Abstract
Background/Aims
Hepatitis C virus (HCV)-specific immune effector responses can cause liver damage in chronic infection. Hepatic stellate cells (HSC) are main effectors of liver fibrosis. We previously identified TGFβ, produced by HCV-specific CD8+ T cells, as key regulatory cytokine modulating HCV-specific effector T cells. Here we studied TGFβ as well as other factors produced by HCV-specific intrahepatic lymphocytes (IHL) and peripheral blood cells in hepatic inflammation and fibrogenesis.
Methods
Cross-sectional study of 2 well-defined groups of HCV-infected subjects with slow (≤0.1 Metavir units/year, n=13) or rapid (n=6) liver fibrosis progression. HCV-specific T cell responses were studied using IFNγ-ELISpot ±mAbs blocking regulatory cytokines, along with multiplex, ELISA and multi-parameter FACS. Effects of IHL stimulated with HCV-core peptides on HSC expression of pro-fibrotic and fibrolytic genes were determined.
Results
Blocking regulatory cytokines significantly raised detection of HCV-specific effector (IFNγ) responses only in slow fibrosis progressors, both in the periphery (p=0.003) and liver (p=0.01). Regulatory cytokine blockade revealed HCV-specific IFNγ responses strongly correlated with HCV-specific TGFβ, measured before blockade (R=0.84, p=0.0003), with only trend to correlation with HCV-specific IL-10. HCV-specific TGFβ was produced by CD8 and CD4 T cells. HCV-specific TGFβ, not IL-10, inversely correlated with liver inflammation (R=-0.63, p=0.008) and, unexpectedly, fibrosis (R=-0.46, p=0.05). In addition, supernatants from HCV-stimulated IHL of slow progressors specifically increased fibrolytic gene expression in HSC and treatment with anti-TGFβ mAb abrogated such expression.
Conclusion
Although TGFβ is considered a major profibrogenic cytokine, local production of TGFβ by HCV-specific T cells appeared to have a protective role in HCV-infected liver, together with other T-cell derived factors, ameliorating HCV liver disease progression.
Keywords: Treg, IL-17, MMP-1, liver fibrosis
Up to 4 million persons in the USA have chronic hepatitis C (CHC) (1). Despite a decline in overall HCV infections, the number of patients with end stage liver disease due to CHC will increase for the next 2 decades (2). Even with highly effective novel therapies, currently 30–50% of infected individuals fail treatment (3). Therefore, a better understanding of mechanisms involved in CHC-related liver disease progression could permit more efficient therapies.
Adaptive effector T cells (frequently assessed by measuring production of prototypic T helper 1 cytokine IFNγ) play an important role in control of HCV infection during the acute phase (4). In CHC, effector HCV-specific T cell immune responses are weak in peripheral blood, although they can be / are present in liver (5–8). It is likely that in chronic infection, persistence of inefficient effector T cell responses causes collateral tissue damage and inflammatory reactions, leading to fibrosis and finally cirrhosis. Regulatory/immunosuppressive T cells (Treg) are apparently involved in HCV pathogenesis, although it remains largely unclear whether they play a detrimental role by suppressing effector T cell responses against HCV or are protective by preventing excessive immunological liver damage.
Treg consist of heterogeneous populations that can be natural or induced, antigen-specific or not. Natural Treg, at least in vitro, function via antigen-independent, contact-dependent, and cytokine-independent mechanisms (9), whereas cytokine-mediated suppression (mostly IL-10 and/or TGFβ) has been established for peripheral adaptive Treg in vivo (10). This heterogeneity leads to ambiguous marker(s) for identifying Treg. Current optimal Treg markers are expression of Foxp3, a transcription factor (11), high levels of CD25 (although both of these markers can also be expressed by activated effector T cells), as well as minimal CD127 (IL-7 receptor) expression (12).
