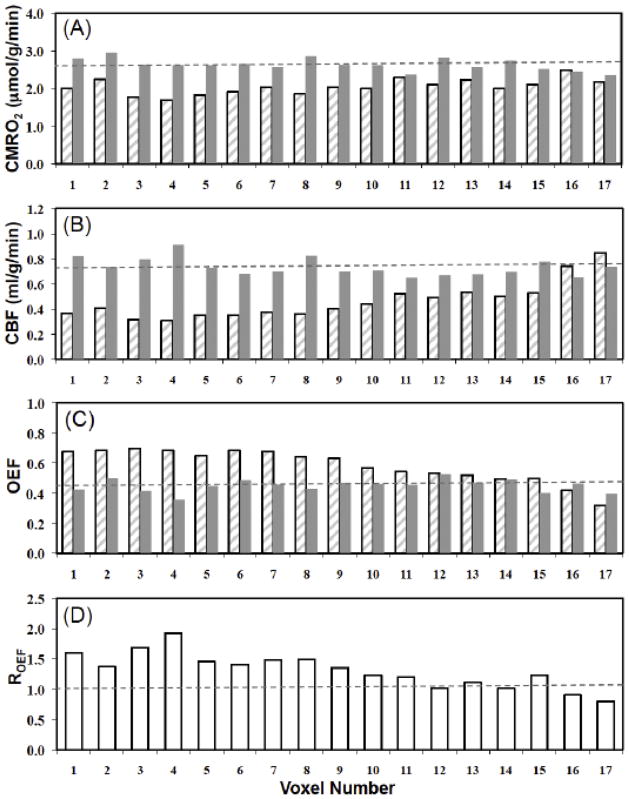
Figure 6 summarizes the quantitative comparison results of the CMRO2, CBF, OEF and OEF ratios in paired-voxels (i.e., the voxels in the MCA occluded hemisphere (hatched bars) vs. those in the contralateral, intact hemisphere (gray bars)) from a representative MCAO mouse brain. A total of 17 pairs of image voxels in the 17O coil sensitive region, covering multiple imaging slices, were selected. The CMRO2 values of the voxels in the contralateral control hemisphere, CMRO2,C, were relatively uniform with mean value of 2.63±0.16 μmol/g/min (see the dashed line in Fig. 6A), which was significantly higher than that of the voxels in the ischemic hemisphere, where mean CMRO2,I value was 2.04±0.20 μmol/g/min (p=4×10−7). The CBF results (see Fig. 6B) show the similar pattern and the mean values of CBFI (0.46±0.15 ml/g/min) measured in the MCAO affected hemisphere was much lower than the CBFC (0.73±0.07 ml/g/min) of the normal hemisphere (p=2×10−5). Figures 6C reports the OEF values of the voxels in the ischemic (OEFI=0.58±0.11) and the control (OEFC=0.45±0.04) hemispheres (p=3×10−4), while Fig. 6D shows the OEF ratios (ROEF=1.3±0.3) between the OEFI and OEFC. The overall results clearly indicate substantial CMRO2 and CBF reductions and an OEF increase in the MCA occluded hemisphere.
Figure 6.
Comparison results of CMRO2, CBF, OEF and OEF ratios among the paired image voxels in the MCAO affected hemisphere (hatched bars) and contralateral, intact hemisphere (gray bars) obtained from a representative MCAO mouse brain. Voxel wise comparison of (A) CMRO2 values, (B) CBF values and (C) OEF values between MCAO affected and normal hemispheres. (D) OEF ratios between OEFI and OEFC. The dashed lines represent mean values of the physiological parameters in the intact hemisphere.
Figure 7 shows T2-weighted 1H MRI of all animals scanned in this study where, in most cases, the stroke lesions were clearly visible. Single or multiple image slices selected from each mouse were displayed and a pair of 17O CSI voxels were identified on each slice with the red circle representing the voxel in the MCA occluded brain regions and the blue circle as the corresponding voxel in the intact brain regions on the contralateral hemisphere. The CMRO2, CBF, OEF values of these paired-voxels as well as their OEF ratios are summarized in Table 1. The paired t-test result shows statistically significant reduction of CMRO2 (CMRO2, I = 1.66±0.29 vs. CMRO2, C = 2.44±0.29 μmol/g/min) and CBF (CBFI = 0.46±0.11 vs. CBFC = 0.88±0.11 ml/g/min), as well as elevation of OEF (OEFI = 0.49±0.19 vs. OEFC = 0.35±0.07) in stroked brain regions as compare to the corresponding normal brain regions.
