Abstract
Recent research has implicated pain-related fear in relation to functional outcomes in children with chronic pain. The current study examined fear of pain, disability, and depression within the context of an intensive pain rehabilitation program. One hundred and forty-five children and adolescents who participated in an intensive interdisciplinary pediatric pain rehabilitation day program were assessed in this study. Patients completed measures of pain intensity, pain-related fears, functional disability, and depressive symptoms at admission, discharge, and on average, two months post-discharge. After controlling for pain intensity, pain-related fear was significantly related to disability and depressive symptoms at all time points. As predicted, a decline in pain-related fear was significantly associated with a decrease in disability and depressive symptoms. Interestingly, high levels of pain-related fears at admission predicted less reduction in functional disability and depression at discharge, suggesting that high levels of pain-related fear may be a risk factor in relation to treatment outcomes. Overall, results indicate that the relationship between fear of pain and changes in disability and depressive symptoms are closely linked, with fear of pain playing an important role in treatment.
Perspective
This paper presents results underscoring the importance of pain-related fear in relation to treatment response for children and adolescents with chronic pain. These findings support the need to develop and implement interventions that target reductions in pain-related fear.
Keywords: chronic pain, child and adolescent, pain-related fear, treatment response, psychological aspects
Introduction
Prolonged pain-related disability in childhood is a common experience with median prevalence rates of 11–38%20 with 3–5% experiencing moderate to severe pain problems17; 10. Pain-related fear and avoidance has been identified as an important psychological factor influencing the development and persistence of pain-related disability among adults, irrespective of pain intensity40; 41; 23. According to the Fear Avoidance Model of Chronic Pain, individuals who develop fears of pain, injury, and/or physical activity experience a number of psychological and physical sequelae, including hypervigilance, muscular reactivity, and guarding behaviors that maintain or exacerbate pain23. Continued avoidance and physical inactivity can lead to disuse, disability and depression19; 39. Conversely, individuals who confront their pain experience and progressively resume physical activities, thereby testing and correcting pain expectations, subsequently experience a recovery in their pain symptoms31.
Few studies have examined pain-related fear in children, but initial findings are supportive of its link to pain-related outcomes. In a small pilot study, Martin and colleagues (2007) found that pain-related fear accounted for 40% of the variance in pain-related disability32. In a more recent study among pediatric patients experiencing acute post-surgical pain, pain-related fear was associated with pain unpleasantness and functional disability two weeks after surgery12. An additional small study found that pain-related fear predicted child physical activity limitations beyond the influence of pain intensity and depressive symptoms49. In a study with patients who presented for a tertiary care pain evaluation36 higher levels of pain-related fear were associated with more frequent physician visits in the previous three months and higher levels of functional disability. In addition, a decrease in pain-related fear over a one-month period following the evaluation was associated with a decrease in functional disability, suggesting that a decline in pain-related fear may correspond with participation in daily activities. Significant evidence in the adult pain literature supports this position4; 25; 26; 50.
The Fear-Avoidance Model suggests that when patients reengage in previously avoided activities without experiencing catastrophic consequences, they correct their fear expectancies leading to improvements in physical and emotional functioning16; 37; 24. Evidence for this relationship in studies among adult chronic pain patients has accumulated14; 48; 15. Only one study with adolescents has examined pain-related fears after participation in an exposure and acceptance based treatment program with declines reported, but investigators did not examine how these changes related to functional outcomes47.
In the current study we sought to examine whether a decrease in fear of pain is associated with functional improvements (i.e., decreased disability and depression) in youth with chronic pain who participate in an intensive pain rehabilitation treatment program. Specifically, we hypothesized that 1) after controlling for pain intensity, pain-related fear would be positively associated with disability and depressive symptoms at each time point. Longitudinally, we hypothesized that 2) pain-related fear, depression, and disability would decrease significantly during the course of pain rehabilitation treatment and at follow-up and 3) decreases in fear of pain would be positively associated with decreases in depression and disability. It is important to note that although we did anticipate that patients in the program would improve, the goal of this investigation is to examine how fear of pain relates to treatment response, rather than evaluate the efficacy of this treatment approach. Answering the latter question is beyond the scope of the current study.
Method
Setting and Participants
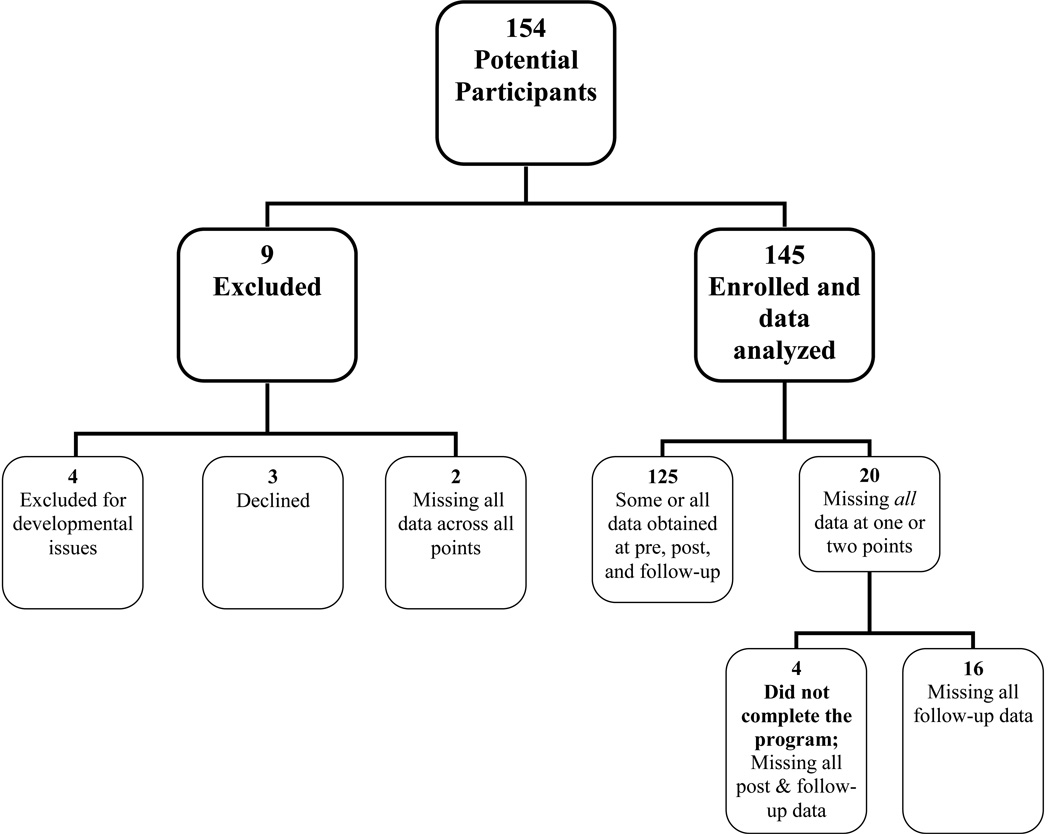
Participants were 145 children and adolescents who completed an intensive interdisciplinary pediatric pain rehabilitation day program from December 2008 to May 2011. In total, 154 patients enrolled in the program during that time. In order to be included in the current analysis, patients did not have a developmental delay, they consented to participate, and provided data at one or more time points (see Figure 1). Four patients were ineligible due to developmental delay, three declined involvement, and two were missing data across all time points thus resulting in 145 participants. The pain rehabilitation program primarily targets patients ages 8–18 with persistent extremity pain with neuropathic features and significant impairment of mobility and limb use (e.g. Complex Regional Pain Syndrome). Program eligibility included failure to progress in conventional outpatient physical and cognitive behavioral therapies. While many patients had psychological challenges, those with active suicidality, psychosis, or an eating disorder prior to enrollment were not eligible. All patients were evaluated through our multidisciplinary outpatient pain treatment clinic prior to admission.