In HCV infection, increased circulating CD4+CD25+Foxp3+ T cells were associated with viral persistence (13, 14) with suppressive activity independent of cytokines and antigen non-specific (15, 16). Histological co-staining of liver infiltrates showed CD4+Foxp3+ cells at high proportion in livers of CHC patients (17), suggesting their involvement in intrahepatic immune regulation, but possibly also amelioration of fibrosis (18). HCV can prime virus-specific CD4+CD25+Foxp3+ Treg with antigen-specific expansion and suppression of HCV-specific CD8+ T cells (19). Treg also include IL-10-producing CD4+ HCV-specific T cells (20), and IL-10 dampens hepatic inflammation, but also leads to increased viral load (21). Peripheral CD4+CD25+ Treg were shown to secrete TGFβ in response to HCV, which was inversely correlated with liver inflammation (22). Suppressive IL-10 producing HCV-specific CD8+ liver infiltrating lymphocytes were also described (23) and have been associated with protection against apoptosis and fibrosis-related laminin production, as CD8 T cells were located in liver areas with both low hepatocyte apoptosis and fibrosis (24). A limitation of previous studies on Treg is use of phenotypic markers to characterize Treg before functional analysis, as opposed to functionally defining relevant Treg first, so as to not miss subsets. We identified in CHC novel blood HCV-specific CD8+CD25-Foxp3- Treg secreting TGFβ, first functionally then phenotypically (25). TGFβ production by CD4+ T cells was also observed in one patient. Suppression of peripheral HCV-specific IFNγ was predominantly mediated by TGFβ rather than IL-10. Presence of HCV-specific CD4 and CD8 T cells producing TGFβ was recently confirmed in PBMC in acute HCV infection (37).
Of note, TGFβ is a multi-functional cytokine with unique ability to direct T cell lineage commitment toward either pro-inflammatory Th17 T cells or anti-inflammatory Treg, depending on presence of additional factors, such as IL-6 (26). Significantly, TGFβ is also a key cytokine driving liver fibrogenesis (27). Disruption of the local balance between opposing effects of TGFβ on liver inflammation and fibrogenesis could underline fibrosis progression in CHC. Here we found that TGFβ produced by HCV specific T cells significantly masks T cell effector response only in those patients that show attenuated fibrosis progression. In addition, TGFβ inversely correlated not only with liver inflammation, but also with liver fibrosis progression and fibrogenic HSC gene expression. It is possible that in chronic HCV infection immunoregulatory and anti-inflammatory functions of TGFβ, produced by certain HCV-specific Treg, ameliorate liver inflammation, whilst limiting the fibrotic process.
SUBJECTS, MATERIALS, AND METHODS
Subjects and samples
Blood and matched liver biopsy samples were assayed from 19 subjects with CHC who were undergoing routine diagnostic evaluation and who had previously another liver biopsy (Table 1). No patients were being treated for HCV infection. All subjects were HCV RNA+, but none were decompensated. Persons with other forms of liver disease, including due to hepatitis B virus or alcohol, other immunosuppressive conditions, or other comorbidity requiring immunosuppressive therapy, were excluded, as were subjects with HIV infection. The protocol was reviewed by BIDMC Investigational Review Board and all subjects gave informed consent.
Table 1. Demographics of studied subjects.
Liver histology scored by Metavir system or modified Ishak converted to Metavir scores. HAI: stage+grade. Progression rate for rapid progressors >0.1 Metavir/yr. ALT: alanine-aminotransferase.
