Abstract
Cellular redox states can regulate cell metabolism, growth, differentiation, motility, apoptosis, signaling pathways, and gene expressions etc. Growing body of literature suggest importance of redox status for cancer progression. While most studies on redox state were done on cells and tissue lysates, it is important to understand the role of redox state in tissue in vivo/ex vivo and image its heterogeneity. Redox scanning is a clinically-translatable method for imaging tissue mitochondrial redox potential with a submillimeter resolution. Redox scanning data in mouse models of human cancers demonstrate a correlation between mitochondrial redox state and tumor metastatic potential. I will discuss the significance of this correlation and possible directions for future research.
Keywords: cancer aggressiveness, fluorescence, redox scanning, NADH, FAD or flavoprotein
Introduction
As a hallmark of cancer, abnormal metabolism has taken the center stage of research in recent years (Pedersen 2007; Christofk et al. 2008; Hsu and Sabatini 2008; Cairns et al. 2011; Hanahan and Weinberg 2011; Koppenol et al. 2011; Lisanti et al. 2011). Most cancers exhibit the Warburg effect – increased glucose consumption even in the presence of oxygen, on which FDG-PET (fluorine-18-2-D-deoxyglucose positron emission tomography) is based to stage tumors and monitor treatment response (Quon and Gambhir 2005; Mac Manus and Hicks 2008). In addition, mitochondrial bioenergetic/genetic abnormalities have been shown to mediate carcinogenesis and tumor progression (King et al. 2006; Modica-Napolitano et al. 2007; Mayevsky 2009; Kaelin and Thompson 2010). Genetic mutations have been identified in cancer patients for certain mitochondrial metabolic enzymes in the TCA cycle including isocitrate dehydrogenase, succinate dehydrogenase and fumarase (Thompson 2009). The expression of genes or activities of proteins known to drive tumor progression such as Myc/HIF1α/p53 have been shown to regulate cellular metabolism including mitochondrial metabolism (Dang 1999; Semenza 2010; Cairns et al. 2011). On the other hand, tumor microenvironment and metabolism may be upstream regulators of signaling pathways (Hsu and Sabatini 2008). Therefore, it has become increasingly important to understand the interwined relationship among tumor signaling pathways, metabolism, and microenvironment.
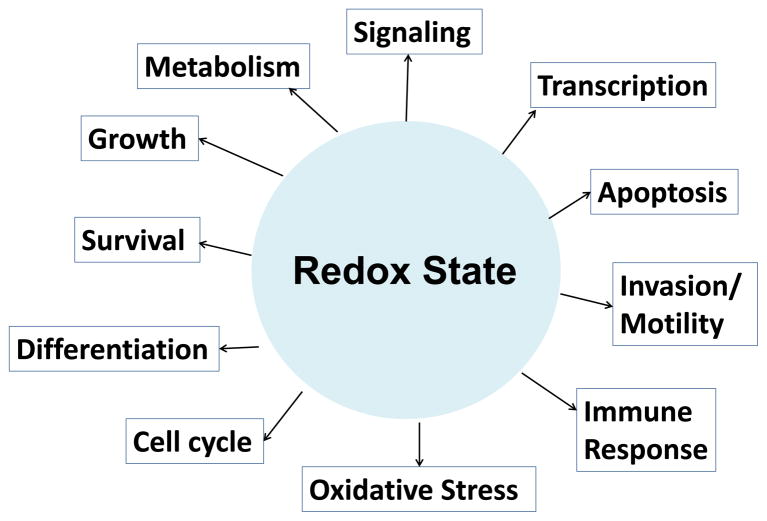
Maintenance of redox state homeostasis has been regarded as important for cancer cells (Dorward et al. 1997; Grek and Tew 2010; Cairns et al. 2011; Locasale and Cantley 2011). As a matter of fact, tremendous research studies (Puppi and Dely 1983; Dorward et al. 1997; Adler et al. 1999; Nkabyo et al. 2002; Weir et al. 2002; Cook et al. 2004; Olschewski et al. 2004; Agarwal and Auchus 2005; Ido 2007; Sattler et al. 2007; Banerjee 2008; Ying 2008; Gough 2009; Maccarrone and Brune 2009; Pani et al. 2009; Sarsour et al. 2009; Grek and Tew 2010; Ishimoto et al. 2011) have demonstrated or implicated redox state as a key mediator of many cellular functions and activities including metabolism, growth, differentiation, cell cycle, motility/invasion, apoptosis, survival, immunological response, oxidative stress, gene transcription, and signaling (Figure 1). Some studies have implied a connection between the redox potentials (or NADH levels) and the metastatic potential of cancers (Zhang et al. 2006; Ishikawa et al. 2008b; Pani et al. 2009; Pelicano et al. 2009; Grek and Tew 2010). Reactive oxygen species (ROS) are known to cause oxidative stress on proteins, lipids, DNA/RNAs and also act as signaling molecules to drive cancer cell motility/invasion and tumor progression. ROS can induce a higher risk of metastasis either by causing more DNA mutagenesis or regulating tumor progressions directly by enhancing cell invasion and metastasis. A mitochondrial DNA mutation encoding a subunit of NADH dehydrogenase (complex I) was shown to control the development of metastasis in animal models by generating more ROS, which, in turn, directly regulates certain nuclear genes that promote metastasis (Ishikawa et al. 2008a; Ishikawa et al. 2008b). However, high level of ROS or oxidants does not necessarily indicate more oxidized redox potential. It has been known that tumors with high levels of ROS are often counter balanced with high levels of reductants such as vitamin C, reduced glutathinone (GSH) and NADPH (Hyodo et al. 2006; Pelicano et al. 2009; Pani et al. 2010; Keshari et al. 2011). It is the balance between oxidants and reductants that define the cellular redox potential. Still redox potential is a complex issue due to multiple intracellular redox systems and their dependence on subcellular compartments (cytosol, nuclear, mitochondrion, etc.). Currently the relationship between cellular redox potential and cancer metastatic potential is far from clear.
Fig. 1.
Important roles of redox state in biology.
Most prior work on redox status was done on the molecular and cellular levels under in vitro conditions or on tissue lysates. To investigate the role of redox potential in tumor progression, it is necessary to image the redox status and its spatial distribution in tissue. The tissue heterogeneity in functional/metabolic/genomic status has been regarded as an important characteristic for malignancy (Gaustad et al. 2005; Schroeder et al. 2005; Gerlinger et al. 2012; Shah et al. 2012). Intra-tumor heterogeneity has been shown to be an important factor for studying tumor metastasis (Nowell 1976; Fidler and Kripke 1977; Fidler and Hart 1982). The heterogeneity in tumor metabolic microenvironment can occur on a small distance < 1mm (Mueller-Klieser et al. 1991; Li et al. 2009b; Xu et al. 2010). Therefore, effective sub-millimeter imaging methods are needed to measure the tumor redox state in vivo/ex vivo. Redox imaging on the basis of the fluorescence signals from NADH and flavoproteins is the only clinically-translatable method that can achieve 3D imaging of the tissue mitochondrial redox state at a submillimeter resolution.
