Abstract
A 124 member norbenzomorphan library has been prepared utilizing a novel multicomponent assembly process (MCAP) followed by a variety of ring-closing reactions to generate norbenzomorphan scaffolds that were readily derivatized via a series of aryl halide cross-coupling and nitrogen functionalization reactions. Biological screening has revealed some novel activities that have not been previously associated with this class of compounds.
Keywords: Combinatorial chemistry, norbenzomorphan, multicomponent assembly process, Heck reaction, cross-coupling reactions
INTRODUCTION
The identification of novel, biologically active molecules is an integral part of programs that are directed toward drug discovery and development of molecular tools that probe biochemical and cellular function. To this end, the high-throughput screening (HTS) of privileged scaffold-based compound libraries has been an effective strategy for obtaining `hits' across a broad range of unrelated biological targets.1 Once the `hit' compound's identity has been confirmed, it is then subjected to sequential rounds of optimization for specificity and potency by manipulation of appended functional groups, substituents, and ring substitution patterns. Since privileged structures are known to exhibit `drug-like' characteristics that include good absorption, membrane permeability, and oral bioavailability,2 chemical libraries based upon these frameworks may have reduced downstream attrition rates making them well-suited for `lead' generation in drug discovery programs.
The 7-methoxy-2-methylnorbenzomorphan (3) was first synthesized in the 1960's during a campaign to discover analgesics more potent than benzomorphan (2), a fundamental structural subunit found in morphine (1) and related opiates (Figure 1).3 A number of members from this new class of heterocycles were identified, including 3, that exhibited analgesic activity in mouse models that was comparable to that of codeine, thus supporting the hypothesis that preparing compounds of this molecular class could lead to the development of novel antinociceptive agents. In a later effort to improve upon the acetylcholinesterase (AChE) inhibitory activity of (−)-physostigmine, a natural product with a wide range of clinical uses,4 a series of norbenzomorphans sharing specific heteroatomic spatial relationships with the natural product were assayed.5 These studies led to the identification of a novel class of norbenzomorphan AChE inhibitors, including 4, that exhibited improved potency and reduced in vivo toxicity relative to (−)-physostigmine. It thus occurred to us that the low molecular weight of the norbenzomorphan scaffold, coupled with its promise as a biologically relevant molecular framework, made it an attractive platform for the construction of a library of novel compounds for use in HTS assays.
Figure 1.
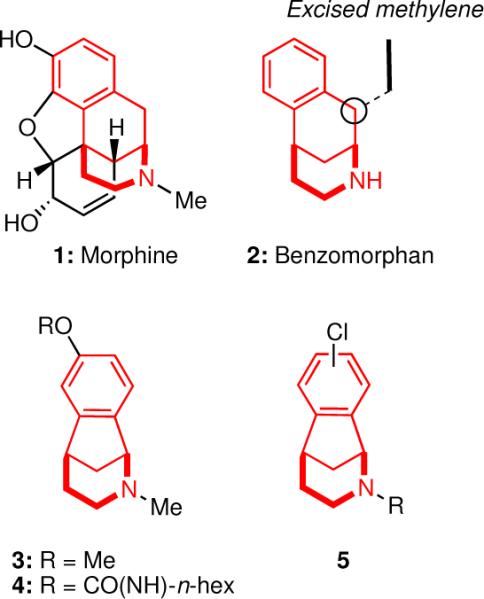
Morphine (1), benzomorphan (2) and norbenzomorphans 3–5.
In order to access a set of diversely substituted norbenzomorphans, a synthetic approach to the azabicyclic skeleton that was both expedient and flexible in terms of the substitution pattern on the aromatic ring and the nitrogen atom was critically important. We elected to prepare scaffolds with aromatic chlorine atoms at various positions (5, Figure 1), because these would serve as functional handles to further derivatize the norbenzomorphan nucleus. The robust nature of the aryl chloride allowed it to be present in the starting material and throughout the scaffold construction sequence, yet it would readily participate in a variety of cross-coupling reactions at the desired stage.
We recently described a general approach to diversity oriented synthesis (DOS) that features a multicomponent assembly process (MCAP) followed by various ring forming reactions.6 The inspiration for this strategy owed its origin to chemistry we had developed in the context of the synthesis of complex alkaloid natural products.7 In the MCAP step of the sequence, three or more reactants are combined to give versatile intermediates, which are endowed with different functional groups that can be paired in a variety of ways to enable cyclizations by numerous ring closing reactions. This basic strategy for DOS has been recently applied to the rapid syntheses of novel benzoxazocines,8 benzazocines, benzodiazepines,9 and tetrahydroisoquinolines,10 as well as isoindolinones,11 tetrahydrobenzonaphtheridines, pyridazines and norbenzomorphans. We now report the application of this strategy to the preparation of a large set of diversely substituted norbenzomorphans based upon 5; we also present select biological screening data that reveal useful activities not previously associated with this class of compounds.