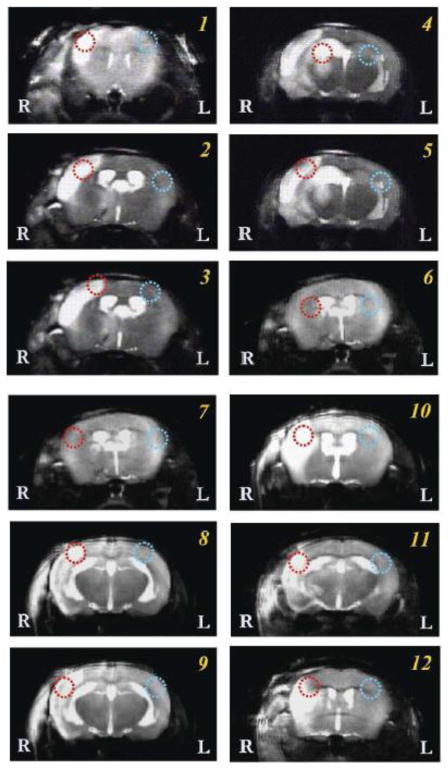
Figure 7.
T2-weighted 1H MR images of all animals scanned in this study. Single or multiple image slices selected from each mouse were displayed with one pair of 17O CSI voxels identified on each image as red and blue circles. The red circles indicate the voxel located in the typical MCA occluded brain regions; and the corresponding blue circles were in the symmetric location of the contralateral hemisphere with intact brain tissue.
Table 1.
Comparison of CMRO2, CBF and OEF values in paired voxels as shown in Fig. 7
| Voxel Pair | CMRO2,I (μmol/g/min) | CMRO2,C (μmol/g/min) | CBF I (ml/g/min) | CBF C (ml/g/min) | OEFI | OEFC | R OEF |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 1.31 | 2.41 | 0.43 | 0.73 | 0.38 | 0.41 | 0.9 |
|
| |||||||
| 2 | 1.30 | 2.45 | 0.53 | 0.96 | 0.31 | 0.32 | 1.0 |
| 3 | 1.35 | 2.44 | 0.37 | 0.71 | 0.45 | 0.43 | 1.1 |
|
| |||||||
| 4 | 1.68 | 2.48 | 0.58 | 0.76 | 0.36 | 0.41 | 0.9 |
|
| |||||||
| 5 | 1.25 | 2.44 | 0.44 | 0.90 | 0.35 | 0.34 | 1.0 |
| 6 | 1.78 | 2.45 | 0.65 | 0.84 | 0.34 | 0.36 | 0.9 |
|
| |||||||
| 7 | 1.84 | 2.02 | 0.54 | 1.03 | 0.42 | 0.24 | 1.7 |
|
| |||||||
| 8 | 2.11 | 2.75 | 0.35 | 0.80 | 0.75 | 0.43 | 1.8 |
|
| |||||||
| 9 | 1.88 | 2.96 | 0.34 | 0.94 | 0.70 | 0.39 | 1.8 |
|
| |||||||
| 10 | 1.91 | 2.67 | 0.26 | 0.94 | 0.91 | 0.36 | 2.6 |
| 11 | 1.90 | 2.32 | 0.51 | 1.02 | 0.46 | 0.28 | 1.6 |
|
| |||||||
| 12 | 1.60 | 1.86 | 0.49 | 0.90 | 0.41 | 0.26 | 1.6 |
|
| |||||||
| Mean | 1.66 | 2.44 | 0.46 | 0.88 | 0.49 | 0.35 | 1.4 |
|
| |||||||
| SD | 0.29 | 0.29 | 0.11 | 0.11 | 0.19 | 0.07 | 0.5 |
Paired t-Test: p < 1.1 × 10−5 (CMRO2, I vs. CMRO2, C); p < 1.0 × 10−6 (CBF I vs . CBF C); p < 0.03 (OEF I vs . OEF C).