In this mini-review we will cover some basic biological roles of NAD(H) and flavins, and the principles and methodology of mitochondrial redox imaging. We will then review the work studying the link of mitochondrial redox potential to tumor metastatic potential using the redox imaging. In the end, we will discuss the significance of these studies in terms of basic research and clinical management for cancer.
NAD(H), flavins and mitochondrial redox imaging
As universal free energy carriers in bioenergetics, NAD+ (oxidized nicotinamide adenine di-nucleotides) and NADH mediate a number of oxidation-reduction reactions along pathways of energy metabolism. By controlling glycolysis in cytosol and the Krebs cycle in mitochondria, the redox potential NAD+/NADH is linked to the phosphorylation potential [ATP]/([ADP]·[Pi]) in living tissues and provides a key parameter for the metabolic control of normal and diseased phenotypes (Veech 2006). In addition, NAD+/NADH is a key component in cellular redox homeostasis as NAD(H) is coupled to NADP(H) by transhydrogenase activity (Lemasters and Nieminen 2001) and, thus, can indirectly affect the oxidation-reduction couples of glutathione and thioredoxin systems as well (Banerjee 2008). These redox couples and related redox-sensitive enzymes may affect almost all major signaling pathways including p53, PI3K and MAPK (Adler et al. 1999; Olovnikov et al. 2009). Accumulating evidence has shown that NAD+ is also a key signaling molecule serving as a precursor to calcium-releasing agents and a substrate for protein modification of transcription factors by PARP (poly-ADP-ribosylation polymerase) (Banerjee 2008). NAD+ can mediate many cellular activities including signaling, reactive oxygen species (ROS) generation, growth, differentiation, survival, and apoptosis (Ziegler 2005; Orrenius et al. 2007; Ying 2008).
In addition to NAD(H), another group of redox-important molecules flavin nucleotides including flavin adenine dinucleotide (FAD) or flavin mononucleotide (FMN) also play important roles in various biological processes including metabolism and signaling events (Lehninger et al. 1993; Taylor et al. 2001; Senda et al. 2009; Becker et al. 2011) FAD or FMN are coenzymes or prosthetic groups for various flavoproteins including the NADH dehydrogenase (complex I) and pyruvate dehydrogenase in mitochondria. These flavoproteins are quite often coupled with NAD+/NADH. FADH2 is also a free energy carrier in electron transport and the FAD-coupled redox potential FAD/FADH2 regulates key reactions in the TCA cycle, oxidative phosphorylation, and fatty acid metabolism (Lehninger et al. 1993). Both FADH2 and NADH levels may regulate the ROS generation in mitochondria. Flavin prosthetic groups also induce redox-dependent conformational and functional changes in flavoproteins which are important for protein transcription, signaling pathways and environmental adaptation (Taylor et al. 2001; Senda et al. 2009; Becker et al. 2011).
The roles of NADH, flavins, and mitochondrial redox potential NAD+/NADH or FAD/FADH2 in tumor progression to metastasis are not clear. However, the fluorescence signals from these molecules (NADH and FAD) enable the development of the mitochondrial redox imaging, a useful tool to investigate these questions.
Dr. Chance and coworkers have pioneered in and made major contributions to using endogenous fluorescence signals of NADH and oxidized flavoproteins (Fp including FAD) to probe mitochondrial metabolic states in isolated mitochondria, intact cells and tissues ex vivo/in vivo since 1950s (Chance and Baltscheffsky 1958; Chance and Jobsis 1959; Chance et al. 1962; Chance 1966; Chance and Schoener 1966; Hassinen and Chance 1968; Chance et al. 1979; Mayevsky and Rogatsky 2007). When excited by UV light (~366nm), NADH emits fluorescence peaked at ~450nm. Oxidized flavoproteins have green fluorescence (~525nm) if excited by blue light (~425nm). The NAD and reduced flavoproteins do not have such fluorescence signals. These fluorescence signals mainly originate from the mitochondrial compartment (Scholz et al. 1969; Rocheleau et al. 2004; Nichols et al. 2005; Mayevsky and Rogatsky 2007; Blinova et al. 2008), and the indices e.g. NADH, Fp, Fp/NADH, NADH/(NADH+Fp), and Fp/(NADH+Fp) have been shown as sensitive to mitochondrial metabolism and redox state (Chance and Williams 1955a; Chance and Williams 1955b; Chance and Baltscheffsky 1958; Chance and Schoener 1966; Fisher et al. 1976; Chance et al. 1979; Masters et al. 1981; Mayevsky et al. 1983; Kitai et al. 1992; Sato et al. 1995). The ratiometirc quantities reduces their sensitivity to hemodynamic artifacts and mitochondrial densities (Chance et al. 1979; Li et al. 2009a).
To image tissue mitochondrial redox state at a high spatial resolution, redox scanning (Quistorff et al. 1985; Gu et al. 2002; Li et al. 2009a; Xu et al. 2009b) a cryogenic NADH/flavoproteins fluorescence imager was developed by the Chance laboratory to provide 3D maps (resolution 50×50×20 μm3) of mitochondrial redox status by acquiring ex vivo the NADH and Fp fluorescence images of frozen organs/tissues in liquid nitrogen. Its submillimeter spatial resolution is suitable for probing the spatial heterogeneity of the tissue metabolic state. Redox scanning employs snap-freezing procedures to maintain the tissue metabolic state same as or similar to the in vivo situation. Tissues mounted in a liquid nitrogen chamber are grounded away at various depths to expose surface planes for a flying-spot scanning with a fiberoptic probe. The fluorescence signal acquisition is time-shared between NADH and Fp channels and recorded by a photomultiplier tube (PMT). CCD-based cryogenic redox imager was also developed by replacing the scanning probe and PMT with a cryogenic microscope and CCD detection system (Li et al. 2009a; Ranji et al. 2009; Xu et al. 2009a). The CCD redox imager can acquire redox images of tissues much faster (tens of minutes versus seconds) and a higher spatial resolution (5 μm vs 50 μm) than the redox scanner.
Redox scanning has been extensively employed to study mitochondrial metabolism and redox state in normal tissues and diseases (Barlow et al. 1979; Mayevsky et al. 1983; Haselgrove et al. 1990; Kitai et al. 1992; Sato et al. 1995; Shiino et al. 1999; Ramanujam et al. 2001; Zhang et al. 2004a; Xu et al. 2010; Xu et al. 2011a; Xu et al. 2011b; Xu et al. 2011c). The redox ratio Fp/NADH of freeze-trapped liver mitochondria was shown to correlate with the oxidation-reduction state modulated by the β-hydroxybutyrate/acetoacetate couple (Chance et al. 1979). The Fp/NADH ratio of frozen liver samples from human subjects as measured by NADH-flavoprotein fluorescence imaging correlated linearly with the blood ketone body ratio (acetoacetate/β-hydroxybutyrate) (Ozawa et al. 1992), which might reflect the NAD+/NADH redox potential coupled via β-hydroxygutyrate dehydrogenase in the mitochondria.