| Rapid progressors | Slow progressors | ||
|---|---|---|---|
| Characteristic | (n = 6) | (n = 13) | P |
| Age: median (range), yr | 57 (38 – 59) | 57 (38 – 66) | 0.67 |
| Race | 5 C; 1 Asian | 10 C; 1 AA; 2 Asian | |
| Male / female sex | 5 / 1 | 9 / 4 | |
| HCV RNA, median (range), copies × 103 IU/mL | 2,025 (594 – 17,800) | 1,210 (31.5 – 14,600) | 0.80 |
| HCV genotype (no.) | 1 (6) | 1 (12), 3 (1) | |
| ALT: median (range) | 81 (34 – 101) | 59 (34 – 417) | 0.83 |
| Liver Inflammation Grade: median (range) | 1 (1 – 3) | 1 (1 – 2) | 0.47 |
| Liver Fibrosis Stage: median (range) | 2 (2 – 4) | 1 (0 – 2) | 0.006 |
| Histological activity index (HAI): median (range) | 3 (3 – 5) | 2 (1 – 4) | 0.009 |
| Fibrosis progression rate: median (range), Metavir/yr | 0.2 (0.1 – 0.67) | 0.0 (−0.167 – 0.074) | 0.0005 |
| Time between 2 biopsies: median (range), yr | 4.9 (3.46 – 5.21) | 4.26 (3.85 – 6.76) | 0.44 |
Histology of adequate liver biopsies were staged and graded by both Ishak and Metavir scoring systems, and histological activity index (HAI) calculated as total score (grade+stage). Liver fibrosis progression rate was calculated based on histological staging as difference in Metavir stage between 2 biopsies divided by years between biopsies. Establishing cutoff rate of liver fibrosis progression at >0.1 Metavir-units/year for relatively rapid progression allowed studying subjects as 2 groups: rapid and slow progressors (Table 1).
Peripheral blood mononuclear cells (PBMC) and intrahepatic lymphocytes (IHL)
Extracted PBMC (25) and expanded IHL (28) were viably cryopreserved for later use. IHL were expanded using CD3 monoclonal antibody (mAb) (gift of J. Wong, MGH/Boston) to uniformly expand T cells. Autologous EBV-transformed B cell lines (B-LCL) were prepared as previously described (28) for use as antigen presenting cells for assaying expanded IHL.
Antigens & media
Two sets of synthetic peptides were used to stimulate PBMC and IHL. Set-1 consisted of 29 18-mer peptides spanning the entire HCV-Core region derived from HCV type 1a strain H77 (BEI resources). While Core protein has been reported to have immunosuppressive properties (29), peptides stimulate CD8 and CD4 cells, but cannot exhibit Core protein function. Choice of HCV-Core peptides was because it has relatively low variability and to economize on cells, since Core is covered by only 29 × 18-mer peptides, as opposed to, for example, >100 for NS3. HCV peptides were split into three pools of ~10 peptides (10µg/ml each peptide within pool). For analysis, results from the pools were analyzed individually and summed. Set-2 (CEF)(NIH), a pool of 23 major histocompatibility complex class-I restricted T-cell 11–18-mer peptides from human CMV, EBV, and influenza virus, was used as a control (2µg/ml each peptide within pool). Positive control was phytohemagglutinin (5µg/ml; Sigma-Aldrich/St.Louis/MO). Negative control was vehicle (DMSO).
Blocking monoclonal antibodies
Effector T cell responses to antigens were studied by IFNγ ELISpot ± blockade of Treg associated cytokines IL-10 and TGFβ, as described (25). Blocking mAbs anti-IL-10 and anti-TGFβ1,2,3 (clone-DII) or immunoglobulin G1 (IgG1) and IgG2b isotype controls (R&D Systems/Minneapolis/MN) were simultaneously added at optimized concentration (10µg/ml).
IFNγ ELISpot
IFNγ ELISpot ± Treg cytokines blocking mAbs were performed, adapted from as previously described (25), to detect presence of suppressive cytokine activity on HCV-specific effector (IFNγ) T cell response. Capture and detection antibodies were used at optimized concentrations of 5µg/ml and 0.2µg/ml for IFNγ (Endogen/Woburn/MA). PBMC (2×105cells/well) or IHL (0.5×105cells/well) were cultured in triplicate for 20h with antigens and in presence or absence of blocking antibodies or isotype controls. Antigen-specific spot-forming cell (SFC) frequencies were measured on automated microscope (Zeiss/Munich/Germany) and expressed after background subtraction (SFC observed with buffer media).