RESULTS AND DISCUSSION
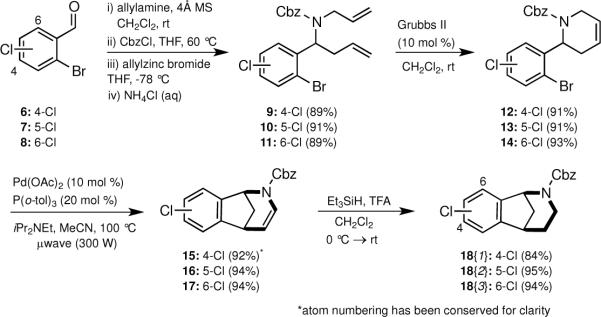
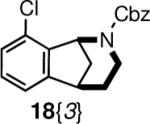
The requisite chloro-regioisomeric norbenzomorphans 18{1–3} were readily prepared in four steps from the known benzaldehydes 6–8 (Scheme 1).12 In the event, sequential treatment of an aldehyde with allylamine, CbzCl and allylzinc bromide provided the diene carbamates 9–11 in good overall yield.11 These diene carbamates 9–11 underwent facile ring closing reactions with Grubbs II catalyst to render tetrahydropyridines 12–14. Subsequent cyclization of 12–14 via an intramolecular Heck reaction, which was promoted under microwave irradiation, furnished the enecarbamates 15–17, which were reduced under ionic conditions to give the chloro norbenzomorphan scaffolds 18{1–3}.
Scheme 1. Synthesis of norbenzomorphans 18{1–3}.
*atom numbering has been conserved for clarity
With multigram quantities of the chloro norbenzomorphans 18{1–3} in hand, we prepared a set of norbenzomorphan derivatives using cross-coupling reactions involving the aryl chloride moiety. Some preliminary screening of conditions was required in order to identify the ideal catalysts, ligands, and reaction parameters that would promote cross-couplings of the aryl chlorides with various amines and boronic acids to provide an assortment of substituted norbenzomorphans 20 with diverse electronic properties and substitution patterns (Scheme 2, Table 1, Figure 2). Under optimized conditions, aniline derivatives 20{1,1–2}, 20{2,1–4} and 20{3,2} were obtained from all three chloro regioisomers 18{1–3} through a Buchwald-Hartwig reaction by pre-mixing Pd(OAc)2 and JohnPhos® prior to addition to the reaction mixture (Table 1).13 When these heterocycles were coupled with piperazine, it was necessary to use an excess (5 equiv) of the diamine to suppress bis-arylation. In all other coupling reactions that involved the use of primary or secondary aliphatic amines as reaction partners, complete consumption of aryl chloride was observed with the use of a slight excess of amine.
Scheme 2. Palladium catalyzed cross-coupling with norbenzomorphan chemset 18.
Conditions: a) 19{1–4}, Pd(OAc)2 (5 mol %), JohnPhos®, NaOtBu, toluene, 100 °C. b) 19{5–11}, Pd(PtBu3)2 (5 mol %), Cs2CO3, 1,4-dioxane, 100 °C.
Table 1.
Cross-coupling reactions with 18{1–3}.
| Entry | Scaffold | Coupling Agent | Product | Yield (%) |
|---|---|---|---|---|
| 1 | 18{1} | 19{1} | 20{1,1} | 75 |
| 2 | 18{1} | 19{2} | 20{1,2} | 88 |
| 3 | 18{1} | 19{5} | 20{1,5} | 60 |
| 4 | 18{2} | 19{1} | 20{2,1} | 72 |
| 5 | 18{2} | 19{2} | 20{2,2} | 97 |
| 6 | 18{2} | 19{3} | 20{2,3} | 67 |
| 7 | 18{2} | 19{4} | 20{2,4} | 66 |
| 8 | 18{2} | 19{6} | 20{2,6} | 98 |
| 9 | 18{2} | 19{7} | 20{2,7} | 86 |
| 10 | 18{2} | 19{8} | 20{2,8} | 94 |
| 11 | 18{2} | 19{9} | 20{2,9} | 89 |
| 12 | 18{2} | 19{10} | 20{2,10} | 94 |
| 13 | 18{2} | 19{11} | 20{2,11} | 73 |
| 14 | 18{3} | 19{2} | 20{3,2} | 69 |
| 15 | 18{3} | 19{7} | 20{3,7} | 88 |
| 16 | 18{3} | 19{8} | 20{3,8} | 86 |
| 17 | 18{3} | 19{6} | 20{3,6} | 82 |
Figure 2.
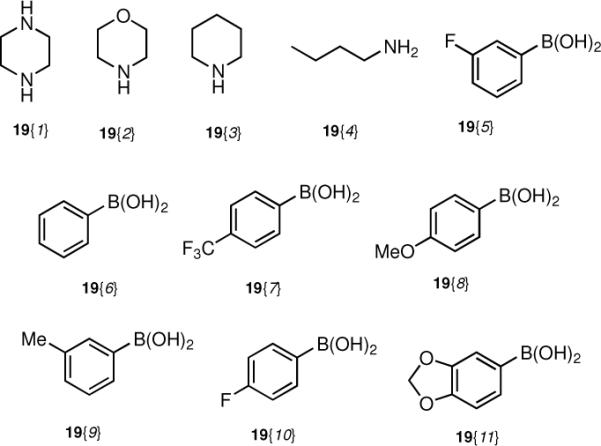
Reagents used for cross-coupling reactions with 18{1–3}.