Discussion
Significance and current state of neuroimaging in acute ischemic stroke
Since the brain consumes oxygen and glucose extensively to support the neuronal activities, the deficit of oxygen supply in the brain tissue due to disturbance in the blood circulation occurring during a stroke attack would result in detrimental consequences. Developing noninvasive neuroimaging modalities capable of imaging the abnormal perfusion and oxygen metabolism in the stroke patients after an acute ischemic attack, in particular, for identifying and differentiating the irreversibly damaged brain tissue and the poorly perfused but potentially salvageable tissue (i.e., the penumbra) would significantly improve the therapeutic intervention of stroke patients (Heiss, 2011).
The combined diffusion-weighted MRI (DWI) for defining the ischemic core and perfusion-weighted MRI (PWI) with contrast agents for identifying adjacent critically hypo-perfused tissue approach is appealing to ultimately identify the ischemic penumbra based on the diffusion-perfusion mismatch (Barber et al., 1998; Heiss, 2011). However, the diffusion-perfusion mismatch approach remains challenging; and it has been reported that the detected diffusion lesion consists of irreversible infract tissues could be reversed if perfusion is restored early in the stroke patients and the measured perfusion lesion may overestimate the penumbra (Davis and Donnan, 2009; Heiss, 2011; Kidwell et al., 2003; Wardlaw, 2010). The indirect approach for detecting stroke tissue pathophysiology resulting from impaired cerebral oxygen metabolism and brain energy deficits may have contributed to this immense challenge (Heiss, 2011). An ideal neuroimaging tool for stroke diagnosis should be able to directly image both CBF and CMRO2, subsequently OEF that reflects the balance between the blood supply and oxygen consumption, and to potentially provide a better assessment of ischemic penumbra in stroke patients (Heiss, 2011).
All three variables (CMRO2, CBF and OEF) have been imaged using PET (Mintun et al., 1984; Ter-Pogossian et al., 1970). However, PET cannot distinguish radioactive signals of the inhaled 15O2 molecules from those emitted by the metabolically generated H215O molecules. Thus, it requires multiple experiments for independently assessing the production rate of the metabolic H215O inside the brain during an 15O2 inhalation; the CBF value with a bolus injection of H215O; the cerebral blood volume (CBV) using an C15O gas inhalation; and the artery 15O-label content to determine the artery input function through the artery blood sampling (Mintun et al., 1984; Zhu et al., 2005). These multiple PET measurements are used to derive the CMRO2 image based on a number of assumptions and a complex quantification model (Mintun et al., 1984). In addition, due to a very short 15O half-life time (~2 minutes), an onsite cyclotron is required for the PET-based CMRO2 measurements. These limitations in methodology complexity, invasiveness and radiation exposure make PET impractical for routine clinical diagnosis despite its value in the assessment of ischemic penumbra (Heiss, 2011).
Advantages of 17O MR imaging technique
The concept behind the in vivo 17O MR-based approach for imaging CMRO2 using a 17O2 tracer is similar to the PET technique that uses 15O2. However, the in vivo 17O imaging approach is characterized by a number of unique properties and merits.
First, the 17O is a non-radioactive, stable isotope and has a very low natural abundance, thus, serves as an ideal metabolic tracer with a small background signal. The inhaled 17O2 is NMR invisible and only the final metabolic product of 17O-labeled water can be detected and imaged; this significantly simplifies the measurement procedure and quantification for imaging CMRO2 in situ (see review articles (Zhu and Chen, 2011; Zhu et al., 2005) and the cited references therein).
Second, several animal studies have suggested that the second term on the right side of Eq. [1] approximately approaches zero during a brief inhalation of 17O2 in the brain of small animals due to rapid blood circulation and air exchange in the lung (Zhang et al., 2004a; Zhu et al., 2005; Zhu et al., 2002), thus, the slope of the brain H217O concentration accumulation during 17O2 inhalation (Phase II) can be used to determine the absolute CMRO2 value according to Eq. [4].
