Abstract
Transgenic (Tg) mice overexpressing human amyloid precursor protein (APP) mutants reproduce features of early Alzheimer's disease (AD) including memory deficit, presence of ß-amyloid (Aß) oligomers, and age-associated formation of amyloid deposits. In this study we used hippocampal microdialysis to characterize the signaling of N-methyl-D-aspartic acid receptors (NMDA-Rs) in awake and behaving AD Tg mice. The NMDA-R signaling is central to hippocampal synaptic plasticity underlying memory formation and several lines of evidence implicate the role of Aß oligomers in effecting NMDA-R dysfunction. CA1 NMDA-Rs were stimulated by NMDA infused through reverse microdialysis while changes in the cyclic guanosine monophosphate (cGMP) concentration in the brain interstitial fluid (ISF) were used to determine NMDA-Rs responsiveness. While four months old wild type C57BL/6 mice mounted robust cGMP response to the NMDA challenge, the same stimulus failed to significantly change the cGMP level in four and 15 months old APPSW and four months old APPSW/PS1L166P Tg mice, which were all on C57BL/6 background. Lack of response to NMDA in AD Tg mice occurred in the absence of changes in expression levels of several synaptic proteins including synaptophysin, NR1 NMDA-R subunit and postsynaptic density protein 95, which indicates lack of profound synaptic degeneration. Aß oligomers were detected in all three AD Tg mice groups and their concentration in the hippocampus ranged from 40.5±3.6ng/g in four months old APPSW mice to 60.8±15.9ng/g in four months old APPSW/PS1L166P mice. Four months old APPSW mice had no Aß amylod plaques, while the other two AD Tg mice groups showed evidence of incipient Aß amyloid plaque formation. Our studies describes a novel approach useful to study the function of NMDA-Rs in awake and behaving AD Tg mice and demonstrate impairment of NMDA-R response in the presence of endogenously formed Aß oligomers but predating onset of Aß amyloidosis.
Keywords: Aß oligomers, AD transgenic mice, cGMP, Hippocampus, Microdialysis, NMDA receptor
1. Introduction
Accumulation of β-amyloid (Aß) peptide in the brain is central to the pathogenesis of Alzheimer's disease (AD) and precedes formation of neurofibrillary tangles and neuronal loss. Aß is a 39–43 amino acid, hydrophobic peptide, derived from enzymatic cleavage of the amyloid precursor protein (APP). Aß is prone to self-aggregation as soluble oligomers or insoluble fibrils, which form plaques and vascular deposits. Transgenic (Tg) mouse models of AD overexpressing mutated form of the amyloid precursor protein (APP) demonstrate impairment of synaptic plasticity and deficit on memory based behavioral tests, which both occur prior to appearance of amyloid plaques (reviewed in Ashe et al., 2010). Toxicity of soluble Aß oligomers is thought to play central role in pathogenesis of early synaptic dysfunction. Various preparations of Aß oligomers including those isolated from brains of AD patients, or from culture media of APP transfected cells, or obtained by in vitro aggregation of synthetic Aß were shown to impair long-term potentiation (LTP) when injected into the ventricles of wild type (WT) rodents or applied into organotypic hippocampal slices (reviewed in Selkoe, 2008). While this effect has mostly been shown with application of exogenous preparations of Aß oligomers there are limited evidence that endogenously formed Aß oligomers produce similar effect in situ.