A number of commonly-used palladium precatalysts such as Pd(OAc)2, Pd(PPh3)4 and Pd2(dba)3 did not promote Suzuki couplings with 18{1–3}, but we eventually discovered that 5 mol% Pd(t-Bu3P)2 was highly effective as a catalyst, delivering a broad range of biaryl norbenzomorphans 20{1,5}, 20{2,6–11} and 20{3,6–8} in generally good yields (Scheme 2, Table 1, Figure 2).14 A variety of amines and boronic acids were coupled to all three chloro regioisomers 18{1–3} to deliver a set of norbenzomorphans adorned with groups having diverse electronic properties and substitution patterns. It is noteworthy that biaryl norbenzomorphans represent a novel class of compounds whose biology has yet to be examined.
Having prepared a collection of diversely substituted amino and aryl norbenzomorphans 20, attention was directed to derivatizing the nitrogen atom of the scaffold. We discovered that a known, but rarely utilized tactic to access benzylamines directly from benzyl carbamates,15 was nicely suited to the rapid generation of a small set of benzylamines. In the event, benzyl carbamates 18 and 20 (Scheme 3, Table 2) were treated with TMSI followed by quenching under basic conditions to give tertiary amines 21; no quaternization was ever observed from the one-pot deprotection/benzylation reaction.
Scheme 3.
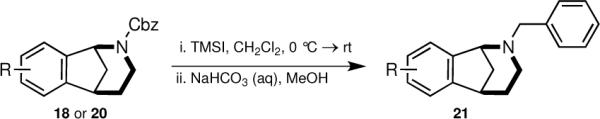
TMSI promoted benzylation.
Table 2.
Benzylamines 21 from carbamates 18 and 20.
| Entry | Carbamate | Benzylamine | Yield (%) |
|---|---|---|---|
| 1 | 18{2} | 21{2} | 88 |
| 2 | 20{1,2} | 21{1,2} | 55 |
| 3 | 20{2,11} | 21{2,11} | 79 |
| 4 | 20{2,7} | 21{2,7} | 91 |
| 5 | 20{2,8} | 21{2,8} | 78 |
| 6 | 20{2,4} | 21{2,4} | 79 |
| 7 | 20{2,3} | 21{2,3} | 96 |
| 8 | 20{2,6} | 21{2,6} | 95 |
| 9 | 20{3,2} | 21{3,2} | 66 |
| 10 | 20{3,7} | 21{3,7} | 72 |
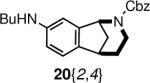
Functionalization of the protected nitrogen atom served as another means to diversify the norbenzomorphans. For example, carbamates 18 and 20 were readily converted to secondary amines by the action of TMSI or Pd(0) and hydrogen (Scheme 4, Table 3). When using TMSI, it was essential to quench the reaction with acid to avoid forming the tertiary benzylamine. Interestingly, Cbz removal under hydrogenolysis conditions with Pd(0) and hydrogen worked well with only a few substrates (Table 3, Entries 6–8); uncharacterized mixtures of compounds were obtained from other starting materials such as 20{2,2} and 20{2,6}.
Scheme 4. Derivatization of 22 and 23.
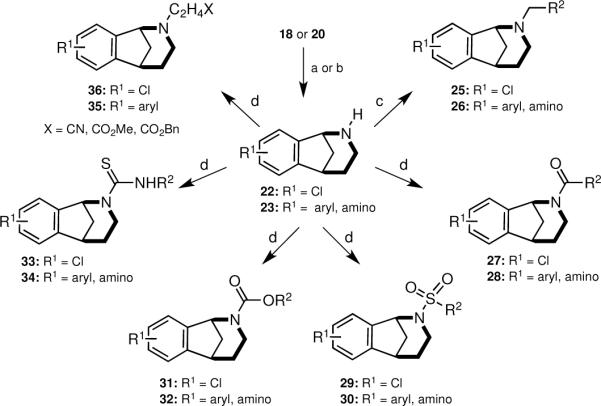
Conditions: a) i. TMSI, CH2Cl2, 0 °C → rt, ii. MeOH/HCl. b) 10% Pd/C, EtOH, H2 (1 atm). c) 24{1–8}, 1,2-dichloroethane, NaBH(OAc)3, AcOH. d) 24{9–42}, NEt3, CH2Cl2.
Table 3.