Third, the metabolic H217O produced in the brain mitochondria can serve as an endogenous perfusion tracer during the post-inhalation period (Phase III) and its decay rate can provide an indirect assessment of CBF (Zhu et al., 2010). This could avoid an additional CBF measurement and the use of exogenous perfusion tracers commonly applied in PET (i.e., a bolus injection of H215O) or in the clinical PWI (i.e., a bolus injection of paramagnetic contrast agent).
Fourth, the 17O detection sensitivity increases substantially at higher magnetic fields according to a square power relation as a function of the field strength (Lu et al., 2012; Thelwall et al., 2003; Zhu et al., 2001). The success of high-field 17O imaging approaches for the assessment of CMRO2 has been evident in animal studies (Zhu et al., 2009; Zhu et al., 2002; Zhu et al., 2007) as well as in some preliminary human brain studies (Atkinson and Thulborn, 2010; Hoffmann et al., 2011; Zhu et al., 2006).
The unique properties of the in vivo 17O imaging approach suggest the possibility for the noninvasive and simultaneous imaging of three important physiology variables: CBF, CMRO2 and OEF from a single, brief inhalation of 17O2.
Feasibility for imaging CMRO2, CBF and OEF in the MCAO mouse brain
Mice are the most common vertebrate animal models in biomedical research and have been used in many preclinical studies of various brain diseases. However, the mouse brain size is very small compared to a human brain posing a major challenge for all in vivo neuroimaging methods owing to the conflict between the needed high imaging spatial resolution and the resulting decreased detection sensitivity or reliability. Despite the progresses of the in vivo 17O-MR methods for imaging CMRO2 in rats (Zhu et al., 2002; Zhu et al., 2007), cats (Pekar et al., 1991; Zhu et al., 2009), and swine (Mellon et al., 2010), a successful application in the mouse brain has yet to be reported prior to the present study.
In this study, we examined MCAO mice at the high field strength of 11.7T in order to achieve higher 17O detection sensitivity. The results indicated that high-field strength affords excellent sensitivity for imaging the natural abundance H217O signal in the mouse brain with superior temporal resolution (~11 s per 3D CSI dataset) and reasonable spatial resolution (~40 μl voxel size) as demonstrated in Fig. 1B. In the central RF field of the 17O surface coil where the 17O detection sensitivity is optimal, the signal-to-noise ratio (SNR) of the 3D CSI voxels could reach 25:1 or better. Thus, the sensitivity gained at 11.7T is essential for reliably detecting the dynamic change of brain H217O signal during the 17O2 inhalation (Phase II in Fig. 2) and the post-inhalation period (Phase III); which is crucial for differentiating the metabolic and perfusion differences in normal and diseased tissues. In addition, the excellent reproducibility of CMRO2 imaging from two repeated measurements in the same animal (Fig. 3), and the ability to detect the slower H217O production and clearance in the MCA occluded hemisphere (Fig. 4) further support this notion. It is interesting to note that we were able to detect the abnormal oxygen metabolism in the MCAO affected brain region even before obvious stroke lesions show up in the T2-weighted 1H MRI (see Fig. 4C, Fig. 7 and Table 1). This result suggests that the 17O-based CMRO2 imaging method may be more sensitive for assessing pathophysiological changes in an early stroke stage compared to conventional structural MRI.
This study is the first attempt to image CBF using the metabolic H217O generated in the mitochondria as an endogenous tracer. These H217O molecules are washed out of the brain cells and enter the blood stream before returning to the heart through blood circulation, thus, the washout rate is expected to reflect the blood perfusion. Although it has been found that the decay rate of the metabolic 17O-water signal is lower than that of conventional tracer measurement following a bolus injection of H217O directly into the brain, presumably due to the limited permeability of water to across the mitochondria membranes (Zhang et al., 2004b; Zhu et al., 2002), the decay constant (D) correlates closely with the CBF value across a wide range of physiology conditions (Zhu et al., 2010). The present study clearly demonstrates the feasibility of imaging CBF during the post-17O2-inhalation period in the same CMRO2 imaging measurement. The results, as illustrated in Fig. 5, show excellent reliability for detecting abnormal CBF and CMRO2 in the MCAO affected brain regions, thus, make it possible to obtain three high-quality images of CBF, CMRO2 and OEF with only one imaging measurement and a short inhalation of 17O2.