Redox scanning may be applied to biopsy samples snap-frozen right after being removed from the body (Ramanujam et al. 2001; Xu et al. 2012). This diagnostic tool requires only a small tissue sample (~1mm×1mm×500 μm) to image. The remaining tissue samples can be further processed with histological assays and correlated with redox images. Another direction of research development is to employ two-photon fluorescence imaging of NADH and Fp (Ramanujan et al. 2005; Skala et al. 2007b) which has deeper tissue penetration depth than single photon fluorescence and can image the mitochondrial redox state in live tissues. But this approach is still limited by the tissue penetration depth of less than 1mm. This technique has been used to study the redox state in precancerous tissues in vivo in animal models (Skala et al. 2007a; Levitt et al. 2011) and can be used for deep tissues in patients if coupled with endoscope or optical biopsy needles (Brown et al. 2009; Zhu et al. 2009).
In summary, the NADH/Fp fluorescence imaging obtains the tissue redox state information which is specific to mitochondrial compartment and at a high spatial resolution. Although invasive, the redox scanning is the major method currently available for 3D imaging of the mitochondrial redox state in tissue with a wide field of view (~cm) and a submillimeter resolution. A number of other methods (Chung and Jue 1992; Mueller-Klieser and Walenta 1993; Matsumoto et al. 2006; Sattlar et al. 2007; Tisdall et al. 2007; Hyodo et al. 2008; Bohndiek et al. 2011; Keshari et al. 2011; Tachtsidis et al. 2011) may measure tissue redox status but they are limited by lack of specificity for redox couples or mitochondrial compartment or having low spatial resolutions. In recent years genetically-encoded redox-sensitive fluorescence proteins (Dooley et al. 2004; Gutscher et al. 2008; Hung et al. 2011) have been developed so that it is feasible to image the redox state with specificity for redox couples and subcellular compartments at a high spatial resolution. However, these approaches require the genetic transfection of cells with fluorescence proteins and clinical translations would be difficult.
Mitochondrial redox state linked to tumor metastatic potential
Mitochondrial redox imaging using NADH/Fp fluorescences has been extensively used in cancer studies (Drezek et al. 2001; Ramanujam et al. 2001; Zhang et al. 2004b; Li et al. 2007; Li et al. 2009a; Xu et al. 2010; Liu et al. 2011; Xu et al. 2011b; Xu et al. 2011c). By using the redox scanner we have demonstrated in mouse models that mitochondrial redox imaging indices may potentially provide sensitive biomarkers for tumor metastatic potential. To date we have not found studies other than our own work to probe the connection between mitochondrial redox state of primary tumors and the risk of metastasis or tumor metastatic potential.
We first conducted a redox scanning study on mouse xenografts of five human melanoma cell lines (Li et al. 2007; Li et al. 2009b). These cell lines have been well characterized in vitro and in vivo regarding their aggressiveness. The invasive potentials of these cell lines as measured by the Boyden chamber method fall in the increasing rank order A375P<A375M<A375P10<A375P5<C8161. The amount of lung metastases of these cell lines in experimental metastasis mouse models have a rank order A375P<A375P5<A375P10<A375M<C8161 (Li et al. 2009a). We implanted these melanoma cells subcutaneously in athymic nude mice and grew tumors within a few weeks. Tumors were then subject to in situ snap-freezing by liquid nitrogen and then excised for redox scanning to obtain multi-slice images at different tissue depths with an in-plane resolution of 80 μm. Significant tissue heterogeneity were identified in these redox images, i.e., NADH, Fp and Fp redox ratio. The aggressive tumors exhibited significant difference between the tumor core and rim, with the cores having higher Fp, low NADH and higher Fp redox ratio. In comparison, the indolent A375P tumors were largely uniform except sporadic “hot” spots with high Fp redox ratios. We found that the Fp redox ratio could differentiate five human melanoma mouse xenografts spanning a full range of aggressiveness. The more metastatic melanomas exhibited a localized area (the tumor core) with higher Fp redox ratios (more oxidized) than the less metastatic melanomas. A highly significant correlation (R2=0.97, p=0.002) was obtained between the Fp redox ratios of tumor cores and the invasive potentials of the corresponding cell lines that were measured by the Boyden chamber method. In comparison, the redox ratios averaged over the whole tumor sections have a less significant correlation (R2=0.63, p=0.1) with the invasive potentials. The redox ratios of the whole-section average can only predict the differences between extremes, i.e., A375P versus other more metastatic xenografts, but not among A375P5, A375P10, A375M and C8161 melanomas (data not published).
We then studied mouse xenografts of two human breast cancer cell lines, the more metastatic MDA-MB-231 line and the less metastatic or indolent MCF-7 line (Xu et al. 2010). Redox scanning has again identified oxidized cores in aggressive tumors and a relatively uniform distribution in the indolent tumors. The redox imaging biomarkers (NADH in the rim, Fp and Fp redox ratio in the core) can readily differentiate between these two types of xenografts with very high statistical significance (p<0.0001). The average redox indices of whole tumor sections do not show statistically significant difference between the two lines, demonstrating the importance of imaging tumor heterogeneity. Furthermore, we have preliminarily observed heterogeneities in mitochondrial redox indices in tumor biopsy samples from breast cancer patients and the redox index differences between cancerous and normal tissues (Xu et al. 2012). The redox index differences between tumor core and rim have also been observed in prostate cancer mouse xenografts as well (Cai et al. 2012).
Note that the more oxidized Fp redox ratio does not necessarily correlate with the high tumor growth rate. In melanoma xenografts of five lines aforementioned, the more invasive ones have faster tumor growth. But the more metastatic MDA-MB-231 breast tumors grow more slowly than the MCF-7 tumors.
Another interesting question is about the metabolic state of cancer cells in the tumor cores. Previously Chance and coworkers (Chance and Williams 1955a; Chance and Williams 1955b; Chance and Baltscheffsky 1958; Chance 1966) have defined 5 metabolic states for mitochondria under different conditions of oxygen, ADP and substrate availability. State 1–4 have adequate oxygen. With low levels of endogeneous substrates and ADP, state 1 represents low levels of oxidative metabolism accompanied by high NADH and low Fp, i.e., low Fp redox ratio. State 2 corresponds to mitochondria starved of substrate but having adequate ADP; this state exhibits low respiratory activity with high levels of Fp and low levels of NADH, i.e., high Fp redox ratio. The State 3 corresponds to adequate levels of substrate and ADP and, hence, high levels of oxidative metabolism. This condition is also indicative of a high Fp redox ratio, a high Fp but not as high as State 2, and a low NADH but not as low as State 2. State 4, typically a state at rest with adequate supplies of substrate but low ADP, exhibits low mitochondrial respiratory activity with a low Fp redox ratio. State 5 corresponds to anoxic conditions with maximum NADH, lowest Fp redox ratio and minimum (~0) respiration rate. In our melanoma xenograft studies, we found the aggressive melanoma (C8161) had a lower blood perfusion and/or vessel permeability in the tumor core compared to rim or to the indolent melanoma (A375P) based on the measurements of dynamic contrast enhanced MRI (DCE-MRI) in vivo. We also found lower microvasculature patency in the aggressive tumor cores compared to the corresponding rim according to Hoechst dye staining of histological tissue sections. These results indicate a possible substrate starvation (State 2) in the aggressive tumor cores with a high Fp redox ratio.