Deprotection of 18 and 20.
| Entry | Carbamate | Free amine | Yield(%) |
|---|---|---|---|
| 1 | 18{2} | 22{2} | 98a |
| 2 | 18{3} | 22{3} | 95a |
| 3 | 20{2,2} | 23{2,2} | 87a |
| 4 | 20{2,3} | 23{2,3} | 88a |
| 5 | 20{2,6} | 23{2,6} | 80a |
| 6 | 20{2,7} | 23{2,7} | 98b |
| 7 | 20{2,8} | 23{2,8} | 94b |
| 8 | 20{2,9} | 23{2,9} | 77b,c |
| 9 | 20{2,10} | 23{2,10} | 87a |
| 10 | 20{2,11} | 23{2,11} | 93a |
| 11 | 20{3,7} | 23{3,7} | 76a |
TMSI
Pd(0)/H2
crude yield
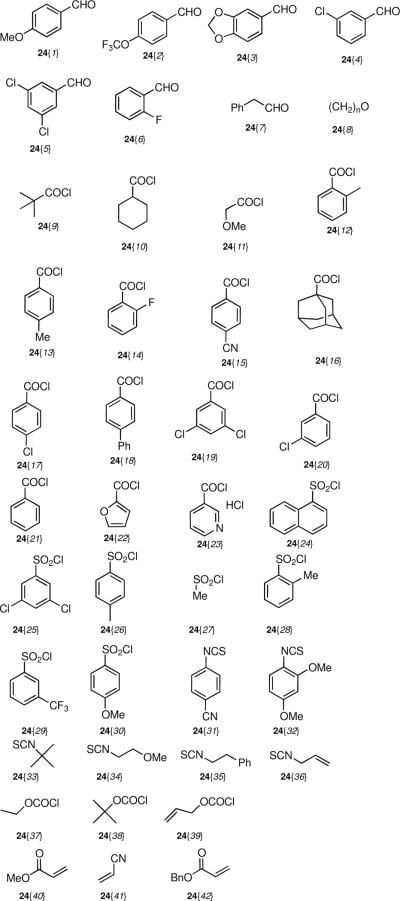
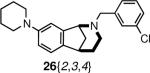
With the free amines 22 and 23 in hand, a series of N-functionalization reactions were conducted to generate libraries of tertiary amines 25 and 26, amides 27 and 28, sulfonamides 29 and 30, carbamates 31 and 32, thioureas 33 and 34, β-amino esters 35 and a β-amino cyanide 36 (Scheme 4, Table 4, Figure 3). Generally, the N-functionalizations of 22 and 23 were performed using a two-fold excess of commercially-available reagents to ensure complete consumption of the starting amine. Both aromatic and aliphatic reagents were employed in diversification reactions to give norbenzomorphan derivatives bearing substituents having a broad spectrum of electronic properties, which will be useful for developing SAR studies following the identification of initial `hits' during screening. In the case of aromatic functionalizing agents, the ring substitution pattern and the electronic nature of the substituents were varied to afford both electron rich and electron poor aromatic analogs. Aliphatic functionalizing agents were chosen to generate norbenzomorphan derivatives having substituents with varying degrees of non-polar surface area. For reductive aminations, the excess aldehyde helped compensate for the loss of a portion of reagent via reduction to the alcohol. The reaction of amines 22 and 23 with acylating agents, isothiocyanates and acrylates were conducted in the presence of triethylamine at room temperature to provide norbenzomorphan derivatives 27–36. It was found that the addition of triethylamine to the reactions of 22 and 23 with acrylates and isothiocyanates gave improved product yields, and it is suggested that the triethylamine helps to facilitate the reaction by acting as a proton transfer agent.
Table 4.
N-Functionalization of 22 and 23.
| Entry | Free amine | Function alizing agent | Product | Yield(%) |
|---|---|---|---|---|
| 1 | 22{2} | 24{1} | 25{2,1} | 72 |
| 2 | 22{2} | 24{9} | 27{2,9} | 88 |
| 3 | 22{2} | 24{9} | 27{2,9} | 40 |
| 4 | 22{2} | 24{11} | 27{2,11} | 71 |
| 5 | 22{2} | 24{12} | 27{2,12} | 95 |
| 6 | 22{2} | 24{24} | 29{2,24} | 87 |
| 7 | 22{2} | 24{25} | 29{2,25} | 77 |
| 8 | 22{2} | 24{26} | 29{2,26} | 68 |
| 9 | 22{2} | 24{37} | 31{2,37} | 74 |
| 10 | 22{2} | 24{38} | 31{2,38} | 88 |
| 11 | 22{2} | 24{40} | 36{2,40} | 81 |
| 12 | 23{2,11} | 24{9} | 28{2,11,9} | 80 |
| 13 | 23{2,11} | 24{10} | 28{2,11,10} | 98 |
| 14 | 23{2,11} | 24{13} | 28{2,11,13} | 55 |
| 15 | 23{2,11} | 24{14} | 28{2,11,14} | 98 |
| 16 | 23{2,11} | 24{18} | 28{2,11,18} | 74 |