In comparison with the rat brain CMRO2 (2.2 μmol/g/min) and CBF (0.53 ml/g/min) values previously measured using the high-field 17O imaging approach (Zhu et al., 2002), the CMRO2 and CBF values measured in the intact mouse hemisphere were found to be significantly higher (see Fig. 6, Fig. 7 and Table 1). This difference is expected considering the inverse relationship between the log of body size and the CMRO2 for different species (Siesjo, 1978).
More importantly, both CMRO2 and CBF in the MCAO affected brain region in one hemisphere were found to be significantly smaller than that of the corresponding intact brain region on the contralateral hemisphere (see Figs. 4–7 and Table 1), indicating the deficits in blood supply and oxygen utilization in the ischemic brain regions. Interestingly, the magnitude of CBF reduction was larger than that of CMRO2 reduction, leading to an OEF increase in the MCAO affected hemisphere as illustrated in Fig. 5, Figs. 6C–6D, Fig. 7 and Table 1. The OEF increase associated with the acute stroke ischemia has been considered as a positive sign of the penumbra that might be treated with therapeutic intervention (Heiss, 2011).
It worth to mention that the same calculation methods (i.e., Eq. [4], Eq. [7] and Eq. [9]) were used to obtain CMRO2, CBF and OEF values for both normal and stroked brain tissues. In the ischemic brain region, the decreases in the oxygen availability are presumably due to the reduction in the blood flow and tissue perfusion rather than the lower oxygen content in the arterial blood since the blood supply of different brain regions was from the same source, the heart. Therefore, the same Ca,O2 value was utilized in the OEF calculation for different brain tissues. The Ca,O2 value in different animals may vary depending on the animal condition, i.e., the hemoglobin concentration and the oxygen saturation of the arterial blood in each animal could be different slightly, which leads to different Ca,O2 values. However, such differences should not change the conclusion of this study because the ischemic brain tissues were compared with the corresponding normal tissue of the same animal brain. Therefore, the simultaneously measured CMRO2, CBF and OEF images as shown in this study could provide comprehensive and reliable assessments for the outcomes of acute ischemic stroke.
Potential impact on stroke patients
Several recent studies have demonstrated the feasibility of imaging the dynamic change of the metabolic H217O either in the human occipital lobe during two-minute 17O2 inhalation at 7T (Zhu et al., 2006), or in the entire human brain with a much longer 17O2 inhalation time at field strength of 1.5T–9.4T (Atkinson and Thulborn, 2010; Fiat et al., 2004; Mellon et al., 2010; Zhu and Chen, 2011). Despite the complication in quantifying CMRO2 in human due to a much larger body size and slower lung exchange between the 17O-labeled and non-labeled oxygen gases, it should still be possible to noninvasively imaging both CMRO2 and CBF in the human brain after establishing a more sophisticated quantification modeling (Atkinson and Thulborn, 2010; Zhang et al., 2004a; Zhu et al., 2002).
An excellent SNR of the natural abundance H217O signal of up to 25:1 was achievable in a 17O-CSI voxel of the mouse brain with a 40-μl voxel size and 11 seconds temporal resolution at 11.7T. This sensitivity led to reliable 3D CMRO2 and CBF imaging as shown in this study. Herein, we estimate the 17O CSI voxel size potentially for the 7T human brain application assuming that a 7.5 cm diameter RF coil will be used and an equivalent SNR can be achieved (Zhu et al., 2006). In this case, the 17O detection sensitivity along the line perpendicular to the coil center is inversely proportional to the coil radius, resulting in 6.3 times of SNR reduction due to the larger human RF coil. In addition, the translation of the 17O imaging from 11.7T to 7T also resulted in additional 2.8 folds of SNR loss based on the field dependence of the 17O detection sensitivity (Lu et al., 2011; Thelwall et al., 2003; Zhu et al., 2001). These led to a total of 18 times in the SNR reduction for the 7T human brain application if the same 17O CSI voxel size of 40 μl were retained. On the other hand, a voxel size of 0.7 ml (=40 μl ×18÷1000) should achieve an identical SNR as observed in the present mouse study. This spatial resolution should be adequate for imaging human brain CMRO2, CBF and OEF in order to identify the abnormal perfusion and oxygen metabolism caused by the ischemic stroke since the human brain size is more than 3000 times larger than that of a mouse brain.