The phenomenon of the hippocampal LTP, which underlies synaptic plasticity and memory formation depends on N-methyl-D-aspartate receptors (NMDA-R) signaling. In this study, we employed in vivo microdialysis to characterize NMDA-R signaling in the hippocampus of awake and behaving AD Tg mice, using a challenge with a selective NMDA-R agonist NMDA, infused through reverse microdialysis, while the concentration of cyclic guanosine monophosphate (cGMP) in the dialysate of the brain interstitial fluid (ISF) was used as a metric of NMDA-R signal transduction. This approach has been previously validated as a sensitive and reliable method to assess NMDA-R signaling in the rat hippocampus and other brain regions in receptor pharmacological studies and in rat model of hepatic encephalopathy (reviewed in Pepicelli et al., 2004). Here for the first time we utilized hippocampal microdialysis to investigate NMDA-R/cGMP signaling in AD Tg mice prior to the appearance of extensive Aß amyloid deposits. Furthermore, we characterized in the hippocampus of AD Tg mice expression levels of several synaptic proteins including NR1 subunit of NMDA-R, postsynaptic density protein (PSD) 95, and synaptophysin and three different pools of Aß: exchangeable Aß (eAß1-X) in the ISF dialysate, which represent monomeric Aß, Aß oligomers in the hippocampal homogenate, and the Aß amyloid plaque load.
2. Material and methods
2.1. Animals
All mouse experimental procedures were carried out in accordance with the National Institutes of Health guide for the care of laboratory animals and were approved by the Institutional Animal Care and Use Committees. When designing the study all efforts were made to minimize animal suffering and reduce the number of animals used.
The study was performed on four months old APPSW/PS1L166P double Tg mice, where a human APP sequence with the double Swedish mutation KM670/671NL and a human PS 1 sequence with the L166P mutation are expressed under the control of a neuron-specific Thy1 promoter and transmitted as a single genetic trait (Radde et al., 2006), and on four and 15 months old male APPSW single Tg mice described previously (Sadowski et al., 2006). Since both APPSW/PS1L166P and APPSW mice were maintained on C57BL/6 background, male C57BL/6 wild type (WT) mice were used as controls. Animal husbandry and genotyping were as described previously (Sadowski et al., 2006).
2.2. Materials and reagents
Unless otherwise specified microdialysis supplies were obtained from Bioanalytical Systems (West Lafayette, IN), whereas chemicals, reagents, and antibodies were obtained from Sigma-Aldrich (St. Louis, MO).
2.3. Hippocampal microdialysis
Mice were anesthetized with an intraperitoneal injection of Ketamine (50mg/kg) and Xylazine (10mg/kg). A BR-10 guide cannula was stereotactically implanted into the right hippocampus (bregma - 3.1mm, 2.4 mm lateral, 12°angle) and four hours later a BR-4 microdialysis probe with 38kDa molecular weight cutoff (MWCO) membrane was introduced through the cannula so that the microdialysis membrane was almost entirely embedded in the strata radiatum and lacunosum-moleculare of the CA1 hippocampal sector (Supplemental Fig 1A). After recovering from anesthesia mice were placed in the Raturn Cage Systems designed to allow unrestricted animal movements without applying pressure to probe assembly (Supplemental Fig 1B). Microdialysis experiments were commenced at least 16hr after the probe placement. The probe was perfused at the constant rate of 1μL/min with artificial cerebrospinal fluid (aCSF) containing 0.15% bovine serum albumin (Cirrito et al., 2003). The ISF dialysate was collected over 30 or 60min intervals into polypropylene tubes containing 4mM Ethylenediaminetetraacetic acid. Samples collected over the first 180min were used to determine concentration of eAß1-X and the baseline cGMP level. Then the probe was perfused over 60min with the aCSF containing NMDA followed by the perfusion with aCSF alone for an additional 120min to determine post-NMDA cGMP level.
2.4. Tissue processing and histology
After the conclusion of the microdialysis mice were killed (150mg/kg sodium pentobarbital) and transcardially perfused as previously described (Sadowski et al., 2006). The right brain hemisphere was sectioned into serial 40μm-thick coronal sections, which were stained with cresyl violet to confirm the microdialysis probe location and Thioflavin-S to determine Aß amyloid plaque load. The hippocampus was dissected from the left hemisphere and homogenized as previously described (Sadowski et al., 2006).
2.5. cGMP determination
The cGMP concentration in the ISF dialysate was determined using cGMP Enzyme Immunoassay Biotrak ELISA kit (GE Healthcare Bio-Sciences Corp., Piscataway, NJ), following the manufacturer's provided manual.