| 17 | 23{2,11} | 24{19} | 28{2,11,19} | 56 |
| 18 | 23{2,11} | 24{24} | 30{2,11,24} | 59 |
| 19 | 23{2,11} | 24{29} | 30{2,11,29} | 57 |
| 20 | 23{2,11} | 24{30} | 34{2,11,30} | 53 |
| 21 | 23{2,11} | 24{31} | 34{2,11,31} | 72 |
| 22 | 23{2,11} | 24{32} | 34{2,11,32} | 53 |
| 23 | 23{2,11} | 24{33} | 34{2,11,33} | 80 |
| 24 | 23{2,11} | 24{34} | 34{2,11,34} | 70 |
| 25 | 23{2,11} | 24{35} | 34{2,11,35} | 98 |
| 26 | 23{2,6} | 24{15} | 28{2,6,15} | 91 |
| 27 | 23{2,6} | 24{17} | 28{2,6,17} | 85 |
| 28 | 23{2,6} | 24{27} | 30{2,6,27} | 61 |
| 29 | 23{2,7} | 24{7} | 26{2,7,7} | 75 |
| 30 | 23{2,7} | 24{8} | 26{2,7,8} | 87 |
| 31 | 23{2,8} | 24{2} | 26{2,8,2} | 77 |
| 32 | 23{2,8} | 24{9} | 28{2,8,9} | 53 |
| 33 | 23{2,8} | 24{10} | 28{2,8,10} | 86 |
| 34 | 23{2,8} | 24{16} | 28{2,8,16} | 51 |
| 35 | 23{2,8} | 24{28} | 30{2,8,28} | 62 |
| 36 | 23{2,8} | 24{39} | 32{2,8,39} | 55 |
| 37 | 23{2,8} | 24{41} | 35{2,8,41} | 84 |
| 38 | 23{2,9} | 24{1} | 26{2,9,1} | 59 |
| 39 | 23{2,9} | 24{3} | 26{2,9,3} | 73 |
| 40 | 23{2,9} | 24{8} | 26{2,9,8} | 66 |
| 41 | 23{2,9} | 24{39} | 32{2,9,39} | 95 |
| 42 | 23{2,9} | 24{40} | 35{2,9,40} | 83 |
| 43 | 23{2,10} | 24{3} | 26{2,10,3} | 71 |
| 44 | 23{2,10} | 24{12} | 28{2,10,12} | 81 |
| 45 | 23{2,10} | 24{15} | 28{2,10,15} | 82 |
| 46 | 23{2,10} | 24{20} | 28{2,10,20} | 67 |
| 47 | 23{2,10} | 24{25} | 30{2,10,25} | 71 |
| 48 | 23{2,3} | 24{4} | 26{2,3,4} | 75 |
| 49 | 23{2,3} | 24{10} | 28{2,3,10} | 61 |
| 50 | 23{2,3} | 24{14} | 28{2,3,14} | 63 |
| 51 | 23{2,3} | 24{15} | 28{2,3,15} | 53 |
| 52 | 23{2,3} | 24{19} | 28{2,3,19} | 56 |
| 53 | 23{2,2} | 24{5} | 26{2,2,5} | 96 |
| 54 | 23{2,2} | 24{6} | 26{2,2,6} | 70 |
| 55 | 23{2,2} | 24{8} | 26{2,2,8} | 92 |
| 56 | 23{2,2} | 24{20} | 28{2,2,20} | 75 |
| 57 | 23{2,2} | 24{30} | 30{2,2,30} | 62 |
| 58 | 22{1} | 24{22} | 28{1,22} | 60 |
| 59 | 22{1} | 24{23} | 28{1,23} | 66 |
| 60 | 22{1} | 24{31} | 33{1,31} | 60 |
| 61 | 22{1} | 24{32} | 33{1,32} | 60 |
| 62 | 22{1} | 24{33} | 33{1,33} | 66 |
| 63 | 22{1} | 24{34} | 33{1,34} | 71 |
| 64 | 22{1} | 24{35} | 33{1,35} | 66 |
| 65 | 22{1} | 24{36} | 33{1,36} | 68 |
| 66 | 22{1} | 24{42} | 36{1,42} | 67 |
| 67 | 23{3,7} | 24{3} | 26{3,7,3} | 55 |
| 68 | 23{3,7} | 24{8} | 26{3,7,8} | 93 |
| 69 | 23{3,7} | 24{11} | 28{3,7,11} | 81 |
| 70 | 23{3,7} | 24{30} | 30{3,7,30} | 85 |
| 71 | 23{3,7} | 24{35} | 34{3,7,35} | 89 |
| 72 | 23{3,7} | 24{39} | 32{3,7,39} | 73 |
Figure 3.
N-Functionalizing agents.
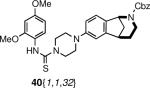
The piperazine-coupled norbenzomorphans 20{1–2,1} represent a new class of compounds, and arylpiperazines are themselves considered to be privileged structures.1f Accordingly, we prepared a small set of derivatives possessing this motif by functionalizing the free nitrogen atom in 20{1–2,1} to afford products that varied in terms of Lewis bacisity as well as the number of hydrogen bond donors and acceptors (Scheme 5, Table 5, Figure 3). An amine (37), amides (38), sulfonamides (39) and thioureas (40) were obtained in moderate to good yield.
Scheme 5. Derivatization of piperazines 20{1–2,1}.