Figure 1.
Flow chart of participants.
Participant Characteristics
Participants in this sample were predominantly adolescent Caucasian females from intact families (see Table 1 for further description). Virtually all patients enrolled in the intensive pain rehabilitation program reported neuropathic pain (93%; remaining patients reported musculoskeletal back pain [3%], musculoskeletal leg or arm pain [2.1%], or muscluskeletal pain in multiple locations [1.4%]), with the majority presenting with solely lower extremity (65%) or a combination of lower and upper extremity (27%) pain complaints. Reports of time since onset of pain ranged widely with the median duration of pain of nine months. The median length of stay in the pain rehabilitation program was four weeks, with follow-up evaluations typically completed two months post-discharge. Length of time from discharge to clinical follow-up visit was not associated with any follow-up variables of interest.
Table 1.
Patient demographic and medical characteristics
| Variable | Range | Mean (SD) | Frequency |
|---|---|---|---|
| Demographic Characteristics | |||
| Age (years) | 8.4–18.3 | 14.5 (2.2) | |
| Gender | |||
| Male | 14.5% | ||
| Female | 85.5% | ||
| Ethnicity | |||
| Caucasian | 97.9% | ||
| Asian | 1.4% | ||
| Hispanic | 0.7% | ||
| Parent Marital Status | |||
| Married | 78.9% | ||
| Single | 9.9% | ||
| Divorced/Separated | 9.9% | ||
| Spouse deceased | 1.4% | ||
| Hollingshead socioeconomic status (SES)+ | 22–66 | 52.5 (10.5) | |
| Medical Characteristics | |||
| Neuropathic Pain | 93% | ||
| Musculoskeletal Pain | 7% | ||
| Affected Limb | |||
| Lower Extremity | 64.8% | ||
| Mixed Upper/Lower Extremity and/or Back | 26.9% | ||
| Upper Extremity | 2.8% | ||
| Torso | 4.8% | ||
| Neck | 0.7% | ||
| Duration of Pain (months) | 0–162 | 9.8 (2.9) median=9 | |
| Length of Stay (weeks) | 2–7 | 4 (median) | |
| Time Frame From Discharge to Follow-Up (weeks) | 2–18 | 10 (median) | |
Note.
SES ranged from laborer (22) to business owner/professional (66).
Intervention
The rehabilitation program entails intensive daily physical, occupational and psychological therapies eight hours a day, five days per week for a typical length of stay of three to four weeks. A typical treatment day begins with a three-hour block of individual physical therapy (PT), occupational therapy (OT), and psychological therapy (one hour each) followed by a two hour academic and lunch period. The subsequent two hours consist of one hour of group physical or occupational therapy and one hour of group psychological therapy. For the final hour of the day, patients participate in either family therapy (twice a week) or parent-observed individual physical or occupational therapy (three times a week). A physician and nurse evaluate patients daily to ensure continued appropriateness for treatment (e.g., continued medical stability) and to address acute or ongoing medical issues. PT, OT, and psychology focuses on helping children return to premorbid levels of functioning through progressively engaging in previously avoided activities and taking a self-management approach to pain.
PT includes promoting increased weight-bearing through the affected limb (stress loading), and improving strength, flexibility, and cardiovascular fitness through a functionally-based program. Tasks are linked to the child’s individualized functional goals such as playing a specific sport.
OT aims to maximize independence and participation in self-care, school, and leisure activities, while promoting normalized use of affected limbs. Progressive, individualized sensory re-education programs, including desensitization and sensory discrimination activities, are utilized to normalize responses to typical daily sensory stimuli, such as wearing a shoe or bathing.
Patients complete physical and occupational therapy home exercise programs each night to promote independence and generalization of skills acquired during the treatment day.
Psychological therapy follows a cognitive-behavioral model, an effective approach for pain rehabilitation46. Patients receive daily individual and group-based cognitive behavioral therapy and families are actively incorporated into the program, with family therapy and parent education provided. Psychological therapy targets include: 1) teaching a self-management approach to pain, 2) addressing negative thinking and fears about pain, 3) engaging in valued activities and relationships in the presence of pain, and 4) reducing parental attention and protective responses to pain. Additional details on the program provided in Logan et a 27; 29l
Follow-up Clinic
Approximately one to two months post-discharge, patients return for a follow-up appointment. At that time they are seen by a physician, psychologist, occupational therapist, and physical therapist individually for one hour each. Following these evaluations, the treatment team meets and provides the family with feedback regarding current clinical status, goal attainment, and goal progression.
Procedures
The study was approved by the institutional review board. For fear of pain, functional disability and depressive symptoms at admission, patients were sent questionnaire packets (that included additional measures) a few days prior to arrival and asked to complete them independently and bring them on admission day. Pain intensity ratings at admission were collected during admission day evaluations. For fear of pain, functional disability, and depressive symptoms at discharge, patients were given questionnaires the day before discharge and asked to complete them at home independently and bring them in the following day. Pain intensity ratings at discharge were collected during discharge evaluations conducted two days prior to the patient’s discharge date. For fear of pain, functional disability, and depressive symptoms at follow-up, patients were sent questionnaire packets a few days prior to their scheduled clinic appointment and asked to complete them independently and bring them to clinic. Pain intensity ratings at follow-up were collected during follow-up clinic evaluations.