2.6. Quantification of synaptic protein expression in the hippocampus
Expression levels of the NR1, PSD-95, and synaptophysin were determined by Western-blot densitometry. The following monoclonal antibodies (mAb) were used for Western-blot signal detection: against the C-terminus of NMDA-R NR1 subunit (1:2,000; Millipore Corp., Billerica, MA), PSD-95 (1:500; Millipore Corp., Billerica, MA), synaptophysin (1:500; Santa Cruz Biotechnology, Santa Cruz, CA), and ß-actin (1:2,500), which was used as a loading control.
2.7. Aß quantification
eAß1-x concentration in the ISF dialysate was quantified using human Aß1-x specific sandwich ELISA utilizing mAb 6E10 (Aß residues 1–16) and biotinylated mAb 4G8 (Aß residues 17–24) as the capture antibody and as the detection antibody, respectively. Aß1-x ELISA detects Aß species regardless of their C-terminal configuration including Aß38, Aß40 and Aß42 (Hong et al., 2011) and it was performed following our published protocols (Sadowski et al., 2006).
Concentration of Aß oligomers in the hippocampal homogenate was determined using Human Aggregated ß-Amyloid ELISA Kit (Invitrogen, Carlsbad, CA) (Calderon-Garciduenas et al., 2009), following the manufacturer's provided manual. This assay utilizes Aß N-terminal mAb both as the capture and detection antibody and four member solid phase sandwiches are formed only when aggregates containing multiple copies of Aß (≥2) are present in the sample.
The load of Aß amyloid deposits in the hippocampus was determined on Thioflavin-S stained sections using unbiased sampling and automated image threshold analysis, as previously described (Sadowski et al., 2006).
2.8. Statistical analysis
Individual data of the ISF cGMP concentrations and optic density of synaptic proteins were converted to percentage values using the mathematical mean of the control group as the 100% value. Repeated measures analysis of variance (ANOVA) was used to analyze differences in cGMP level before, during and after the NMDA challenge in various animal groups. Expression levels of synaptic protein were analyzed using one-way ANOVA. Bonferroni test was used for post-hoc ANOVA analysis. Differences in the concentrations of eAß1-x and Aß oligomers were analyzed using Mann-Whitney U test. Statistical analysis was performed using GraphPad Prism v5.02 (GraphPad Software, Inc., San Diego).
3. Results
3.1. Impairment of NMDA-R/cGMP response in AD Tg mice
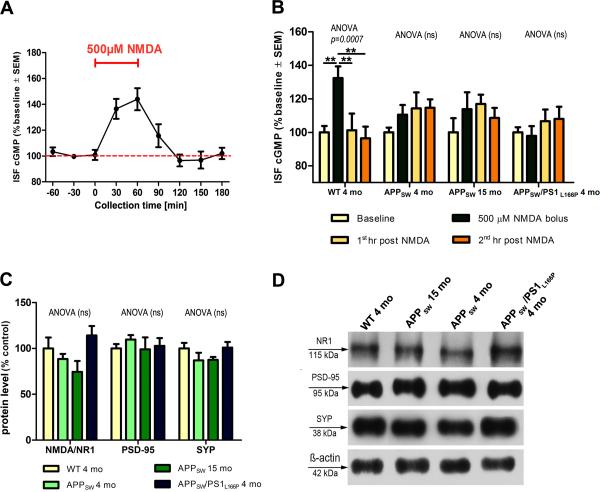
In four months old WT mice 500μM NMDA challenge increased cGMP level to 136.5±5.9% and 144±8.6% of the baseline during the first and the second 30min interval after commencing the infusion, respectively (Fig 1A). Within 60min after concluding the NMDA infusion cGMP level returned to its baseline value. In subsequent experiments comparing the cGMP response to 500μM NMDA challenge between WT and AD Tg mice the ISF cGMP level were averaged over one hour intervals. Again in WT mice the challenge with 500μM NMDA caused elevation of cGMP level upto 132.4±8% (p<0.01) of the baseline value, followed by its return to the baseline during the first hour after the infusion (Fig 1B). In APPSW Tg mice the NMDA challenge induced only a modest increase in the cGMP level reaching 110.5±5.7% and 113.8±11.5% of the baseline, in four and 15 months old mice, respectively (differences not statistically significant). Unlike in WT mice, the cGMP level in APPSW mice, did not return to baseline after concluding the NMDA challenge, but remained modestly elevated without reaching statistical significance. In APPSW/PS1L166P mice the 500μM NMDA challenge failed to induce the cGMP response at all. To determine whether lack of response in AD Tg mice is dose dependent we challenged WT and APPSW/PS1L166P mice with lower and higher NMDA concentrations. One micromole of NMDA did not produce significant response in either WT and APPSW/PS1L166P mice, whereas 1mM of NMDA raised the cGMP level to 123.3±22.4% (p<0.05) of the baseline in WT mice, while it failed to change cGMP level in APPSW/PS1L166P mice (Supplemental Fig 2).