Conclusion
In summary, this study demonstrates that, with a brief 17O2 inhalation, the high-field 17O MR imaging approach is capable of simultaneous and completely noninvasive imaging of three important physiology variables: CMRO2, CBF and OEF in a small mouse brain. In contrast to the established PET technique, the 17O imaging approach is advantageous for its noninvasiveness, robustness, simplicity, and rapid measurement; thus, it should be more suitable for survival and longitudinal animal studies. The overall results clearly indicate the high sensitivity of this method for mapping abnormal CMRO2, CBF and OEF changes in the ischemic brain regions and for discerning that from the intact brain tissue located in the contralateral hemisphere.
The findings from this study also suggest the possibility of extending the 17O-MR based CMRO2, CBF and OEF imaging approach to the healthy human subjects and stroke patients, potentially as a clinical diagnosis tool. Finally, this new neuroimaging modality should be valuable for investigating normal brain functions and brain diseases beyond stroke (e.g., brain tumor and many degenerative diseases), or for studying the oxygen metabolism and perfusion in other organs such as heart.
Highlights.
The stroke brain of MCAO mouse was studied using a new in vivo 17O MR imaging method.
CMRO2, CBF and OEF were simultaneously and noninvasively imaged in mouse.
CMRO2 and CBF were significantly lower in the MCAO affected brain region
In contrast, OEF was significantly higher in the MCAO affected brain region.
17O-MRI method is promising for studying abnormal oxygen metabolism and perfusion.
Acknowledgments
This work was supported in part by National Institute of Health grants: NS047592, NS057560, NS041262, NS070839, P01 NS059560, P41 RR08079 and P41 EB015894. The authors would like to thank Professor Kamil Ugurbil (University of Minnesota) and Professor Joseph Ackerman (Washington University) for their support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arai T, Mori K, Nakao S, Watanabe K, Kito K, Aoki M, Mori H, Morikawa S, Inubushi T. In vivo oxygen-17 nuclear magnetic resonance for the estimation of cerebral blood flow and oxygen consumption. Biochem Biophys Res Commun. 1991;179:954–961. doi: 10.1016/0006-291x(91)91911-u. [DOI] [PubMed] [Google Scholar]
- Atkinson IC, Thulborn KR. Feasibility of mapping the tissue mass corrected bioscale of cerebral metabolic rate of oxygen consumption using 17-oxygen and 23-sodium MR imaging in a human brain at 9.4 T. Neuroimage. 2010;51:723–733. doi: 10.1016/j.neuroimage.2010.02.056. [DOI] [PubMed] [Google Scholar]
- Barber PA, Darby DG, Desmond PM, Yang Q, Gerraty RP, Jolley D, Donnan GA, Tress BM, Davis SM. Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI. Neurology. 1998;51:418–426. doi: 10.1212/wnl.51.2.418. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Bland JM, Altman DG. Measuring agreement in method comparison studies. Statistical Methods in Medical Research. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- Davis SM, Donnan GA. MR mismatch and thrombolysis: appealing but validation required. Stroke. 2009;40:2910. doi: 10.1161/STROKEAHA.109.552893. [DOI] [PubMed] [Google Scholar]
- Delapaz R, Gupte P. Potential application of 17O MRI to human ischemic stroke. Adv Exp Med Biol. 2011;701:215–222. doi: 10.1007/978-1-4419-7756-4_29. [DOI] [PubMed] [Google Scholar]
- Fiat D, Hankiewicz J, Liu S, Trbovic S, Brint S. 17O magnetic resonance imaging of the human brain. Neurol Res. 2004;26:803–808. doi: 10.1179/016164104X5156. [DOI] [PubMed] [Google Scholar]