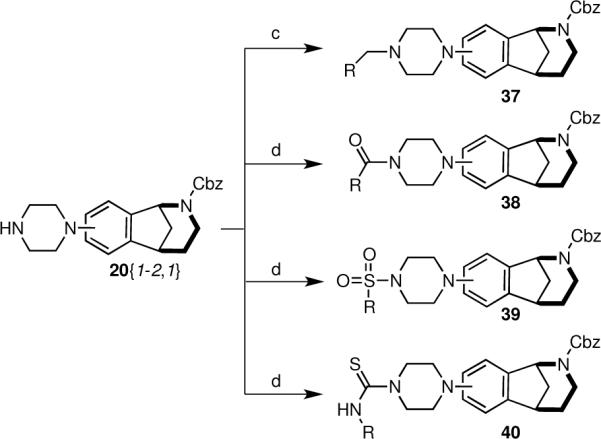
Conditions: See scheme 4.
Table 5.
Library derived from 20{1–2,1}.
| Entry | Piperazine | N-functionali zing Reagent | Product | Yield (%) |
|---|---|---|---|---|
| 1 | 20{1,1} | 24{3} | 37{1,1,3} | 58 |
| 2 | 20{1,1} | 24{21} | 38{1,1,21} | 54 |
| 3 | 20{1,1} | 24{32} | 40{1,1,32} | 68 |
| 4 | 20{1,1} | 24{9} | 38{2,1,9} | 96 |
| 5 | 20{2,1} | 24{13} | 38{2,1,13} | 93 |
| 6 | 20{2,1} | 24{18} | 38{2,1,18} | 92 |
| 7 | 20{2,1} | 24{20} | 39{2,1,20} | 97 |
| 8 | 20{2,1} | 24{35} | 40{2,1,35} | 60 |
| 9 | 20{2,1} | 24{25} | 39{2,1,25} | 68 |
| 10 | 20{2,1} | 24{29} | 39{2,1,29} | 53 |
| 11 | 20{2,1} | 24{30} | 39{2,1,30} | 54 |
BIOLOGICAL ACTIVITY
Biological screening of the 124 member norbenzomorphan library is currently underway in the NIH's Molecular Library Probe Production Center Network (MLPCN), the National Institute of Mental Health's (NIMH) Psychoactive Drug Screening Program (PDSP), and Eli Lilly's Open Innovation Drug Discovery (OIDD) Program in order to identify biological probes and potential leads for new therapeutics. The assays are comprised of both phenotypic and target-based modules, which are useful for interrogating complex cellular systems as well as assessing enzymatic activity and receptor-ligand binding, respectively. These collaborations have unveiled some interesting biological activities which have not been previously associated with the norbenzomorphan class of compounds. Some of these data obtained from the MLPCN are detailed herein (Table 6).
Table 6.
Biological activity of select norbenzomorphans.
| Entry | Compound | Activity* | Potency |
|---|---|---|---|
| 1 |
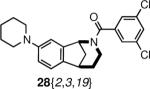
|
human M1 muscarinic receptor antagonist18 | 69% (3uM) |
| 2 |
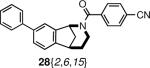
|
striatal-enriched protein tyrosine phosphatase (STEP) inhibitor16 | 69% (20 uM) |
| 3 |
|
fatty acid synthase inhibitor17 | 57% (15uM) |
| 4 |
|
Y. pestis topoisomerase I inhibitor19 | 61% (10 uM) |
| 5 |
|
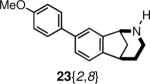
mycobacterium tuberculosis bioA enzyme inhibitor20 | 91% (10 uM) |
| 6 |
|
microphthalmia-associated transcription factor (MITF) activator21 | 3.3 uM (AC40) |
| 7 |
|
serotonin 5A receptor (5HTR5A) inverse agonist22 | 27% (9.3 uM) |
an `active' compound is defined individually by each assay provider; see references 16–22.
It is noteworthy that inhibitors of both striatal-enriched protein tyrosine phosphatase (STEP)16 and fatty acid synthase (FAS)17 are being investigated as potential therapeutics for the treatment of Alzheimer's Disease (AD) and cancer, respectively (Table 6). STEP is a brain specific tyrosine phosphatase that is elevated in AD patients. Recent work suggests that decreasing STEP levels in the prefrontal cortex can mitigate the cognitive deficits from AD. Furthermore, FAS is overexpressed in many cancers and is believed to be essential for the growth of solid tumors. It has been demonstrated that inhibition of FAS can induce apoptosis in cancer cells. Accordingly, compounds that exhibit selective inhibition of these targets could lead to advances in the development of drugs relevant to these diseases.
SUMMARY
We have prepared a 124 membered library of novel norbenzomorphans by employing a sequential MCAP/RCM/Heck reaction sequence to generate the unsaturated azabicycle core, which was subsequently reduced to the target scaffold under ionic conditions. Utilizing a series of palladium catalyzed cross-coupling reactions and nitrogen functionalizations, the synthesis of a diverse set of norbenzomorphans bearing functional groups with varying electronic properties and substitution patterns was achieved. A complete Lipinski analysis of a representative group of 40 compounds expressing maximal diversity of substituents and aromatic substitution patterns was performed (see supporting information). Only one compound was found to violate more than one Lipinski parameter, suggesting that the majority of library members are expected to have desirable physiochemical properties.23 Preliminary biological screening has identified `hits' that could serve as starting points for `lead' development. The wide range of biological activities associated with members of this library lends support to the `privileged scaffold' approach towards the identification of novel drug leads across a range of biological targets. SAR studies involving select `hit' compounds are ongoing to facilitate the development of `leads' for small molecule probes and therapeutics. Further applications of this and related approaches to the synthesis of medicinally relevant compound libraries are in progress, and the results of these investigations will be reported in due course.