Figure 1.
NMDA-R/cGMP microdialysis and synaptic protein expression level. (A) A detailed time course of the ISF cGMP response to 500μM of NMDA in four month old WT mice (n=6). Spontaneous fluctuations in the ISF cGMP concentration in samples taken before and after NMDA challenge were <5% of the mean baseline value. (B) A comparison of the cGMP response to 500μM of NMDA between four month old WT mice (n=6), four (n=6) and 15 month old (n=3) APPSW mice and four months old APPSW/PS1L166P mice (n=8). (C) Densitometric analysis of NR1 NMDAR subunit, PSD-95, and synaptophysin expression level. (D) Example Western-blots of synaptic proteins in the hippocampal homogenate with ß-actin as a loading control. ns=non significant, **p<0.01, SEM=standard error of the mean.
3.2. Quantification of synaptic protein expression level in the hippocampus
Expression levels of the NR1 NMDA-R subunit in the hippocampal homogenate in four months old WT and APPSW Tg mice were comparable (Fig 1C, D). In 15 months old APPSW and in APPSW/PS1L166P Tg mice 17% decrease and a 17.2% increase comparing to WT mice were observed respectively, which did not reach statistical significance. Expression level of PSD-95 and synaptophysin in the hippocampus was closely comparable between WT mice and all three Tg mice groups.
3.3. eAß1-x level in the hippocampal ISF
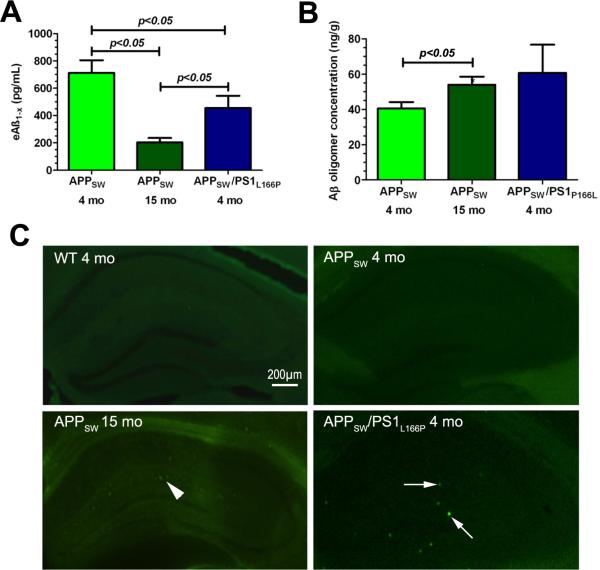
The mean eAß1-x concentration in the dialysate of hippocampal ISF in four months old APPSW Tg mice was 712.5±92 pg/mL (Fig 2A), whereas in 15 months old APPSW mice its concentration was significantly lower 201.7±34.9 pg/mL (p<0.05). In APPSW/PS1L166P mice the mean eAß1-x concentration was 455.5±88.7pg/mL, which was significantly lower than that in four months old APPSW mice but significantly higher than that in 15 months old APPSW mice (p<0.05).