Measures
Basic demographic (e.g, age, gender) and medical information (e.g., pain location) was collected from patient charts.
Covariate
Pain intensity
During admission, discharge, and follow-up evaluations, children were asked to provide their average pain intensity rating on a standard 11-point numeric rating scale43 from 0 (no pain) to 10 (most pain possible).
Predictor variable
Pain-related fear
The Fear of Pain Questionnaire (FOPQ-C)36 assesses self-reported perceptions of child pain-related fears and avoidance behaviors. Specific items include: “I avoid making plans because of my pain.” and “I worry when I am in pain.” It is comprised of 24 items rated on a 5-point scale from 0= strongly disagree to 4= strongly agree. Items are summed to derive a total score. Higher scores indicate higher levels of pain-related fear. Construct validity for this measure is supported with significant relations found for the FOPQ-C with child somatization, anxiety, and catastrophizing. This measure has been validated with children 8–17 years. Internal consistency of the total score in this sample was .93, .95, and .95 across admission, discharge, and follow-up.
Outcome variables
Functional disability
The Functional Disability Inventory (FDI)45 is a child-completed scale that assesses difficulty in physical and psychosocial functioning due to physical health. The instrument consists of 15 items concerning perceptions of activity limitations during the past two weeks; total scores are computed by summing the items. Higher scores indicate greater disability. The FDI has good reliability and validity7. Internal consistency of this measure was .89, .91, and .93 across admission, discharge, and follow-up.
Depressive symptoms
The Children’s Depression Inventory (CDI) is a valid and reliable 27-item child self-report measure of depressive symptoms. The CDI has good reliability and validity and is widely used in assessments of children with medical conditions. Higher scores indicate higher levels of depression22. This measure has been validated with children 7–17 years. Internal consistency of this measure was .89, .91, and .92 across admission, discharge, and follow-up.
Statistical Analyses
Preliminary analyses were first conducted in SPSS version 19. Data were screened to ensure that all SEM requirements for normality were met (i.e., skewness <3.0; kurtosis<10.0;21). Descriptive statistics and correlations were conducted to determine whether the pattern of relationships among the variables was consistent with the hypothesized model. Correlations were also conducted to determine whether there were associations among demographic variables (age, SES, gender, ethnicity), pain variables (pain duration, affected limb, presence of neuropathic pain, pain intensity), and the study variables (fear of pain, functional disability, and depression); demographic and/or pain variables that were significantly correlated with study variables were controlled for in all subsequent analyses.
Path analysis conducted within a structural equation modeling (SEM) framework with Mplus software33, was employed to evaluate the study hypotheses. SEM was considered superior to other analytic techniques, such as multiple regression, because it is possible to simultaneously evaluate the overall fit of complex models as well as the significance of individual model pathways, to reduce measurement error, to compare alternative models, and to include cases with missing data in the model33. Full information maximum likelihood estimation (FIML) was employed to account for missing data. Based on recommendations by Bentler and Bonett2 and Ullman38, the following statistics were used to evaluate model fit: χ2, χ2/df (<2 acceptable); Comparative Fit Index (CFI; >.90 acceptable, >.95 excellent); and Root Mean Square Error of Approximation (RMSEA; <.08 acceptable, <.05 excellent). A sample size of 100–200 subjects is generally considered adequate for testing complex models in SEM21.
We tested separate models for each of our outcomes of interest, depression and disability. For all SEM analyses, pain intensity at each time point was included as a control variable in order to examine the influence of pain-related fear above and beyond the influence of pain intensity on disability and depressive symptoms. We specified auto-regressive, longitudinal regression models in which pain severity was specified as a control variable at each time point and fear of pain was the primary predictor of the outcome of interest (i.e., depression or disability) cross-sectionally. To evaluate change in each variable over time and to account for prior values on each variable in the model, we included paths from each variable to the same variable at each subsequent time point (e.g., from fear at admission to fear at discharge, from fear at discharge to fear at follow-up). Thus, with exception of the first time point, each variable in the model represents change in that variable since the previous time point. We also included longitudinal regression pathways, controlling for pain intensity and allowing fear of pain at one time point to predict the outcome of interest at a subsequent time point (e.g., fear of pain at admission was allowed to predict disability at discharge). We then evaluated the fit of the models and the strength and direction of individual model parameters.
Results
Preliminary and Descriptive Analyses
We examined the relations between demographic and pain characteristics (affected limb, duration of pain) and all variables of interest in this study (pain intensity, fear of pain, disability, depressive symptoms). A shorter duration of pain was associated with higher levels of pain intensity (r = −.21, p < .05) and disability at admission (r = −.19, p < .05). No other demographic or pain characteristics were associated with variables of interest in this study. Duration of pain was controlled for in the SEM models.
Using repeated measures ANOVA we examined whether there were significant changes over time across all variables of interest (Table 2). As anticipated, pain intensity, fear of pain, functional disability, and depressive symptoms significantly decreased during the course of the rehabilitation treatment. Using Bonferroni post-hoc analyses we examined changes from admission to discharge and from discharge to follow-up. Significant decreases in pain intensity and pain-related fears were observed across all time points. Functional disability and depressive symptoms significantly decreased from admission to discharge, but no further changes were observed from discharge to follow-up.
Table 2.
Changes in pain, fear of pain, functional disability, and depressive symptoms across time
| Variables | Pre Mean (SD) |
Post Mean (SD) |
Pre to Post T-test |
Follow-up Mean (SD) |
Post to Follow-up T-test |
|---|---|---|---|---|---|
| Pain | 6.42 (2.15) | 5.16 (2.75) | 7.44** | 3.54 (3.0) | 7.92** |
| Fear of Pain | 47.9 (18.0) | 27.3 (17.5) | 11.0** | 19.6 (16.7) | 4.00** |
| Functional Disability | 32.0 (10.3) | 9.76 (8.29) | 22.8** | 7.69 (9.22) | 2.46* |
| Depressive Symptoms | 12.5 (8.18) | 7.89 (7.82) | 7.40** | 6.88 (7.81) | .58 |
Note.
p< .05;
p<.01. N for Pain = 141 for Pre to Post, 122 for Post to Follow-up. N for Fear of Pain = 113 for Pre to Post, 96 for Post to Follow-up. N for Functional Disability= 115 for Pre, to Post, 97 for Post to Follow-up. N for Depressive Symptoms = 112 for Pre to Post, 96 for Post to Follow-up.
Correlations were conducted between pain intensity, pain-related fear, functional disability, and depression within and across time points (Table 3). Pain intensity and fear of pain were unrelated at admission. All other variables were associated concurrently across time points.
Table 3.