Supplementary Material
ACKNOWLEDGEMENT
We thank the National Institutes of Health (GM 86192 and GM 24539) and the Robert A. Welch Foundation (F-0652) for their generous support of this work. We are also grateful to Dr. Richard Pederson (Materia, Inc.) for catalyst support.
Footnotes
SUPPORTING INFORMATION Experimental procedures, full characterization data, and tabulated Lipinski parameters for representative compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).(a) Evans BE, Rittle KE, Bock MG, Dipardo RM, Freidinger RM, Whitter WL, Lundell GF, Veber DF, Anderson PS, Chang RSL, Lotti VJ, Cerino DJ, Chen TB, Kling PJ, Kunkel KA, Springer JP, Hirshfield J. Methods for drug discovery: Development of potent, selective, orally effective cholecystokinin antagonists. J. Med. Chem. 1988;31:2235–2246. doi: 10.1021/jm00120a002. [DOI] [PubMed] [Google Scholar]; (b) Horton DA, Bourne GT, Smythe ML. The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem. Rev. 2003;103:893–930. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]; (c) DeSimone RW, Currie KS, Mitchell SA, Darrow JW, Pippin DA. Privileged structures: Applications in drug discovery. Comb. Chem. High Throughput Screening. 2004;7:473–493. doi: 10.2174/1386207043328544. [DOI] [PubMed] [Google Scholar]; (d) Costantino L, Barlocco D. Privileged structures as leads in medicinal chemistry. Curr. Med. Chem. 2006;13:65–85. [PubMed] [Google Scholar]; (e) Duarte CD, Barreiro EJ, Fraga CAM. Privileged structures: A useful concept for the rational design of new lead candidates. Mini-Rev. Med. Chem. 2007;7:1108–1119. doi: 10.2174/138955707782331722. [DOI] [PubMed] [Google Scholar]; (f) Welsch ME, Snyder SA, Stockwell BR. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 2010;14:347–361. doi: 10.1016/j.cbpa.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; For reviews on privileged structures, see:
- (2).Han C, Zhang J, Zheng M, Xiao Y, Li Y, Liu G. An integrated drug-likeness study for bicyclic privileged structures: from physiochemical properties to in vitro ADME properties. Mol. Divers. 2011;15:857–876. doi: 10.1007/s11030-011-9317-2. [DOI] [PubMed] [Google Scholar]
- (3).(a) Jacobsen AE, Mokotoff M. Azabicyclo Chemistry. I. Synthesis of 1,5-methano-7-methoxy-2,3,4,5-tetrahydro-1H-2-benzazepines. B-Norbenzomorphans. J. Med. Chem. 1970;13:7–9. doi: 10.1021/jm00295a002. [DOI] [PubMed] [Google Scholar]; (b) Kanematsu K, Takeda M, Jacobson AE, May EL. Synthesis of 6,7-benzomorphan and related nonquaternary carbon structures with marked analgesic activity. J. Med. Chem. 1969;12:405–408. doi: 10.1021/jm00303a015. [DOI] [PubMed] [Google Scholar]
- (4).(a) Greig N,H, Pei XF, Soncrant TT, Ingram DK, Brossi A. Phenserine and ring C heteroanalogs: Drug candidates for the treatment of Alzheimer's Disease. Med. Res. Rev. 1995;15:3–31. doi: 10.1002/med.2610150103. [DOI] [PubMed] [Google Scholar]; (b) Lauven P, Stoeckel H. Flumazenil (Ro 15–1788) and physostigmine. Resuscitation. 1988;16:S41–S48. doi: 10.1016/0300-9572(88)90004-4. [DOI] [PubMed] [Google Scholar]
- (5).Chen YL, Liston D, Nielson J, Chapin D, Dunaiskis A, Hedberg K, Ives J, Johnson J, Jones S. Synthesis and anticholinesterase activity of tetrahydrobenzazepine carbamates. J. Med. Chem. 1994;37:1996–2000. doi: 10.1021/jm00039a013. [DOI] [PubMed] [Google Scholar]
- (6).(a) Sunderhaus JD, Dockendorff C, Martin SF. Applications of multicomponent reactions for the synthesis of diverse heterocyclic scaffolds. Org. Lett. 2007;9:4223–4226. doi: 10.1021/ol7018357. [DOI] [PubMed] [Google Scholar]; (b) Sunderhaus JD, Dockendorff C, Martin SF. Synthesis of diverse heterocyclic scaffolds via tandem additions to imine derivatives and ring-forming reactions. Tetrahedron. 2009;65:6454–6469. doi: 10.1016/j.tet.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Donald JR, Granger BA, Hardy S, Sahn JJ, Martin SF. Applications of multicomponent assembly processes to the facile syntheses of diversely functionalized nitrogen heterocycles. Heterocycles. 2010;84:1089–1112. doi: 10.3987/COM-11-S(P)92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Martin SF, Benage B, Hunter JE. A concise strategy for the syntheses of indole alkaloids of the heteroyohimboid and corynatheioid families. Total syntheses of (±)-tetrahydroalstonine, (±)-cathenamine and (±)-geissoschizine. J. Am. Chem. Soc. 1988;110:5925–5927. [Google Scholar]