Although the H&E staining of aggressive tumors often appear morphologically different (more reddish) in the core than in the rim, the tumor cores (not necessarily in the geometrical center) with oxidized redox state may not be regarded as complete necrotic centers for the following several reasons: 1) The tumor regions with comparable high redox ratios were also observed in tumors of small size (4~6mm in diameter) which are less likely to develop necrotic centers (data not published); 2) MRI of these melanomas and breast tumors seldom showed hyperintensity on T1-weighted images, a feature commonly observed for necrotic tissues (data not published); 3) Another MRI imaging technique sensitive to tissue necrosis, i.e., magnetization transfer MRI indicated viable tissues in some aggressive breast tumor cores with oxidized redox states (data not published); 4) Microscopic observation of melanoma tissue slides stained with DAPI and TUNEL indicated existence of viable cells with intact nuclei (DAPI) and low apoptosis in those core regions (Xu et al. 2009c); 5) Fluorescence imaging of the pyro-2-deoxy-glucose uptake in MDA-MB-231 tumors indicates heterogeneity in the cores with some areas having high glucose uptake and thus probably not substrate limited (Xu et al. 2011c). The high redox ratio for those regions might indicate high respiratory activities (State 3). Based on all these observations, it is quite possible that the biological states and micro-environment of those cancer cells in the oxidized tumor cores are heterogeneous spatially and temporally. More investigations are needed to understand the significance of these heterogeneities.
The existence of viable cells in the aggressive tumor cores possibly under inhospitable environment such as starvation led to the hypothesis that those cancer cells may be in the state of autophagy. Autophagy may facilitate the survival of cancer cells under environmental/nutritional stresses and is expected to increase the success rate of metastasis (Lum et al. 2005; Amaravadi et al. 2007). In collaboration with Julian Lum, Ravi Amaravadi and Xiaohong Ma et al., we demonstrated that the more invasive/aggressive melanomas exhibit higher autophagy than less invasive/aggressive melanoma in 3D cell spheroids and mouse xengorafts, higher autophagy indices predict shorter progression-free survival and overall survival in melanoma patients, and high autophagy activity predicts drug resistance in melanomas (Ma et al. 2011). Further research is needed to investigate the link between mitochondrial redox state, autophagy and tumor metastatic potential.
Significance
Although the underlying mechanism is not clear, our redox scanning studies on cancer mouse models indicate a possibly fundamental connection between mitochondrial redox state and tumor progression to metastasis. Our work also indicates the importance of characterizing the tumor redox state heterogeneity by high resolution imaging methods to predict tumor aggressiveness. It is the redox ratios in the oxidized tumor cores not the whole tumor average that can differentiate better between aggressive and indolent tumors.
With the results across cancer types, we may wonder whether mitochondrial redox potential can be a general mediator in tumor progression to metastasis. As the Warburg effect has been demonstrated as a hallmark in majority of cancers, further studies about the role of mitochondrial redox state in tumor progression to metastasis might open new pages in cancer research.
On the other hand, redox imaging biomarkers can be useful for clinical cancer management. A major challenge in cancer research is to develop surrogate biomarkers for the risk of tumor metastasis. With ~90% cancer patients die of tumor metastasis, the prognosis of cancer patients is largely determined by the risk of metastasis rather than the tumor size. Compared with other biomarkers that may only provide binary differentiation of malignant from benign lesions, the “scaling” of neoplasia on the basis of the redox imaging indices has an advantage of providing continuously quantitative biomarkers for tumor metastatic potential, which, if successfully established, are expected to facilitate personalized clinical cancer management.
Acknowledgments
The author would like to acknowledge the valuable discussion with Dr. He N. Xu during the manuscript preparation, and the grant support from Susan G. Komen Research Foundation (KG081069) and National Institute of Health (R01CA155348).
References
- Adler V, Yin ZM, Tew KD, Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18(45):6104–6111. doi: 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- Agarwal AK, Auchus RJ. Minireview: Cellular redox state regulates hydroxysteroid dehydrogenase activity and intracellular hormone potency. Endocrinology. 2005;146(6):2531–2538. doi: 10.1210/en.2005-0061. [DOI] [PubMed] [Google Scholar]
- Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, Thomas-Tikhonenko A, Thompson CB. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117(2):326–336. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R. Redox Biochemistry. Hoboken, New Jersey: John Wiley & Sons; 2008. [Google Scholar]
- Barlow CH, Harden WR, 3rd, Harken AH, Simson MB, Haselgrove JC, Chance B, O’Connor M, Austin G. Fluorescence mapping of mitochrondrial redox changes in heart and brain. Crit Care Med. 1979;7(9):402–406. doi: 10.1097/00003246-197909000-00011. [DOI] [PubMed] [Google Scholar]
- Becker DF, Zhu W, Moxley MA. Flavin Redox Switching of Protein Functions. Antioxidants & Redox Signaling. 2011;14(6):1079–1091. doi: 10.1089/ars.2010.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinova K, Levine RL, Boja ES, Griffiths GL, Shi ZD, Ruddy B, Balaban RS. Mitochondrial NADH fluorescence is enhanced by Complex I binding. Biochemistry. 2008;47(36):9636–9645. doi: 10.1021/bi800307y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohndiek SE, Kettunen MI, Hu D-e, Kennedy BWC, Boren J, Gallagher FA, Brindle KM. Hyperpolarized [1-13C]-Ascorbic and Dehydroascorbic Acid: Vitamin C as a Probe for Imaging Redox Status in Vivo. Journal of the American Chemical Society. 2011;133(30):11795–11801. doi: 10.1021/ja2045925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JQ, Wilke LG, Geradts J, Kennedy SA, Palmer GM, Ramanujam N. Quantitative optical spectroscopy: a robust tool for direct measurement of breast cancer vascular oxygenation and total hemoglobin content in vivo. Cancer Res. 2009;69(7):2919–2926. doi: 10.1158/0008-5472.CAN-08-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Cai K, *Xu HN, Singh A, Haris M, Reddy R, Li LZ, (*Equal contribution) Characterizing prostate tumor mouse xenografts with CEST & MT MRI and redox scanning. Adv Exp Med Biol. 2012;765:39–45. doi: 10.1007/978-1-4614-4989-8_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. [10.1038/nrc2981] Nat Rev Cancer. 2011;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Chance B. Spectrophotometric and kinetic studies of flavoproteins in tissues, cell suspensions, mitochondria and their fragments. In: Slater EC, editor. Flavins and Flavoproteins. Amsterdam: Elsevier; 1966. pp. 498–510. [Google Scholar]