Intercorrelations, means, and standard deviations for all study variables at admission, discharge, and follow-up
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | N | Mean | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Admission | |||||||||||||||
| 1. Pain (average) | -- | .15 | .01 | .49** | .69** | .12 | −.07 | .17 | .44** | .08 | .04 | .07 | 145 | 6.47 | 2.16 |
| 2. Fear of Pain (FOPQ) | -- | .51** | .41** | .13 | .39** | .22* | .19* | .10 | .41** | .20* | .08 | 134 | 49.0 | 18.1 | |
| 3. Depressive Symptoms (CDI) | -- | .31** | .12 | .28** | .67** | .25** | .18 | .43** | .60** | .27** | 133 | 12.6 | 8.26 | ||
| 4. Functional Disability (FDI) | -- | .37** | .08 | .19* | .40** | .30** | .03 | .03 | .22* | 138 | 31.8 | 10.7 | |||
| Discharge | |||||||||||||||
| 5. Pain (average) | -- | .30** | .09 | .43** | .71** | .27** | .13 | .31** | 141 | 5.16 | 2.75 | ||||
| 6. Fear of Pain (FOPQ) | -- | .47** | .54** | .21* | .49** | .30** | .25* | 122 | 27.6 | 17.6 | |||||
| 7. Depressive Symptoms (CDI) | -- | .50** | .16 | .37** | .76** | .34** | 121 | 7.94 | 8.00 | ||||||
| 8. Functional Disability (FDI) | -- | .43** | .30** | .26* | .58** | 123 | 10.2 | 8.74 | |||||||
| Follow-up | |||||||||||||||
| 9. Pain (average) | -- | .43** | .31** | .59** | 122 | 3.54 | 2.96 | ||||||||
| 10. Fear of Pain (FOPQ) | -- | .59** | .46** | 110 | 20.1 | 16.5 | |||||||||
| 11. Depressive Symptoms (CDI) | -- | .46** | 108 | 7.32 | 8.08 | ||||||||||
| 12. Functional Disability (FDI) | -- | 110 | 7.70 | 9.12 | |||||||||||
Note.
p< .05;
p<.01.
Path Analysis
All variables met the SEM requirements for normality. There was some missing data (see Figure 1). Of the 145 patients enrolled in the study 125 participants had some or all data across all three time points, while 20 participants were missing all data at one or more time points (n=4 did not complete the program, thus had no post or follow-up data; n=16 did not return for follow-up). With 2.7% of patients not completing the program and an additional 11% not returning for follow-up, we explored baseline differences between this subset of 20 patients and the larger sample using one-way ANOVAs. There were no significant differences across pain intensity, fear of pain, functional disability, and depressive symptoms, thus data were considered missing completely at random for SEM analyses. Model parameters reported represent standardized values. Pain duration was controlled for in both models by regressing the relevant exogenous variable (i.e., pain intensity at admission) on pain duration.
Functional Disability
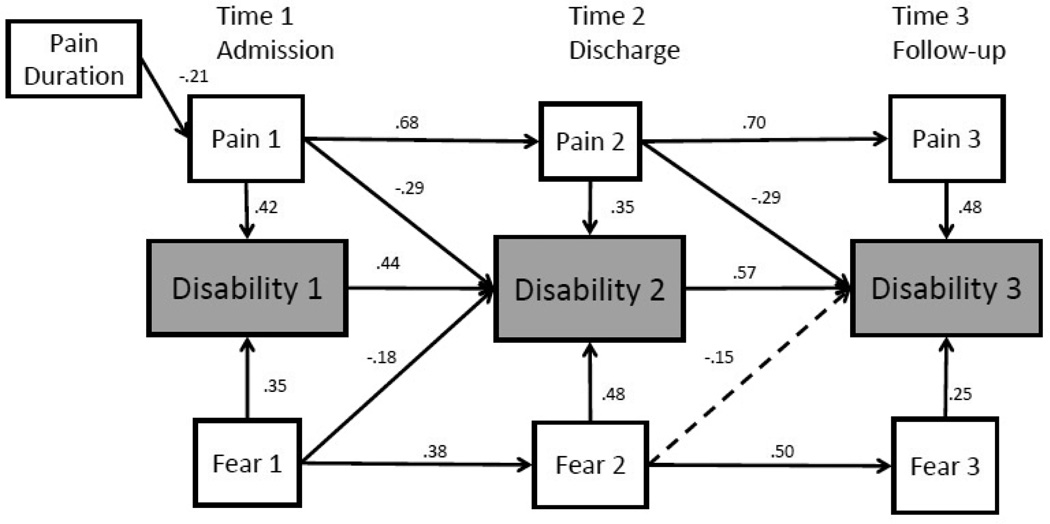
For functional disability, the longitudinal regression model provided poor fit to the data [χ2(27) = 62.33, p < .01; χ2/ df = 2.31; CFI = .92, RMSEA = 0.10 (90% CI = 0.07–0.13)]. To test whether it would be possible to improve model fit, we altered the model based on modification indices provided by the Mplus program33. A modification index indicates the amount the χ2 is expected to improve if the suggested pathway is included in the model. SEM models should be based on theory, and therefore only modifications that can be theoretically justified may be included. The use of modification indices to improve model fit in SEM is common practice in health psychology3. Based on a review of the modification indices, we determined that model fit would be improved if we included error covariances between pain severity and fear of pain at each time point (e.g., error covariances for pain severity at admission and fear of pain at admission, pain severity at discharge and fear of pain at discharge, and pain severity at follow-up and fear of pain at follow-up). These modifications were considered theoretically sound because both variables were assessed at the same time point in the course of treatment, were child self-report, and were indicative of the child’s perceptions and beliefs about their pain at the time of assessment. Thus, it is likely that there is some covariation between these variables that was not accounted for by the original model. The error covariances were added to the model and the modified model provided excellent fit to the data [χ2(24) = 30.88, p = .16; χ2/ df = 1.29; CFI = .98, RMSEA = 0.04 (90% CI = 0.00–0.09)].
Results of the modified longitudinal regression model indicate that, after controlling for pain severity at each time point, fear of pain was concurrently associated with disability at all three time points, as predicted (see Figure 2). Specifically, fear of pain at admission was associated with disability at admission (β = .35, p < .05, 95% CI = .21−.48), decreases in fear of pain at discharge was associated with decreases in disability at discharge (β = .48, p < .05, 95% CI = .35−.62), and decreases in fear of pain at follow-up was associated with decreases in disability at follow-up (β = .25, p < .05, 95% CI = .08−.41), as predicted. Error covariances between pain severity and fear of pain at each time point were as follows: admission (Θ= .13, ns); discharge (Θ= .29, p<.001); and follow-up (Θ= .37, p<.001).