- Fiat D, Ligeti L, Lyon RC, Ruttner Z, Pekar J, Moonen CT, McLaughlin AC. In vivo 17O NMR study of rat brain during 17O2 inhalation. Magn Reson Med. 1992;24:370–374. doi: 10.1002/mrm.1910240218. [DOI] [PubMed] [Google Scholar]
- Frietsch T, Maurer MH, Vogel J, Gassmann M, Kuschinsky W, Waschke KF. Reduced cerebral blood flow but elevated cerebral glucose metabolic rate in erythropoietin overexpressing transgenic mice with excessive erythrocytosis. J Cereb Blood Flow Metab. 2007;27:469–476. doi: 10.1038/sj.jcbfm.9600360. [DOI] [PubMed] [Google Scholar]
- Heiss WD. The ischemic penumbra: correlates in imaging and implications for treatment of ischemic stroke. The Johann Jacob Wepfer award 2011. Cerebrovasc Dis. 2011;32:307–320. doi: 10.1159/000330462. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Sobesky J. Comparison of PET and DW/PW-MRI in acute ischemic stroke. Keio J Med. 2008;57:125–131. doi: 10.2302/kjm.57.125. [DOI] [PubMed] [Google Scholar]
- Hendrich K, Hu X, Menon R, Merkle H, Camarata P, Heros R, Ugurbil K. Spectroscopic imaging of circular voxels with a two-dimensional Fourier-Series Window technique. J Magn Reson. 1994;105:225–232. doi: 10.1006/jmrb.1994.1128. [DOI] [PubMed] [Google Scholar]
- Hoffmann SH, Begovatz P, Nagel AM, Umathum R, Schommer K, Bachert P, Bock M. A measurement setup for direct 17O MRI at 7 T. Magn Reson Med. 2011;66:1109–1115. doi: 10.1002/mrm.22871. [DOI] [PubMed] [Google Scholar]
- Kidwell CS, Alger JR, Saver JL. Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003;34:2729–2735. doi: 10.1161/01.STR.0000097608.38779.CC. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Hopkins AL, Belliveau JW, Chesler DA, Porkka LM, McKinstry RC, Finelli DA, Hunter GJ, Moore JB, Barr RG, et al. Proton NMR imaging of cerebral blood flow using H217O. Magn Reson Med. 1991;22:154–158. doi: 10.1002/mrm.1910220116. [DOI] [PubMed] [Google Scholar]
- Lu M, Zhang Y, Ugurbil K, Chen W, Zhu XH. In vitro and In vivo Studies of 17O NMR Sensitivity at 9.4 and 16.4 T. Mag Reson Med. 2012 doi: 10.1002/mrm.24386. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateescu GD. Functional oxygen-17 magnetic resonance imaging and localized spectroscopy. Adv Exp Med Biol. 2003;510:213–218. doi: 10.1007/978-1-4615-0205-0_35. [DOI] [PubMed] [Google Scholar]
- Mateescu GD, LaManna JC, Lust WD, Mars LM, Tseng J. Oxygen-17 magnetic resonance: in vivo detection of nascent mitochondrial water in animals breathing 17O2 enriched air. Soc Magn Reson Med. 1991:1031. [Google Scholar]
- Mateescu GD, Yvars GM, LaManna JC, Lust WD, Sudilovsky D. Oxygen-17 MRS: In vivo Evalution of water uptake and residence time in the mouse brain after injection of O-17 labelled water. Proc Inter Soc Magn Reson Med. 1990:1236. [Google Scholar]
- Mellon EA, Beesam RS, Elliott MA, Reddy R. Mapping of cerebral oxidative metabolism with MRI. Proc Natl Acad Sci U S A. 2010;107:11787–11792. doi: 10.1073/pnas.1006951107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintun MA, Raichle ME, Martin WR, Herscovitch P. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J Nucl Med. 1984;25:177–187. [PubMed] [Google Scholar]