- Chance B, Baltscheffsky H. Respiratory Enzymes in Oxidative Phosphorylation.7. Binding of Intramitochondrial Reduced Pyridine Nucleotide. Journal of Biological Chemistry. 1958;233(3):736–739. [PubMed] [Google Scholar]
- Chance B, Cohen P, Jobsis F, Schoener B. Intracellular oxidation-reduction states in vivo. Science. 1962;137:499–508. doi: 10.1126/science.137.3529.499. [DOI] [PubMed] [Google Scholar]
- Chance B, Jobsis F. Changes in Fluorescence in a Frog Sartorius Muscle Following a Twitch. Nature. 1959;184(4681):195–196. [Google Scholar]
- Chance B, Schoener B. Fluorometric studies of flavin component of the respiratory chain. In: Slater EC, editor. Flavins and Flavoproteins. Amsterdam: Elsevier; 1966. pp. 510–519. [Google Scholar]
- Chance B, Schoener B, Oshino R, Itshak F, Nakase Y. Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples: NADH and flavoprotein fluorescence signals. J Biol Chem. 1979;254(11):4764–4771. [PubMed] [Google Scholar]
- Chance B, Williams GR. A method for the localization of sites for oxidative phosphorylation. Nature. 1955a;176(4475):250–254. doi: 10.1038/176250a0. [DOI] [PubMed] [Google Scholar]
- Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. III. The steady state. Journal of Biological Chemistry. 1955b;217(1):409–428. [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. [10.1038/nature06734] Nature. 2008;452(7184):230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- Chung Y, Jue T. 1H NMR observation of redox potential in liver. Biochemistry. 1992;31(45):11159–11165. doi: 10.1021/bi00160a029. [DOI] [PubMed] [Google Scholar]
- Cook JA, Gius D, Wink DA, Krishna MC, Russo A, Mitchell JB. Oxidative stress, redox, and the tumor microenvironment. Seminars in Radiation Oncology. 2004;14(3):259–266. doi: 10.1016/j.semradonc.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Dang CV. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol. 1999;19:1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. Journal of Biological Chemistry. 2004;279(21):22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- Dorward A, Sweet S, Moorehead R, Singh G. Mitochondrial contributions to cancer cell physiology: Redox balance, cell cycle, and drug resistance. Journal of Bioenergetics and Biomembranes. 1997;29(4):385–392. doi: 10.1023/a:1022454932269. [DOI] [PubMed] [Google Scholar]
- Drezek R, Brookner C, Pavlova I, Boiko I, Malpica A, Lotan R, Follen M, Richards-Kortum R. Autofluorescence microscopy of fresh cervical-tissue sections reveals alterations in tissue biochemistry with dysplasia. Photochemistry and Photobiology. 2001;73(6):636–641. doi: 10.1562/0031-8655(2001)073<0636:AMOFCT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Fidler IJ, Hart IR. Biological Diversity in Metastatic Neoplasms - Origins and Implications. Science. 1982;217(4564):998–1003. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- Fidler IJ, Kripke ML. Metastasis Results from Preexisting Variant Cells within a Malignant-Tumor. Science. 1977;197(4306):893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- Fisher AB, Furia L, Chance B. Evaluation of redox state of isolated perfused rat lung. Am J Physiol. 1976;230(5):1198–1204. doi: 10.1152/ajplegacy.1976.230.5.1198. [DOI] [PubMed] [Google Scholar]
- Gaustad JV, Benjaminsen IC, Graff BA, Brurberg KG, Ruud EBM, Rofstad EK. Intratumor heterogeneity in blood perfusion in orthotopic human melanoma xenografts assessed by dynamic contrast-enhanced magnetic resonance imaging. Journal of Magnetic Resonance Imaging. 2005;21(6):792–800. doi: 10.1002/jmri.20321. [DOI] [PubMed] [Google Scholar]
- Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. New England Journal of Medicine. 2012;366(10):883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough NR. Focus Issue: The Long and Short of Redox Signaling. Sci Signal. 2009;2(90):eg12. [Google Scholar]
- Grek CL, Tew KD. Redox metabolism and malignancy. Current Opinion in Pharmacology. 2010;10(4):362–368. doi: 10.1016/j.coph.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Qian Z, Chen J, Blessington D, Ramanujam N, Chance B. High-resolution three-dimensional scanning optical image system for intrinsic and extrinsic contrast agents in tissue. Review of Scientific Instruments. 2002;73(1):172–178. [Google Scholar]
- Gutscher M, Pauleau AL, Marty L, Brach T, Wabnitz GH, Samstag Y, Meyer AJ, Dick TP. Real-time imaging of the intracellular glutathione redox potential. Nature Methods. 2008;5(6):553–559. doi: 10.1038/nmeth.1212. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Haselgrove JC, Bashford CL, Barlow CH, Quistorff B, Chance B, Mayevsky A. Time resolved 3-dimensional recording of redox ratio during spreading depression in gerbil brain. Brain Res. 1990;506(1):109–114. doi: 10.1016/0006-8993(90)91205-u. [DOI] [PubMed] [Google Scholar]
- Hassinen I, Chance B. Oxidation-reduction properties of the mitochondrial flavoprotein chain. Biochem Biophys Res Commun. 1968;31(6):895–900. doi: 10.1016/0006-291x(68)90536-6. [DOI] [PubMed] [Google Scholar]
- Hsu PP, Sabatini DM. Cancer Cell Metabolism: Warburg and Beyond. Cell. 2008;134(5):703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Hung Yin P, Albeck John G, Tantama M, Yellen G. Imaging Cytosolic NADH-NAD+ Redox State with a Genetically Encoded Fluorescent Biosensor. Cell Metabolism. 2011;14(4):545–554. doi: 10.1016/j.cmet.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo F, Matsumoto K, Matsumoto A, Mitchell JB, Krishna MC. Probing the intracellular redox status of tumors with magnetic resonance imaging and redox-sensitive contrast agents. Cancer Research. 2006;66(20):9921–9928. doi: 10.1158/0008-5472.CAN-06-0879. [DOI] [PubMed] [Google Scholar]
- Hyodo F, Murugesan R, Matsumoto K, Hyodo E, Subramanian S, Mitchell JB, Krishna MC. Monitoring redox-sensitive paramagnetic contrast agent by EPRI, OMRI and MRI. Journal of Magnetic Resonance. 2008;190(1):105–112. doi: 10.1016/j.jmr.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ido Y. Pyridine nucleotide redox abnormalities in diabetes. Antioxidants & Redox Signaling. 2007;9(7):931–942. doi: 10.1089/ars.2007.1630. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Koshikawa N, Takenaga K, Nakada K, Hayashi JI. Reversible regulation of metastasis by ROS-generating mtDNA mutations. Mitochondrion. 2008a;8(4):339–344. doi: 10.1016/j.mito.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008b;320(5876):661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 Variant Regulates Redox Status in Cancer Cells by Stabilizing the xCT Subunit of System xc and Thereby Promotes Tumor Growth. Cancer Cell. 2011;19(3):387–400. doi: 10.1016/j.ccr.2011.01.038. [DOI] [PubMed] [Google Scholar]