Figure 2.
Model with Functional Disability as Outcome. Bold lines are significant at p < .05; dashed lines are not significant. Error covariances were included in the model but were not included in the figure for simplicity and interpretability.
Regarding longitudinal pathways, after controlling for pain intensity at admission, fear of pain at admission was negatively related to decreases in disability at discharge (β = −.18, p<.05, 95% CI = −.33–−.03). Thus, increased fear of pain at admission was associated with a smaller decrease in disability from admission to discharge. There was no direct relation between decreases in fear of pain at discharge and decreases in disability at follow-up; however, tests of indirect effects demonstrated that decreases in fear of pain at discharge was indirectly related to decreases in disability at follow-up via decreases in fear of pain at follow-up (indirect effect = .12, p <.01, 95% CI = .03−.21).
To further evaluate whether the proposed longitudinal regression model best represented the relations among the variables, we also tested an alternative model for comparison. Specifically, we tested an indirect effect model where pain severity was associated with fear of pain, both concurrently and longitudinally, and fear of pain was associated with disability, both concurrently and longitudinally, but pain severity was not directly associated with disability, thus fear of pain served as the mediator between pain and disability. This model provided poor fit to the data [χ2(28) = 96.30, p <.001; χ2/ df = 3.44; CFI = .85, RMSEA = 0.13 (90% CI = 0.10– 0.16)], providing further support that the longitudinal regression model best depicted the relations among the variables.
Depression
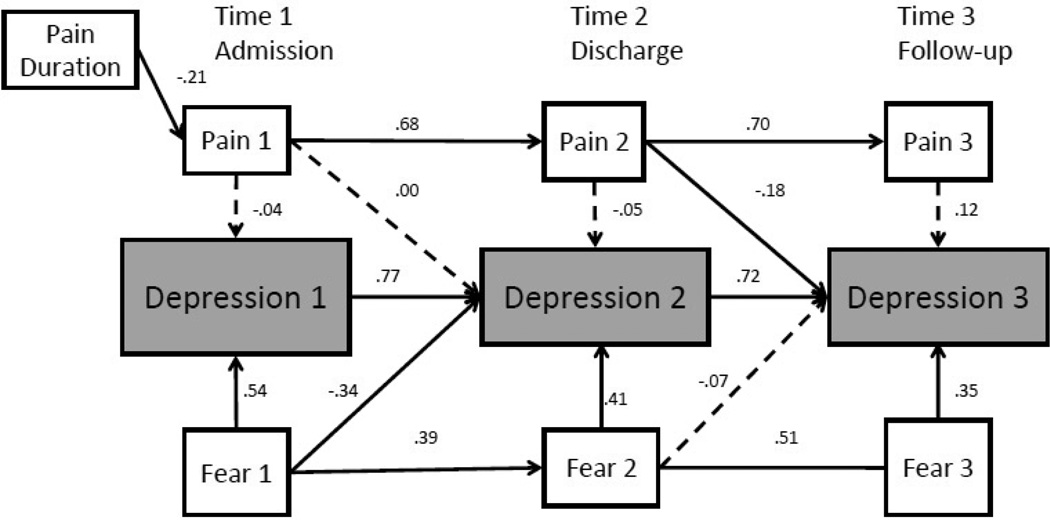
For depression, the longitudinal regression model provided poor fit to the data [χ2(27) = 67.59, p < .05; χ2/ df = 2.50; CFI = .92, RMSEA = 0.11 (90% CI = 0.08–0.14)]. The concurrent error covariances between pain severity and fear of pain at the same time point were again recommended by modification indices, and because these modifications were again considered theoretically sound, these error covariances were added to the model, resulting in improved fit [χ2(24) = 39.38, p < .05; χ2/ df = 1.64; CFI = .97, RMSEA = 0.07 (90% CI = 0.02–0.10)].
Cross-sectionally, fear of pain was significantly related to depression at all three time points (see Figure 3). Thus, fear of pain at admission was associated with depression at admission (β = .54, p < .05, 95% CI = .42−.66), decreases in fear of pain at discharge was associated with decreases in depression at discharge (β = .41, p < .05, 95% CI = .29−.54), and decreases in fear of pain at follow-up was associated with decreases in depression at follow-up (β = .35, p < .05, 95% CI =.22−.50). Although pain intensity was included in the model to control for its severity, it was not associated with depression cross-sectionally at any time point. Error covariances between pain severity and fear of pain at each time point were as follows: admission (Θ= .13, ns); discharge (Θ= .28, p<.01); and follow-up (Θ= .38, p<.001).
Figure 3.
Model with Depression as Outcome. Bold lines are significant at p < .05; dashed lines are not significant. Error covariances were included in the model but were not included in the figure for simplicity and interpretability.
Fear of pain at admission was negatively associated with declines in depression at discharge (β = −.34, p < .05), indicating that high fear of pain at admission predicted a smaller decrease in depression from admission to discharge. There was no direct relation between decreases in fear of pain at discharge and decreases in depression at follow-up; however, tests of indirect effects demonstrated that decreases in fear of pain at discharge was indirectly related to decreases in depression at follow-up via decreases in fear of pain at follow-up (indirect effect = .18, p <.001, 95% CI = .09−.27).
We also tested an indirect effect model with depression as the outcome for comparison, where fear of pain was the hypothesized mediator between pain and depressive symptoms. This alternative model also provided good fit to the data [χ2(28) = 44.62, p <.05; χ2/ df = 1.60; CFI = .97, RMSEA = 0.07 (90% CI = 0.02–0.10)]. Given the unexpected goodness of fit observed for this alternative model, we conducted tests of indirect effects. We found a significant indirect effect of pain on depressive symptoms via fear of pain at discharge (indirect effect = .42, p <001) and at follow-up (indirect effect = .48, p<.001).