Figure 2.
Characterization of various Aß pools in the hippocampus of AD Tg mice. (A) Concentration of eAβ1−x representing the pool of monomeric Aß species contained in the ISF. (B) Concentration of Aβ oligomers in the hippocampal homogenate. (C) Aß amyloid deposits in the hippocampus revealed by Thioflavin-S staining. Infrequent, sparse and small Aß plaques consistent with incipient stage of Aß amyloid deposition were seen 15 months old APPSW mice (arrowhead). Rare and small, but more conspicuous Aß plaques found in APPSW/PS1L166P mice are indicated by arrows. No plaques were seen in four months old WT and APPSW Tg mice.
3.4. Aß oligomer concentration and Aß amyloid plaque load in the hippocampus
The concentration of Aß oligomers in the hippocampal homogenate of four and 15 months old APPSW mice were 40.5±3.6ng/g and 54±4.6ng/g of wet brain tissue (p<0.05), respectively (Fig 2B). The concentration of Aß oligomers in APPSW/PS1L166P mice was 60.8 ±15.9ng/g. Thioflavin-S staining revealed no amyloid Aß plaques in the hippocampus in four months old APPSW Tg mice (Fig 2C). Infrequent, sparse and small Aß plaques consistent with incipient stage of Aß amyloid deposition were seen in 15 months old APPSW mice. Rare and small, but more conspicuous Aß plaques were seen APPSW/PS1L166P double Tg mice. The load of Aß amyloid deposits in APPSW/PS1L166P mice was 0.045±0.038%, whereas in 15 months old APPSW mice it was too low to be reliably quantified. No signal on eAß1-x ELISA, aggregated Aß ELISA or positive Thioflavin-S staining were identified in any of WT mice.
4. Discussion
NMDA-R/cGMP microdialysis constitutes an indirect yet very specific approach to determine NMDA-R signal transduction in vivo. NMDA-Rs form transmembrane Ca2+ channels, which activation by a physiological agonist glutamate or NMDA, results in Ca2+ influx into neurons. Rapid raise in the cytosolic Ca2+ level activates nitric oxide synthase (NOS) causing generation of nitric oxide (NO), which in turn stimulates soluble guanylyl cyclase (sGC) to produce cGMP. Upon diffusion to the ISF cGMP can be measured there through a microdialysis probe to determine NMDA-R signal transduction. The microdialysis approach assessing the NMDA-R signaling through determination cGMP release has been widely validated in numerous physiological and disease rat models, and it was shown to be abolished in wild type rodents by administration of NMDA-R specific antagonists like MK-801 (reviewed in Fedele et al., 1999 and Pepicelli et al., 2004). Using NMDA-R/cGMP microdialysis we demonstrated here for the first time impairment of the NMDA-R signal transduction in the hippocampus of awake and behaving AD Tg mice. Impairment of NMDA-R/cGMP response was found in the absence of extensive Aß amyloid deposits and preceded onset of memory deficit demonstrable by behavioral testing the earliest at the age of eight months in APPSW/PS1L166P mice (Radde et al., 2006) and at the age of nine months and APPSW mice (Ashe et al., 2010).