- Pekar J, Ligeti L, Ruttner Z, Lyon RC, Sinnwell TM, van Gelderen P, Fiat D, Moonen CT, McLaughlin AC. In vivo measurement of cerebral oxygen consumption and blood flow using 17O magnetic resonance imaging. Magn Reson Med. 1991;21:313–319. doi: 10.1002/mrm.1910210217. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronen I, Merkle H, Ugurbil K, Navon G. Imaging of H217O distribution in the brain of a live rat by using proton-detected 17O MRI. Proc Natl Acad Sci U S A. 1998;95:12934–12939. doi: 10.1073/pnas.95.22.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjo BK. Brain energy metabolism. Wiley; New York: 1978. [Google Scholar]
- Ter-Pogossian MM, Eichling JO, Davis DO, Welch MJ. The measure in vivo of regional cerebral oxygen utilization by means of oxyhemoglobin labeled with radioactive oxygen-15. J Clin Invest. 1970;49:381–391. doi: 10.1172/JCI106247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelwall PE, Blackband SJ, Chen W. Field dependence of 17O T1, T2 and SNR - in vitro and in vivo studies at 4.7, 11 and 17.6 Tesla. Proc. Intl. Soc. Mag. Reson. Med; Toronto. 2003. p. 504. [Google Scholar]
- Wardlaw JM. Neuroimaging in acute ischaemic stroke: insights into unanswered questions of pathophysiology. J Intern Med. 2010;267:172–190. doi: 10.1111/j.1365-2796.2009.02200.x. [DOI] [PubMed] [Google Scholar]
- Zhang N, Zhu XH, Lei H, Ugurbil K, Chen W. Simplified methods for calculating cerebral metabolic rate of oxygen based on 17O magnetic resonance spectroscopic imaging measurement during a short 17O2 inhalation. J Cereb Blood Flow Metab. 2004a;24:840–848. doi: 10.1097/01.WCB.0000125885.54676.82. [DOI] [PubMed] [Google Scholar]
- Zhang NY, Zhu XH, Chen W. A Quantitative Model to Estimate Limited Permeability of Mitochondrial Membrane to Water in Brain. Proc. Intl. Soc. Mag. Reson. Med; Kyoto, Japan. 2004b. p. 73. [Google Scholar]
- Zhu XH, Chen W. In vivo oxygen-17 NMR for imaging brain oxygen metabolism at high field. Progress in NMR Spectroscopy. 2011;59:319–335. doi: 10.1016/j.pnmrs.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XH, Merkle H, Kwag JH, Ugurbil K, Chen W. 17O relaxation time and NMR sensitivity of cerebral water and their field dependence. Magn Reson Med. 2001;45:543–549. doi: 10.1002/mrm.1073. [DOI] [PubMed] [Google Scholar]
- Zhu XH, Zhang N, Zhang Y, Ugurbil K, Chen W. New insights into central roles of cerebral oxygen metabolism in the resting and stimulus-evoked brain. J Cereb Blood Flow Metab. 2009;29:10–18. doi: 10.1038/jcbfm.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XH, Zhang N, Zhang Y, Zhang X, Ugurbil K, Chen W. In vivo 17O NMR approaches for brain study at high field. NMR Biomed. 2005;18:83–103. doi: 10.1002/nbm.930. [DOI] [PubMed] [Google Scholar]
- Zhu XH, Zhang XL, Zhang NY, Zhang Y, Strupp J, Ugurbil K, Chen W. High-field 17O Study of 3D CMRO2 Imaging in human visual cortex. Proc. Intl. Soc. Mag. Reson. Med; Seattle. 2006. p. 409. [Google Scholar]
- Zhu XH, Zhang Y, Tian RX, Lei H, Zhang N, Zhang X, Merkle H, Ugurbil K, Chen W. Development of 17O NMR approach for fast imaging of cerebral metabolic rate of oxygen in rat brain at high field. Proc Natl Acad Sci U S A. 2002;99:13194–13199. doi: 10.1073/pnas.202471399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XH, Zhang Y, Wiesner H, Ugurbil K, Chen W. Estimation of CBF Based on the Metabolic H217O Decay Rate in CMRO2 Measurement using In Vivo 17O MR Approach. Proc. Intl. Soc. Mag. Reson. Med; Stockholm, Sweden. 2010. p. 716. [Google Scholar]
- Zhu XH, Zhang Y, Zhang N, Ugurbil K, Chen W. Noninvasive and three-dimensional imaging of CMRO2 in rats at 9.4 T: reproducibility test and normothermia/hypothermia comparison study. J Cereb Blood Flow Metab. 2007;27:1225–1234. doi: 10.1038/sj.jcbfm.9600421. [DOI] [PubMed] [Google Scholar]