Discussion
Within the context of intensive pain rehabilitation for children and adolescents with chronic pain we found that decreases in pain-related fear were associated with concurrent improvements in disability and depression, that intensive pain rehabilitation led to improvements in pain-related fears, and that greater fear of pain at admission predicted fewer improvements during treatment. These results indicate that fear of pain is closely linked to changes in disability and depressive symptoms, with fear of pain playing an important role in treatment outcomes across multiple time points
In order to explore how fear of pain was associated with functional improvements we first examined whether there was a sufficient change in functioning achieved. We observed significant decreases in pain intensity, pain-related fears, functional disability, and depressive symptoms of medium to large effect sizes; thus allowing us to examine fear of pain in relation to improvements in functioning. We found that after controlling for pain severity across time points, fear of pain was significantly associated with disability and depressive symptoms concurrently. After treatment and at follow-up, a decrease in fear of pain was associated with improvements in functional disability and depressive symptoms. These findings are consistent with the theorized Fear Avoidance Model41 and support the potential application of this model to pediatric pain patients. In testing an alternative model, we examined whether fear of pain fully mediated the relationship between pain and outcomes. For functional disability, this alternative model was a poor fit to the data, suggesting that pain and pain-related fear each exert direct influences on functional disability. This pattern is consistent with our hypothesis. For depressive symptoms, the alternative model fit as adequately as our hypothesized model. Through tests of indirect effects we observed that pain was associated with depressive symptoms via fear of pain, thus explaining the goodness of the alternative model’s fit. These results provide further support for our hypothesized model as there was no direct relationship between pain and depressive symptoms observed Given evidence in the literature that has supported the direct link between depressive symptoms and pain previously18; 19; 28, further exploration of these variables in relation to one another in additional patient samples is warranted.
Strikingly, we found that higher levels of fear of pain at admission predicted fewer improvements in disability and depressive symptoms at discharge. Thus it is likely that a higher baseline level of fear is a potential risk factor in relation to treatment outcomes. In addition, it suggests that patients who present with high levels of pain-related fears may benefit from targeted treatment to potentially ameliorate this obstacle to treatment response, either within the context of intensive pain rehabilitation or prior to enrollment. The current treatment model does not explicitly include an exposure-specific paradigm for patients. Incorporating treatment approaches that deliberately expose patients to previously avoided movements and experiences, such graded in-vivo exposure8, graded exercise13, and acceptance and commitment therapy44 have been found to improve disability and reduce pain-related fear25; 1 and may help to overcome the risks of high pain related fear at the outset of treatment. Additionally, it would be beneficial to determine if there is a clinical threshold of baseline pain-related fear that incrementally increases a patient’s risk for less treatment response. Future research studies should explore potential clinical cut-offs for pain-related fears in children as have been established for adults34.
Fear of pain at discharge did not predict disability or depressive symptoms at follow-up. This may be due to little to no change observed in functional disability and depressive symptoms from discharge to follow-up, potentially representing maintenance of treatment gains, while fear of pain continued to decline. Although functional improvements are marked during the intensive treatment program (e.g., a formerly wheelchair bound patient can now walk the length of a football field) and state-dependent mood difficulties are likely alleviated as patients regain functionality, many previously avoided activities cannot be confronted until the patient returns home. This may be why the majority of treatment studies that target pain-related fears typically occur on an outpatient basis42; 13, thus facilitating engagement in activities within the individual’s day-to-day life.. Worthy of consideration, tests of indirect effects demonstrated that decreases of fear of pain at discharge were indirectly associated with decreases in disability and depressive symptoms at follow-up via fear of pain at follow-up. Not surprisingly this finding suggests that changes in pain-related fear at discharge influence changes in fear at follow-up, which in turn exert an influence on changes observed in disability and depressive symptoms at follow-up. Thus levels of pain-related fear at each juncture appear to influence subsequent outcomes.
Although these data provide the first glimpses of the role of pain-related fear among children and adolescents within the context of a pain rehabilitation program, there are limitations. Data collection in this study occurred across multiple time points, including admission, discharge, and initial follow-up, however, there are no data collection points during the treatment itself. Such data collection would provide more specific information about the timing of changes in pain-related fear and their associations with disability and depressive symptoms as it is also likely that improvements in functioning would yield greater opportunities for the patient to expose themselves to previously avoided activities thus leading to decreased pain-related fearsAdditionally, it is potentially important to include additional variables such as pain catastrophizing, a cognitive antecedent to pain-related fear48, and parent pain-related fears36 and catastrophizing6 to further our understanding of how the Fear Avoidance Model of Pain operates in children within the social context. Given multiple findings that indicate that protective parent responses impact child outcomes35; 30, it is likely that parent willingness or reluctance to encourage their child to engage in previously avoided activities would have a profound impact on the child’s pain-related fear and associated functional outcomes.
Additionally, these finding most accurately represent pediatric patients suffering from persistent neuropathic pain and as with most studies in tertiary pain care settings, the patients were predominantly Caucasian and from a higher socioeconomic class11; 5. This differs from the majority of research literature examining pain-related fear among adults with chronic low back pain. What these results do indicate is that the impact of pain-related fears appear to be applicable to broader scope of patient populations, such as patients with neuropathic pain. This is reinforced with recent findings among adults with complex regional pain syndrome9. Our data did not include information regarding Axis I psychological disorders for patients enrolled in the pain rehabilitation program. Although it is not clear whether these disorders could be considered pre-existing conditions or sequelae of living with chronic pain, availability of this information would have allowed us to examine the influence of a comorbid psychological condition on changes observed. Lastly, we did not collect specific data regarding number of previous treatments attempts and duration of treatments, thus we could not examine the potential influence that these variables may have had on our results. Despite this limitation, we know that all patients enrolled in the intensive rehabilitation program have had at least one course of physical therapy and psychological treatment, as failure to progress in these outpatient treatments is a prerequisite for eligibility for the day hospital program.
With regards to future research directions, routine assessment of pain-related fear in the context of pediatric pain rehabilitation is warranted. It is likely beneficial in an intensive day hospital setting as well as within the context of outpatient treatment as high levels of pain-related fear can serve as a barrier to treatment progress. Although examining pain-related fear among children and adolescents is a relatively new area of inquiry, these results suggest its importance among youth with neuropathic pain. Hence, it would likely be advantageous to examine this construct closely among other pediatric pain populations, particularly youth with low back pain as the bulk of the literature examining the Fear Avoidance Model of Chronic Pain has centered on this patient group. Lastly, as noted in our limitations, it is imperative to include social contextual factors, such as parent beliefs and behaviors, as they likely play an influential role on subsequent child behavior.
The results of this study validate the hypothesis that fear of pain plays a significant role in relation to functional disability and depressive symptoms in the context of pediatric chronic pain. Fear of pain appears to play both a facilitative and inhibitory role in relation to treatment response. Specifically, a high level of pain-related fear at admission may hinder improvements in disability and depressive symptoms, yet declines in pain-related fear are strongly associated with positive functional outcomes. These results suggest that interventions that specifically target pain-related fear, such as in-vivo graded exposure, warrant adaptation and testing in pediatric patients with chronic pain. In summary, the current study provides evidence for the role of pain-related fear in association with improvements in disability and depression following treatment in a pain rehabilitation setting. Results reflect the complex relationship of pain-related fear to disability and depressive symptom reduction and the importance of developing targeted interventions for pain-related fear in children.