- (8).Sahn JJ, Martin SF. Facile syntheses of substituted, conformationally-constrained benzoxazocines and benzazocines via sequential multicomponent assembly and cyclization. Tetrahedron Lett. 2011;52:6855–6858. doi: 10.1016/j.tetlet.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).(a) Donald JR, Martin SF. Synthesis and diversification of 1,2,3-triazole-fused 1,4-benzodiazepine scaffolds. Org. Lett. 2011;13:852–855. doi: 10.1021/ol1028404. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Donald JR, Wood RR, Martin SF. Application of a sequential multicomponent assembly process/Huisgen cycloaddition strategy to the preparation of libraries of 1,2,3-triazole-fused 1,4-benzodiazepines. ACS Comb. Sci. 2012;14:135–143. doi: 10.1021/co2002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) Granger BA, Kaneda K, Martin SF. Multicomponent assembly strategies for the synthesis of diverse tetrahydroisoquinoline scaffolds. Org. Lett. 2011;13:4542–4545. doi: 10.1021/ol201739u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Granger BA, Kaneda K, Martin SF. Libraries of 2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinoline-2-amine derivatives via a multicomponent assembly process/1,3-dipolar cycloaddition strategy. ACS Comb. Sci. 2012;14:75–79. doi: 10.1021/co2001636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Sahn JJ, Su JY, Martin SF. Facile and unified approach to skeletally diverse, privileged scaffolds. Org. Lett. 2011;13:2590–2593. doi: 10.1021/ol200709h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).(a) Rawalpally T, Ji Y, Shankar A, Edwards W, Allen J, Jiang Y, Cleary TP, Pierce ME. A practical method for stabilizing lithiated halogenated aromatic compounds. Org. Proc. Res. Dev. 2008;12:1293–1298. [Google Scholar]; (b) Spring DR, Krishnan S, Blackwell HE, Schreiber SL. Diversity-oriented synthesis of biaryl-containing medium rings using a one bead/one stock solution platform. J. Am. Chem. Soc. 2002;124:1354–1363. doi: 10.1021/ja017248o. [DOI] [PubMed] [Google Scholar]; (c) Comins DL, Brown JD. Ortho metalation directed by α-amino alkoxides. J. Org. Chem. 1984;49:1078–1083. [Google Scholar]
- (13).Wolfe JP, Buchwald SL. A highly active catalyst for the room-temperature amination and Suzuki coupling of aryl chlorides. Angew. Chem., Int. Ed. Engl. 1999;38:2413–2416. doi: 10.1002/(sici)1521-3773(19990816)38:16<2413::aid-anie2413>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- (14).Littke AF, Dai C, Fu GC. Versatile catalysts for the Suzuki cross-coupling of arylboronic acids with aryl and vinyl halides and triflates under mild conditions. J. Am. Chem. Soc. 2000;122:4020–4028. [Google Scholar]
- (15).D'Andrea SV, Michalson ET, Freeman JP, Chidester CG, Szmuskzkovicz J. trans-3,4-Diaminopiperidines. Azacyclohexane congeners of k agonist U50488. J. Org. Chem. 1991;56:3133–3137. [Google Scholar]
- (16).(a) Goebel-Goody SM, Baum M, Paspalas CD, Fernandez SM, Carty NC, Kurup P, Lombroso PJ. Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol. Rev. 2012;64:65–87. doi: 10.1124/pr.110.003053. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=588621.
- (17).(a) Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202–208. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]; (b) Wang Y, Kuhajda FP, Li JN, Pizer ES, Han WF, Sokoll LJ, Chan DW. Fatty acid synthase (FAS) expression in human breast cancer cell culture supernatants and in breast cancer patients. Cancer Lett. 2001;167:99–104. doi: 10.1016/s0304-3835(01)00464-5. [DOI] [PubMed] [Google Scholar]; (c) http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=602261.
- (18).(a) Waelbroeck M, Tastenoy M, Camus J, Christophe J. Binding of selective antagonists to four muscarinic receptors (M1 to M4) in rat forebrain. Mol. Pharmacol. 1990;38:267–273. [PubMed] [Google Scholar]; (b) http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=588852.
- (19).(a) Tse-Dinh Y. Bacterial topoisomerase I as a target for discovery of antibacterial compounds. Nucleic Acids Res. 2009;37:731–737. doi: 10.1093/nar/gkn936. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=504884.
- (20). See: http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=602481.
- (21). See: http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=540258.
- (22). See: http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=504634.
- (23).Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.