Toxicity of Aß oligomers has been implicated in the early stages of synaptic dysfunction in AD models. Treatment of primary neuronal cultures with exogenous Aß oligomers was shown to cause rapid reduction of membrane expression of NMDA-Rs (Snyder et al., 2005), while in other in vitro experiments treatment of hippocampal slices with exogenous Aß oligomers was found to trigger even more profound synaptic pathology including reduction in synaptic spine density and LTP deficit (reviewed in Selkoe, 2008). There is also evidence provided by studies on primary neuronal cultures derived from AD Tg mice, that intrasynaptic accumulation and oligomerization of Aß can cause down-regulation of NMDA-R expression and dysfunction of glutamatergic synapses (reviewed in Tampellini et al., 2010). In this study we found the impairment of NMDA-R/cGMP hippocampal response in the presence of endogenously formed Aß oligomers. Their concentration measured in the hippocampal homogenate likely reflects a combination of both extracellular and intracellular Aß oligomeric pools. Apart from synaptotoxic effect of extracellular and intracellular Aß oligomers impairment of NMDA-R/cGMP signaling can be also linked to disrupted Ca2+ homeostasis detectable early in AD Tg mice. A study utilizing hippocampal slices from 3xTg mice found evidence for increased baseline SK channel Ca2+ currents reducing neuronal excitability and increasing the threshold for induction of synaptic plasticity (Chakroborty et al., 2012). We also observed that following NMDA challenge the cGMP level in AD Tg mice did not return to its baseline like in WT mice but it remained modestly elevated. The rate of cGMP production by sGC is regulated by NOS. Upregulation of NOS activity was shown in AD Tg models and is believed to have protective effect against Aß pathology (Colton et al., 2006). Therefore, increased endogenous nitrinergic tone in AD Tg mice could underlie protracted low level cGMP response, after cessation of NMDA infusion.
No evidence of global down-regulation of synaptophysin, which is a major synaptic vesicle protein, NR1 subunit of NMDA-R and PSD-95, which is physiologically and structurally, associated with NMDA-R was found in the hippocampus of AD Tg mice despite impairment of NMDA-R/cGMP response. In vitro studies have shown that exposure of primary neuronal cultures to Aß oligomers can cause a range of toxic effects from the reduction of membrane expression of NMDA-Rs and other receptor proteins, through alteration in synaptic spine morphology to profound loss of spine density (Lacor et al., 2007). Reduction of membrane expression of NMDA-Rs is not associated with global reduction of NMDA-R expression in neurons, what indicates that Aß oligomers initially induce translocation of NMDA-Rs from synaptic membrane to the cytosol (Snyder et al., 2005). Lack of significant changes, in the expression level of synaptic proteins indicates that impairment of NMDA-R/cGMP signaling reflects rather earlier stages of Aß oligomer related synaptic pathology associated with translocation of NMDA-Rs and possibility focal and discrete loss of synaptic spines, but not caused by profound and widespread synaptic loss.
Along with cGMP detection we also analyzed the concentration of eAß1−X in the ISF microdialysate. Although, a probe with 38kDa MWCO membrane would theoretically allow for a passage of Aß monomers and aggregates smaller than 38kDa, however previous studies have demonstrated that only Aß monomers enter the probe (Hong et al., 2011). The eAß1−x concentration in four months old APPSW mice was significantly higher than that in 15 months old APPSW mice and in four months old APPSW/PS1L166P mice. Similar age-associated reduction in eAß1−x level was demonstrated in hAPP mice (Hong et al., 2011). Since eAß1−x levels are closely related to synaptic activity its reduction in older APPSW and in four month old APPSW/PS1L166P mice, that represent a more rapidly progressing model of Aß pathology can be interpreted as reduced ability of synapses to secret Aß to the ISF, which evidence was recently provided from studies on primary neuronal cultures derived from Tg2576 mice (Tampellini et al., 2011).
5. Conclusion
NMDA-R/cGMP microdialysis reveals novel evidence for early dysfunction of NMDA-R signal transduction and synaptic plasticity in the hippocampus of APPSW and APPSW/PS1L166P mice, which occur in the presence of endogenously formed Aß oligomers, but prior to the onset of Aß amyloid deposition. The coordinated NMDA-R/cGMP-eAß microdialysis technique developed in this study is a new research method, which can be helpful in characterization of new therapeutic approaches in AD Tg mouse models.