Acknowledgements
The authors wish to thank Anne Pauler, BS, Elizabeth Carpino, MS, and Melissa Pielech, BA for their work on this study.
This investigation was supported by the American Pain Society Future Leaders in Pain Small Grants Program (LS), Children’s Hospital Boston Career Development Fellowship Award (LS), NIH grant HD067202 (LS), Deborah Monroe Noonan Grant (DL), the Sara Page Mayo Endowment for Pediatric Pain Research and Treatment, and the Department of Anesthesiology, Perioperative and Pain Medicine at Children’s Hospital Boston.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: There are no conflicts of interest to report.
References
- 1.Bailey KM, Carleton RN, Vlaeyen JW, Asmundson GJ. Treatments addressing pain-related fear and anxiety in patients with chronic musculoskeletal pain: a preliminary review. Cogn Behav Ther. 2010;39:46–63. doi: 10.1080/16506070902980711. [DOI] [PubMed] [Google Scholar]
- 2.Bentler PM, Bonett DG. Significance tests and goodness-of-fit in the analysis of covariance structures. Psychological Bulletin. 1980;88:588–600. [Google Scholar]
- 3.Bol Y, Duits AA, Lousberg R, Hupperts RM, Lacroix MH, Verhey FR, Vlaeyen JW. Fatigue and physical disability in patients with multiple sclerosis: a structural equation modeling approach. Journal of behavioral medicine. 2010;33:355–363. doi: 10.1007/s10865-010-9266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns JW, Glenn B, Bruehl S, Harden RN, Lofland K. Cognitive factors influence outcome following multidisciplinary chronic pain treatment: a replication and extension of a cross-lagged panel analysis. Behaviour research and therapy. 2003;41:1163–1182. doi: 10.1016/s0005-7967(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 5.Bursch B, Tsao JC, Meldrum M, Zeltzer LK. Preliminary validation of a self-efficacy scale for child functioning despite chronic pain (child and parent versions) Pain. 2006;125:35–42. doi: 10.1016/j.pain.2006.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caes L, Vervoort T, Eccleston C, Vandenhende M, Goubert L. Parental catastrophizing about child's pain and its relationship with activity restriction: the mediating role of parental distress. Pain. 2011;152:212–222. doi: 10.1016/j.pain.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 7.Claar RL, Walker LS. Functional assessment of pediatric pain patients: psychometric properties of the functional disability inventory. Pain. 2006;121:77–84. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jong JR, Vlaeyen JW, Onghena P, Cuypers C, den Hollander M, Ruijgrok J. Reduction of pain-related fear in complex regional pain syndrome type I: the application of graded exposure in vivo. Pain. 2005;116:264–275. doi: 10.1016/j.pain.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 9.de Jong JR, Vlaeyen JW, de Gelder JM, Patijn J. Pain-related fear, perceived harmfulness of activities, and functional limitations in complex regional pain syndrome type I. J Pain. 2011;12:1209–1218. doi: 10.1016/j.jpain.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Dunn KM, Jordan KP, Mancl L, Drangsholt MT, Le Resche L. Trajectories of pain in adolescents: a prospective cohort study. Pain. 2011;152:66–73. doi: 10.1016/j.pain.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eccleston C, Crombez G, Scotford A, Clinch J, Connell H. Adolescent chronic pain: Patterns and predictors of emotional distress in adolescents with chronic pain and their parents. Pain. 2004;108:221–229. doi: 10.1016/j.pain.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Gabrielle Page M, Campbell F, Isaac L, Stinson J, Martin-Pichora AL, Katz J. Reliability and validity of the Child Pain Anxiety Symptoms Scale (CPASS) in a clinical sample of children and adolescents with acute postsurgical pain. Pain. 2011 doi: 10.1016/j.pain.2011.02.053. [DOI] [PubMed] [Google Scholar]
- 13.George SZ, Wittmer VT, Fillingim RB, Robinson ME. Comparison of graded exercise and graded exposure clinical outcomes for patients with chronic low back pain. J Orthop Sports Phys Ther. 2010;40:694–704. doi: 10.2519/jospt.2010.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gheldof EL, Vinck J, Van den Bussche E, Vlaeyen JW, Hidding A, Crombez G. Pain and pain-related fear are associated with functional and social disability in an occupational setting: evidence of mediation by pain-related fear. Eur J Pain. 2006;10:513–525. doi: 10.1016/j.ejpain.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Gheldof EL, Crombez G, Van den Bussche E, Vinck J, Van Nieuwenhuyse A, Moens G, Mairiaux P, Vlaeyen JW. Pain-related fear predicts disability, but not pain severity: a path analytic approach of the fear-avoidance model. Eur J Pain. 2010;14:e871–879. doi: 10.1016/j.ejpain.2010.01.003. 870. [DOI] [PubMed] [Google Scholar]
- 16.Goubert L, Crombez G, Danneels L. The reluctance to generalize corrective experiences in chronic low back pain patients: a questionnaire study of dysfunctional cognitions. Behaviour research and therapy. 2005;43:1055–1067. doi: 10.1016/j.brat.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Huguet A, Miro J. The severity of chronic pediatric pain: an epidemiological study. J Pain. 2008;9:226–236. doi: 10.1016/j.jpain.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Kashikar-Zuck S, Goldschneider KR, Powers SW, Vaught MH, Hershey AD. Depression and functional disability in chronic pediatric pain. Clin J Pain. 2001;17:341–349. doi: 10.1097/00002508-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Kashikar-Zuck S, Vaught MH, Goldschneider KR, Graham TB, Miller JC. Depression, coping, and functional disability in juvenile primary fibromyalgia syndrome. J Pain. 2002;3:412–419. doi: 10.1054/jpai.2002.126786. [DOI] [PubMed] [Google Scholar]
- 20.King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, MacDonald AJ. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152:2729–2738. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 21.Kline RB. Principles and Practice of Structural Equation Modeling. New York: Guilford Press; 2010. [Google Scholar]
- 22.Kovacs M. The Children's Depression, Inventory (CDI) Psychopharmacology bulletin. 1985;21:995–998. [PubMed] [Google Scholar]
- 23.Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. Journal of behavioral medicine. 2007;30:77–94. doi: 10.1007/s10865-006-9085-0. [DOI] [PubMed] [Google Scholar]