- Kaelin WG, Thompson CB. Q&A: Cancer: Clues from cell metabolism. Nature. 2010;465(7298):562–564. doi: 10.1038/465562a. [DOI] [PubMed] [Google Scholar]
- Keshari KR, Kurhanewicz J, Bok R, Larson PEZ, Vigneron DB, Wilson DM. Hyperpolarized (13)C dehydroascorbate as an endogenous redox sensor for in vivo metabolic imaging. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(46):18606–18611. doi: 10.1073/pnas.1106920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene. 2006;25(34):4675–4682. doi: 10.1038/sj.onc.1209594. [DOI] [PubMed] [Google Scholar]
- Kitai T, Tanaka A, Tokuka A, Ozawa K, Iwata S, Chance B. Changes in the redox distribution of rat liver by ischemia. Anal Biochem. 1992;206(1):131–136. doi: 10.1016/s0003-2697(05)80022-3. [DOI] [PubMed] [Google Scholar]
- Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. [10.1038/nrc3038] Nat Rev Cancer. 2011;11(5):325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- Lehninger AL, Nelson DL, Cox MM. Principles of Biochemistry. 2. New York: Worth Publishers; 1993. [Google Scholar]
- Lemasters JJ, Nieminen AL. Mitochondria in Pathogenesis. New York: Kluwer Academic/Plenum Publishers; 2001. [Google Scholar]
- Levitt JM, McLaughlin-Drubin ME, Munger K, Georgakoudi I. Automated Biochemical, Morphological, and Organizational Assessment of Precancerous Changes from Endogenous Two-Photon Fluorescence Images. Plos One. 2011;6(9) doi: 10.1371/journal.pone.0024765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LZ, Xu HN, Ranji M, Nioka S, Chance B. Mitochondrial redox imaging for cancer diagnostic and therapeutic studies. Journal of Innovative Optical Health Sciences. 2009a;2:325–341. doi: 10.1142/S1793545809000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LZ, Zhou R, Xu HN, Moon L, Zhong TX, Kim EJ, Qiao H, Reddy R, Leeper D, Chance B, Glickson JD. Quantitative magnetic resonance and optical imaging biomarkers of melanoma metastatic potential. Proceedings of the National Academy of Sciences of the United States of America. 2009b;106(16):6608–6613. doi: 10.1073/pnas.0901807106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LZJ, Zhou R, Zhong TX, Moon L, Kim EJ, Qiao H, Pickup S, Hendrix MJ, Leeper D, Chance B, Glickson JD. Predicting melanoma metastatic potential by optical and magnetic resonance imaging. In: Maguire DJ, Bruley DF, Harrison DK, editors. Oxygen Transport to Tissue Xxviii. Vol. 599. Berlin: Springer-Verlag Berlin; 2007. pp. 67–78. Advances in Experimental Medicine and Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti MP, Martinez-Outschoorn UE, Lin Z, Pavlides S, Whitaker-Menezes D, Pestell RG, Howell A, Sotgia F. Hydrogen peroxide fuels aging, inflammation, cancer metabolism and metastasis The seed and soil also needs “fertilizer”. Cell Cycle. 2011;10(15):2440–2449. doi: 10.4161/cc.10.15.16870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Grant G, Li JJ, Zhang Y, Hu FY, Li SQ, Wilson C, Chen K, Bigner D, Tuan VD. Compact point-detection fluorescence spectroscopy system for quantifying intrinsic fluorescence redox ratio in brain cancer diagnostics. Journal of Biomedical Optics. 2011;16(3) doi: 10.1117/1.3558840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale Jason W, Cantley Lewis C. Metabolic Flux and the Regulation of Mammalian Cell Growth. Cell Metabolism. 2011;14(4):443–451. doi: 10.1016/j.cmet.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120(2):237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Ma X-H, Piao S, Wang D, McAfee QW, Nathanson KL, Lum JJ, *Li LZ, *Amaravadi RK, (Equal contribution) Measurements of tumor cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clinical Cancer Research. 2011;17(10):3478–3489. doi: 10.1158/1078-0432.CCR-10-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Manus M, Hicks RJ. The Use of Positron Emission Tomography (PET) in the Staging/Evaluation, Treatment, and Follow-up of Patients with Lung Cancer: a Critical Review. International Journal of Radiation Oncology Biology Physics. 2008;72(5):1298–1306. doi: 10.1016/j.ijrobp.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Brune B. Redox regulation in acute and chronic inflammation. Cell Death Differ. 2009;16(8):1184–1186. doi: 10.1038/cdd.2009.65. [DOI] [PubMed] [Google Scholar]
- Masters BR, Falk S, Chance B. In vivo flavoprotein redox measurements of rabbit corneal normoxic-anoxic transitions. Curr Eye Res. 1981;1(10):623–627. doi: 10.3109/02713688109001865. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Hyodo F, Matsumoto A, Koretsky AP, Sowers AL, Mitchell JB, Krishna MC. High-resolution mapping of tumor redox status by magnetic resonance imaging using nitroxides as redox-sensitive contrast agents. Clinical Cancer Research. 2006;12(8):2455–2462. doi: 10.1158/1078-0432.CCR-05-2747. [DOI] [PubMed] [Google Scholar]
- Mayevsky A. Mitochondrial function and energy metabolism in cancer cells: Past overview and future perspectives. Mitochondrion. 2009;9(3):165–179. doi: 10.1016/j.mito.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Mayevsky A, Rogatsky GG. Mitochondrial function in vivo evaluated by NADH fluorescence: from animal models to human studies. Am J Physiol Cell Physiol. 2007;292(2):C615–640. doi: 10.1152/ajpcell.00249.2006. [DOI] [PubMed] [Google Scholar]
- Mayevsky A, Zarchin N, Kaplan H, Haveri J, Haselgroove J, Chance B. Brain metabolic responses to ischemia in the mongolian gerbil: in vivo and freeze trapped redox scanning. Brain Res. 1983;276(1):95–107. doi: 10.1016/0006-8993(83)90551-6. [DOI] [PubMed] [Google Scholar]
- Modica-Napolitano JS, Kulawiec M, Singh KK. Mitochondria and human cancer. Current Molecular Medicine. 2007;7(1):121–131. doi: 10.2174/156652407779940495. [DOI] [PubMed] [Google Scholar]
- Mueller-Klieser W, Kroeger M, Walenta S, Rofstad EK. Comparative Imaging of Structure and Metabolites in Tumors. Int J Radiat Biol. 1991;60(1–2):147–159. doi: 10.1080/09553009114551741. [DOI] [PubMed] [Google Scholar]
- Mueller-Klieser W, Walenta S. Geographical mapping of metabolites in biological tissue with quantitative bioluminescence and single photon imaging. The Histochemical Journal. 1993;25(6):407–420. doi: 10.1007/BF00157805. [DOI] [PubMed] [Google Scholar]