Supplementary Material
Acknowledgements
Supported by NIH/NIA grants: R01 AG031221 and K02 AG034176. The authors would like to acknowledge Dr. Mathias Jucker from Hertie-Institute for Clinical Brain Research, University of Tübingen (Tübingen, Germany) for providing APPSW/PS1L166P mice and Dr. Richard Kascsak from the New York State Institute for Basic Research (Staten Island, NY) for providing 6E10 and 4G8 mAbs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashe KH, Zahs KR. Probing the biology of Alzheimer's disease in mice. Neuron. 2010;66:631–645. doi: 10.1016/j.neuron.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Mora-Tiscareno A, Gomez-Garza G, Carrasco-Portugal MC, Perez-Guille B, Flores-Murrieta FJ, Perez-Guille G, Osnaya N, Juarez-Olguin H, Monroy ME, Monroy S, Gonzalez-Maciel A, Reynoso-Robles R, Villarreal-Calderon R, Patel SA, Kumarathasan P, Vincent R, Henriquez-Roldan C, Torres-Jardon R, Maronpot RR. Effects of a cyclooxygenase-2 preferential inhibitor in young healthy dogs exposed to air pollution: a pilot study. Toxicol. Pathol. 2009;37:644–660. doi: 10.1177/0192623309340277. [DOI] [PubMed] [Google Scholar]
- Chakroborty S, Kim J, Schneider C, Jacobson C, Molgo J, Stutzmann GE. Early presynaptic and postsynaptic calcium signaling abnormalities mask underlying synaptic depression in presymptomatic Alzheimer's disease mice. J. Neurosci. 2012;32:8341–8353. doi: 10.1523/JNEUROSCI.0936-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, May PC, O'Dell MA, Taylor JW, Parsadanian M, Cramer JW, Audia JE, Nissen JS, Bales KR, Paul SM, DeMattos RB, Holtzman DM. In vivo assessment of brain interstitial fluid with microdialysis reveals plaque-associated changes in amyloid-beta metabolism and half-life. J. Neurosci. 2003;23:8844–8853. doi: 10.1523/JNEUROSCI.23-26-08844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA, Vitek MP, Wink DA, Xu Q, Cantillana V, Previti ML, van Nostrand WE, Weinberg JB, Dawson H. NO synthase 2 (NOS2) deletion promotes multiple pathologies in a mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12867–12872. doi: 10.1073/pnas.0601075103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele E, Raiteri M. In vivo studies of the cerebral glutamate receptor/NO/cGMP pathway. Prog. Neurobiol. 1999;58:89–120. doi: 10.1016/s0301-0082(98)00077-x. [DOI] [PubMed] [Google Scholar]
- Hong S, Quintero-Monzon O, Ostaszewski BL, Podlisny DR, Cavanaugh WT, Yang T, Holtzman DM, Cirrito JR, Selkoe DJ. Dynamic analysis of amyloid beta-protein in behaving mice reveals opposing changes in ISF versus parenchymal Abeta during age-related plaque formation. J. Neurosci. 2011;31:15861–15869. doi: 10.1523/JNEUROSCI.3272-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepicelli O, Raiteri M, Fedele E. The NOS/sGC pathway in the rat central nervous system: a microdialysis overview. Neurochem. Int. 2004;45:787–797. doi: 10.1016/j.neuint.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Radde R, Bolmont T, Kaeser SA, Coomaraswamy J, Lindau D, Stoltze L, Calhoun ME, Jaggi F, Wolburg H, Gengler S, Haass C, Ghetti B, Czech C, Holscher C, Mathews PM, Jucker M. A beta 42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. Embo Reports. 2006;7:940–946. doi: 10.1038/sj.embor.7400784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski MJ, Pankiewicz J, Scholtzova H, Mehta PD, Prelli F, Quartermain D, Wisniewski T. Blocking the apolipoprotein E/amyloid-beta interaction as a potential therapeutic approach for Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2006;103:18787–18792. doi: 10.1073/pnas.0604011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav. Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat. Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Tampellini D, Gouras GK. Synapses, synaptic activity and intraneuronal abeta in Alzheimer's disease. Front Aging Neurosci. 2010;2:13. doi: 10.3389/fnagi.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampellini D, Rahman N, Lin MT, Capetillo-Zarate E, Gouras GK. Impaired beta-amyloid secretion in Alzheimer's disease pathogenesis. J. Neurosci. 2011;31:15384–15390. doi: 10.1523/JNEUROSCI.2986-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.