- 24.Leeuw M, Houben RM, Severeijns R, Picavet HS, Schouten EG, Vlaeyen JW. Pain-related fear in low back pain: a prospective study in the general population. Eur J Pain. 2007;11:256–266. doi: 10.1016/j.ejpain.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Leeuw M, Goossens ME, van Breukelen GJ, de Jong JR, Heuts PH, Smeets RJ, Koke AJ, Vlaeyen JW. Exposure in vivo versus operant graded activity in chronic low back pain patients: results of a randomized controlled trial. Pain. 2008;138:192–207. doi: 10.1016/j.pain.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Linton SJ, Boersma K, Jansson M, Overmeer T, Lindblom K, Vlaeyen JW. A randomized controlled trial of exposure in vivo for patients with spinal pain reporting fear of work-related activities. Eur J Pain. 2008;12:722–730. doi: 10.1016/j.ejpain.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Logan D, Chiang G, Condon M, Firn E, Gaughan V, Hogan M, Leslie D, Olson K, Simons L, Berde C. Development of an intensive pain rehabilitation program for children and adolescents with complex regional pain syndrome. Pediatric Pain Letter. 2010;12:1–6. [Google Scholar]
- 28.Logan DE, Simons LE, Kaczynski KJ. School functioning in adolescents with chronic pain: the role of depressive symptoms in school impairment. Journal of pediatric psychology. 2009;34:882–892. doi: 10.1093/jpepsy/jsn143. [DOI] [PubMed] [Google Scholar]
- 29.Logan DE, Carpino EA, Chiang G, Condon M, Firn E, Gaughan VJ, Hogan M, Leslie DS, Olson K, Sager S, Sethna N, Simons LE, Zurakowski D, Berde CB. A Day-hospital Approach to Treatment of Pediatric Complex Regional Pain Syndrome: Initial Functional Outcomes. Clin J Pain. 2012 doi: 10.1097/AJP.0b013e3182457619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Logan DE, Simons LE, Carpino EA. Too sick for school? Parent influences on school functioning among children with chronic pain. Pain. 2012;153:437–443. doi: 10.1016/j.pain.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lohnberg JA. A review of outcome studies on cognitive-behavioral therapy for reducing fear-avoidance beliefs among individuals with chronic pain. Journal of Clinical Psychology in Medical Settings. 2007;14:113–122. [Google Scholar]
- 32.Martin AL, McGrath PA, Brown SC, Katz J. Anxiety sensitivity, fear of pain and pain-related disability in children and adolescents with chronic pain. Pain Res Manag. 2007;12:267–272. doi: 10.1155/2007/897395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muthen LK, Muthen BO. User’s guide. Los Angeles: Muthen & Muthen; 2004. Mplus: The comprehensive modeling program for applied researchers. [Google Scholar]
- 34.Roelofs J, van Breukelen G, Sluiter J, Frings-Dresen MH, Goossens M, Thibault P, Boersma K, Vlaeyen JW. Norming of the Tampa Scale for Kinesiophobia across pain diagnoses and various countries. Pain. 2011;152:1090–1095. doi: 10.1016/j.pain.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 35.Sieberg CB, Williams S, Simons LE. Do parent protective responses mediate the relation between parent distress and child functional disability among children with chronic pain? Journal of pediatric psychology. 2011;36:1043–1051. doi: 10.1093/jpepsy/jsr043. [DOI] [PubMed] [Google Scholar]
- 36.Simons LE, Sieberg CB, Carpino E, Logan D, Berde C. The Fear of Pain Questionnaire (FOPQ): assessment of pain-related fear among children and adolescents with chronic pain. J Pain. 2011;12:677–686. doi: 10.1016/j.jpain.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Smeets RJ, Wittink H, Hidding A, Knottnerus JA. Do patients with chronic low back pain have a lower level of aerobic fitness than healthy controls?: are pain, disability, fear of injury, working status, or level of leisure time activity associated with the difference in aerobic fitness level? Spine. 2006;31:90–97. doi: 10.1097/01.brs.0000192641.22003.83. discussion 98. [DOI] [PubMed] [Google Scholar]
- 38.Ullman JB. Structural equation modelling. Boston: Allyn and Bacon; 2001. [Google Scholar]
- 39.Verbunt JA, Seelen HA, Vlaeyen JW, van der Heijden GJ, Knottnerus JA. Fear of injury and physical deconditioning in patients with chronic low back pain. Archives of physical medicine and rehabilitation. 2003;84:1227–1232. doi: 10.1016/s0003-9993(03)00132-1. [DOI] [PubMed] [Google Scholar]
- 40.Vlaeyen JW, Kole-Snijders AM, Boeren RG, van Eek H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62:363–372. doi: 10.1016/0304-3959(94)00279-N. [DOI] [PubMed] [Google Scholar]
- 41.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 42.Vlaeyen JW, de Jong J, Geilen M, Heuts PH, van Breukelen G. The treatment of fear of movement/(re)injury in chronic low back pain: further evidence on the effectiveness of exposure in vivo. Clin J Pain. 2002;18:251–261. doi: 10.1097/00002508-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 43.von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children's self-reports of pain intensity. Pain. 2009;143:223–227. doi: 10.1016/j.pain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Vowles KE, McCracken LM. Acceptance and values-based action in chronic pain: a study of treatment effectiveness and process. J Consult Clin Psychol. 2008;76:397–407. doi: 10.1037/0022-006X.76.3.397. [DOI] [PubMed] [Google Scholar]
- 45.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16:39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- 46.Wetherell JL, Afari N, Rutledge T, Sorrell JT, Stoddard JA, Petkus AJ, Solomon BC, Lehman DH, Liu L, Lang AJ, Hampton Atkinson J. A randomized, controlled trial of acceptance and commitment therapy and cognitive-behavioral therapy for chronic pain. Pain. 2011;152:2098–2107. doi: 10.1016/j.pain.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 47.Wicksell RK, Melin L, Lekander M, Olsson GL. Evaluating the effectiveness of exposure and acceptance strategies to improve functioning and quality of life in longstanding pediatric pain--a randomized controlled trial. Pain. 2009;141:248–257. doi: 10.1016/j.pain.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Wideman TH, Adams H, Sullivan MJ. A prospective sequential analysis of the fear-avoidance model of pain. Pain. 2009;145:45–51. doi: 10.1016/j.pain.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 49.Wilson AC, Lewandowski AS, Palermo TM. Fear-avoidance beliefs and parental responses to pain in adolescents with chronic pain. Pain Res Manag. 2011;16:178–182. doi: 10.1155/2011/296298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woods MP, Asmundson GJ. Evaluating the efficacy of graded in vivo exposure for the treatment of fear in patients with chronic back pain: a randomized controlled clinical trial. Pain. 2008;136:271–280. doi: 10.1016/j.pain.2007.06.037. [DOI] [PubMed] [Google Scholar]