- Nichols MG, Barth EE, Nichols JA. Reduction in DNA Synthesis During Two-photon Microscopy of Intrinsic Reduced Nicotinamide Adenine Dinucleotide Fluorescence. Photochemistry and Photobiology. 2005;81(2):259–269. doi: 10.1562/2004-08-05-RA-263. [DOI] [PubMed] [Google Scholar]
- Nkabyo YS, Ziegler TR, Gu LH, Watson WH, Jones DP. Glutathione and thioredoxin redox during differentiation in human colon epithelial (Caco-2) cells. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2002;283(6):G1352–G1359. doi: 10.1152/ajpgi.00183.2002. [DOI] [PubMed] [Google Scholar]
- Nowell PC. Clonal Evolution of Tumor-Cell Populations. Science. 1976;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Olovnikov IA, Kravchenko JE, Chumakova PM. Homeostatic functions of the p53 tumor suppressor: Regulation of energy metabolism and antioxidant defense. Seminars in Cancer Biology. 2009;19(1):32–41. doi: 10.1016/j.semcancer.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olschewski A, Hong ZG, Peterson DA, Nelson DP, Porter VA, Weir EK. Opposite effects of redox status on membrane potential, cytosolic calcium, and tone in pulmonary arteries and ductus arteriosus. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2004;286(1):L15–L22. doi: 10.1152/ajplung.00372.2002. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Gogvadze A, Zhivotovsky B. Mitochondrial oxidative stress: Implications for cell death. Annual Review of Pharmacology and Toxicology. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Chance B, Tanaka A, Iwata S, Kitai T, Ikai I. Linear Correlation between Acetoacetate Beta-Hydroxybutyrate in Arterial Blood and Oxidized Flavoprotein Reduced Pyridine-Nucleotide in Freeze-Trapped Human Liver-Tissue. Biochimica Et Biophysica Acta. 1992;1138(4):350–352. doi: 10.1016/0925-4439(92)90014-e. [DOI] [PubMed] [Google Scholar]
- Pani G, Galeotti T, Chiarugi P. Metastasis: cancer cell’s escape from oxidative stress. Cancer Metastasis Rev. 2010;29:351–378. doi: 10.1007/s10555-010-9225-4. [DOI] [PubMed] [Google Scholar]
- Pani G, Giannoni E, Galeotti T, Chiarugi P. Redox-Based Escape Mechanism from Death: The Cancer Lesson. Antioxidants & Redox Signaling. 2009;11:2791–2806. doi: 10.1089/ars.2009.2739. [DOI] [PubMed] [Google Scholar]
- Pedersen PL. Warburg, me and hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the “Warburg Effect”, i.e., elevated glycolysis in the presence of oxygen. Journal of Bioenergetics and Biomembranes. 2007;39(3):211–222. doi: 10.1007/s10863-007-9094-x. [DOI] [PubMed] [Google Scholar]
- Pelicano H, Lu W, Zhou Y, Zhang W, Chen Z, Hu Y, Huang P. Mitochondrial Dysfunction and Reactive Oxygen Species Imbalance Promote Breast Cancer Cell Motility through a CXCL14-Mediated Mechanism. Cancer Research. 2009;69(6):2375–2383. doi: 10.1158/0008-5472.CAN-08-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppi A, Dely M. Tissue redox-state potential (E0) -- As regulator of physiological processes. Acta Biologica Hungarica. 1983;34:323–350. [PubMed] [Google Scholar]
- Quistorff B, Haselgrove JC, Chance B. High resolution readout of 3-D metabolic organ structure: An automated, low-temperature redox ratio-scanning instrument. Anal Biochem. 1985;148:389–400. doi: 10.1016/0003-2697(85)90244-1. [DOI] [PubMed] [Google Scholar]
- Quon A, Gambhir SS. FDG-PET and Beyond: Molecular Breast Cancer Imaging. J Clin Oncol. 2005;23(8):1664–1673. doi: 10.1200/JCO.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Ramanujam N, Richards-Kortum R, Thomsen S, Mahadevan-Jansen A, Follen M, Chance B. Low Temperature Fluorescence Imaging of Freeze-trapped Human Cervical Tissues. Opt Express. 2001;8(6):335–343. doi: 10.1364/oe.8.000335. [DOI] [PubMed] [Google Scholar]
- Ramanujan VK, Zhang JH, Biener E, Herman B. Multiphoton fluorescence lifetime contrast in deep tissue imaging: prospects in redox imaging and disease diagnosis. Journal of Biomedical Optics. 2005;10(5) doi: 10.1117/1.2098753. [DOI] [PubMed] [Google Scholar]
- Ranji M, Nioka S, Xu N, Wu B, Li LZ, Jaggard DL, Chance B. Fluorescent images of mitochondrial redox states in in situ mouse hypoxic ischemic intestines. Journal of International Optical Health Sciences. 2009;2:365–374. [Google Scholar]
- Rocheleau JV, Head WS, Piston DW. Quantitative NAD(P)H/flavoprotein autofluorescence imaging reveals metabolic mechanisms of pancreatic islet pyruvate response. J Biol Chem. 2004;279(30):31780–31787. doi: 10.1074/jbc.M314005200. [DOI] [PubMed] [Google Scholar]
- Sarsour EH, Kumar MG, Chaudhuri L, Kalen AL, Goswami PC. Redox Control of the Cell Cycle in Health and Disease. Antioxid Redox Signal. 2009;11:2985–3011. doi: 10.1089/ars.2009.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato B, Tanaka A, Mori S, Yanabu N, Kitai T, Tokuka A, Inomoto T, Iwata S, Yamaoka Y, Chance B. Quantitative analysis of redox gradient within the rat liver acini by fluorescence images: effects of glucagon perfusion. Biochim Biophys Acta. 1995;1268(1):20–26. doi: 10.1016/0167-4889(95)00035-q. [DOI] [PubMed] [Google Scholar]
- Sattlar UGA, Walenta S, Mueller-Klieser W. A bioluminescence technique for quantitative and structure-associated imaging of pyruvate. Laboratory Investigation. 2007;87(1):84–92. doi: 10.1038/labinvest.3700493. [DOI] [PubMed] [Google Scholar]
- Sattler UGA, Walenta S, Mueller-Klieser W. Lactate and redox status in malignant tumors. Anaesthesist. 2007;56(5):466–469. doi: 10.1007/s00101-007-1164-2. [DOI] [PubMed] [Google Scholar]
- Scholz R, Thurman RG, Williamson JR, Chance B, Bucher T. Flavin and Pyridine Nucleotide Oxidation-Reduction Changes in Perfused Rat Liver. I. ANOXIA AND SUBCELLULAR LOCALIZATION OF FLUORESCENT FLAVOPROTEINS. J Biol Chem. 1969;244(9):2317–2324. [PubMed] [Google Scholar]
- Schroeder T, Yuan H, Viglianti BL, Peltz C, Asopa S, Vujaskovic Z, Dewhirst MW. Spatial heterogeneity and oxygen dependence of glucose consumption in R3230Ac and fibrosarcomas of the Fischer 344 rat. Cancer Research. 2005;65(12):5163–5171. doi: 10.1158/0008-5472.CAN-04-3900. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Current Opinion in Genetics & Development. 2010;20(1):51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senda T, Senda M, Kimura S, Ishida T. Redox Control of Protein Conformation in Flavoproteins. Antioxidants & Redox Signaling. 2009;11(7):1741–1766. doi: 10.1089/ars.2008.2348. [DOI] [PubMed] [Google Scholar]
- Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, Turashvili G, Ding J, Tse K, Haffari G, Bashashati A, Prentice LM, Khattra J, Burleigh A, Yap D, Bernard V, McPherson A, Shumansky K, Crisan A, Giuliany R, Heravi-Moussavi A, Rosner J, Lai D, Birol I, Varhol R, Tam A, Dhalla N, Zeng T, Ma K, Chan SK, Griffith M, Moradian A, Cheng SWG, Morin GB, Watson P, Gelmon K, Chia S, Chin S-F, Curtis C, Rueda OM, Pharoah PD, Damaraju S, Mackey J, Hoon K, Harkins T, Tadigotla V, Sigaroudinia M, Gascard P, Tlsty T, Costello JF, Meyer IM, Eaves CJ, Wasserman WW, Jones S, Huntsman D, Hirst M, Caldas C, Marra MA, Aparicio S. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012 doi: 10.1038/nature10933. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiino A, Haida M, Beauvoit B, Chance B. Three-dimensional redox image of the normal gerbil brain. Neuroscience. 1999;91(4):1581–1585. doi: 10.1016/s0306-4522(98)00670-8. [DOI] [PubMed] [Google Scholar]
- Skala MC, Riching KM, Gendron-Fitzpatrick A, Eickhoff J, Eliceiri KW, White JG, Ramanujam N. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. [Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, Non-P.H.S.] Proc Natl Acad Sci U S A. 2007a;104(49):19494–19499. doi: 10.1073/pnas.0708425104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skala MC, Riching KM, Gendron-Fitzpatrick A, Eickhoff J, Eliceiri KW, White JG, Ramanujam N. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. Proceedings of the National Academy of Sciences of the United States of America. 2007b;104(49):19494–19499. doi: 10.1073/pnas.0708425104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachtsidis I, Tisdall MM, Pritchard C, Leung TS, Ghosh A, Elwell CE, Smith M. Analysis of the Changes in the Oxidation of Brain Tissue Cytochrome-c-Oxidase in Traumatic Brain Injury Patients during Hypercapnoea A Broadband NIRS Study. In: LaManna JC, Puchowicz MA, Xu K, Harrison DK, Bruley DF, editors. Oxygen Transport to Tissue Xxxii. Vol. 701. Berlin: Springer-Verlag Berlin; 2011. pp. 9–14. Advances in Experimental Medicine and Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BL, Rebbapragada A, Johnson MS. The FAD-PAS Domain as a Sensor for Behavioral Responses in Escherichia coli. Antioxidants & Redox Signaling. 2001;3(5):867–879. doi: 10.1089/15230860152665037. [DOI] [PubMed] [Google Scholar]
- Thompson CB. Metabolic Enzymes as Oncogenes or Tumor Suppressors. N Engl J Med. 2009;360(8):813–815. doi: 10.1056/NEJMe0810213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdall MM, Tachtsidis I, Leung TS, Elwell CE, Smith M. Near-infrared spectroscopic quantification of changes in the concentration of oxidized cytochrome c oxidase in the healthy human brain during hypoxemia. Journal of Biomedical Optics. 2007;12(2) doi: 10.1117/1.2718541. [DOI] [PubMed] [Google Scholar]
- Veech RL. The determination of the redox states and phosphorylation potential in living tissues and their relationship to metabolic control of disease phenotypes. Biochemistry and Molecular Biology Education. 2006;34(3):168–179. doi: 10.1002/bmb.2006.49403403168. [DOI] [PubMed] [Google Scholar]
- Weir EK, Hong ZG, Porter VA, Reeve HL. Redox signaling in oxygen sensing by vessels. Respiratory Physiology & Neurobiology. 2002;132(1):121–130. doi: 10.1016/s1569-9048(02)00054-x. [DOI] [PubMed] [Google Scholar]
- Xu HN, Addis RC, Goings DF, Nioka S, Chance B, Gearhart JD, Li LZ. Imaging redox state heterogeneity within individual embryonic stem cell colonies. Journal of Innovative Optical Health Sciences. 2011a;4:279–288. doi: 10.1142/s1793545811001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HN, Nioka S, Chance B, Li LZ. Heterogeneity of mitochondrial redox state in premalignant pancreas in a PTEN null transgenic mouse model. Adv Exp Med Biol. 2011b;701:207–213. doi: 10.1007/978-1-4419-7756-4_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HN, Nioka S, Chance B, Zheng G, Li LZ. Advances in Experimental Medicine and Biology. Vol. 737. New York: Springer; 2011c. High-resolution simultaneous mapping of mitochondrial redox state and glucose uptake in human breast tumor xenografts; pp. 175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HN, Nioka S, Glickson J, Chance B, Li LZ. Quantitative Mitochondrial Redox Imaging of Breast Cancer Metastatic Potential. Journal of Biomedical Optics. 2010;15:036010. doi: 10.1117/1.3431714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HN, Tchou J, Chance B, Li LZ. Imaging the Redox States of Human Breast Cancer Core Biopsies. Adv Exp Med Biol. 2012;765:343–349. doi: 10.1007/978-1-4614-4989-8_48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HN, Wu B, Nioka S, Chance B, Li LZ. Calibration of CCD-based redox imaging for biological tissues. Paper presented at the Proceedings of SPIE - Medical Imaging 2009: Biomedical Applications in Molecular, Structural, and Functional Imaging; Lake Buena Vista, FL, USA. 2009a. [Google Scholar]
- Xu HN, Wu B, Nioka S, Chance B, Li LZ. Quantitative redox scanning of tissue samples using a calibration procedure. Journal of Innovative Optical Health Sciences. 2009b;2:375–385. doi: 10.1142/S1793545809000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HN, Zhou R, Nioka S, Chance B, Glickson JD, Li LZ. Histological basis of MR/optical imaging of human melanoma mouse xenografts spanning a range of metastatic potentials. Advances in Experimental Medicine and Biology. 2009c;645:247–253. doi: 10.1007/978-0-387-85998-9_37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying WH. NAD(+)/NADH and NADP(+)/NADPH in cellular functions and cell death: Regulation and biological consequences. Antioxidants & Redox Signaling. 2008;10(2):179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Wang SY, Nottke AC, Rocheleau JV, Piston DW, Goodman RH. Redox sensor CtBP mediates hypoxia-induced tumor cell migration. Proc Natl Acad Sci U S A. 2006;103(24):9029–9033. doi: 10.1073/pnas.0603269103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Blessington D, Li H, Busch TM, Glickson J, Luo Q, Chance B, Zheng G. Redox ratio of mitochondria as an indicator for the response of photodynamic therapy. J Biomed Opt. 2004a;9(4):772–778. doi: 10.1117/1.1760759. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Li H, Liu Q, Zhou LL, Zhang M, Luo QM, Glickson J, Chance B, Zheng G. Metabolic imaging of tumors using intrinsic and extrinsic fluorescent markers. Biosensors & Bioelectronics. 2004b;20(3):643–650. doi: 10.1016/j.bios.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Zhu C, Burnside ES, Sisney GA, Salkowski LR, Harter JM, Yu B, Ramanujam N. Fluorescence spectroscopy: an adjunct diagnostic tool to image-guided core needle biopsy of the breast. IEEE Trans Biomed Eng. 2009;56(10):2518–2528. doi: 10.1109/TBME.2009.2015936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler M. A vital link between energy and signal transduction. Regulatory functions of NAD(P) FEBS Journal. 2005;272(18):4561–4564. doi: 10.1111/j.1742-4658.2005.04893.x. [DOI] [PubMed] [Google Scholar]