Multi-parameter intracytoplasmic cytokine staining (ICS)
ICS was performed on PBMC after 6hr. of stimulation, as described (25). The following fluorochrome-labeled antibodies were used: FITC-CD8, PE-Cy5-CD3, APC-TGFβ (BD Biosciences Pharmingen), PerCP-Cy5.5-CD4, Alexa-Fluor 700-IFNγ, PE-Cy7-IL-10 (Biolegend) and Pacific blue-viability (eBiosciences). Cells were analyzed using LSR-II multicolor flow cytometer (BD Biosciences Pharmingen) and FlowJo software (version 9.4.5; TreeStar).
ELISA and Searchlight multiplex assays
Supernatants from cultured cells ± HCV peptide stimulation were harvested. Cytokines released upon antigen stimulation were measured using standardized methods: TGFβ and IL-17 by ELISA (Quantikine) and, 11 additional Th1 and Th2 cytokines (IL-1α/IL-1β/IL-2/IL-4/ IL-5/ IL-6/ IL-8/ IL-10/ IL-12/IL-13/TNF-α) using multiplex cytokine array (Endogen).
Quantification of fibrosis-related gene expression in hepatic stellate cells (HSC) by Real-Time quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR)
IHL were stimulated with media or HCV-Core pool-1, pool-2 or pool-3 peptides in presence of autologous B-LCL. Fibrogenic / fibrolytic effects of conditioned supernatants (dilution 1:20) from these stimulated IHL or direct co-culture were assessed using human LX-2 HSC (30) cultured in DMEM plus 2% FBS, in triplicates. HSC transcripts for procollagen-α1 (COL1A1) and matrix metalloproteinase-1 (MMP-1) were quantified, using β2-microglobulin for normalization on a LightCycler 1.5 instrument (Roche/Mannheim/Germany) using TaqMan methodology, as described (31). 1 µl of cDNA (derived from 1µg of total RNA in 20 µl reaction volume) was used per reaction. Taqman 5’-FAM/3’-TAMRA dual-labeled probes and forward/reverse primers were: COL1A1 (5'-TCGATGGCTGCACGAGTCACACC-3' and 5'-CAGCCGCTTCACCTACAGC-3'/5'-TCAATCACTGTCTTGCCCCA-3'); MMP-1 (5'-CATCCAAGCCATATATGGACGTTCCCAAA-3' and 5'-CAGTGGTGATGTTCAGCTAGCTCA-3'/5'-GCCGATGGGCTGGACA-3'). Effect of supernatants from B-LCL alone on HSC showed no significant difference from effect of media control (IHL+B-LCL+media)(not shown).
Statistical analysis
Non-parametric tests were used: Wilcoxon sign rank test to compare each subject results before and after addition of blocking mAbs; Mann-Whitney U test to compare results between 2 groups of subjects; Spearman rank tests for correlations. Unpaired Student’s t test was used to compare gene expression in HSC treated with HCV-stimulated IHL or supernatants versus control-treated HSC. Tests were performed using STATview (Cary, N.C./version 6.0l). p <0.05 considered significant.
RESULTS
Subject characteristics
Subjects’ characteristics at the time of study entry are shown in Table 1. Subjects were split into 2 groups according to the liver fibrosis progression rate: 13 slow progressors (SP) and 6 rapid progressors (RP) with >0.1 Metavir/yr. As expected, liver fibrosis stage and progression rate were significantly higher in RP group (p<0.006). No difference was observed in alanine-aminotransfrease (ALT) serum levels, nor in liver inflammation, while the histological activity index (HAI), a score combining both liver inflammation and fibrosis, was significantly higher in RP (p=0.009). The 2 groups were comparable in terms of age, race, gender, HCV genotype, RNA levels, and number of years separating liver biopsies.
Presence of T cell regulatory activity in PBMC and liver, with greater suppression of HCV-specific effector T cell responses in slow progressors
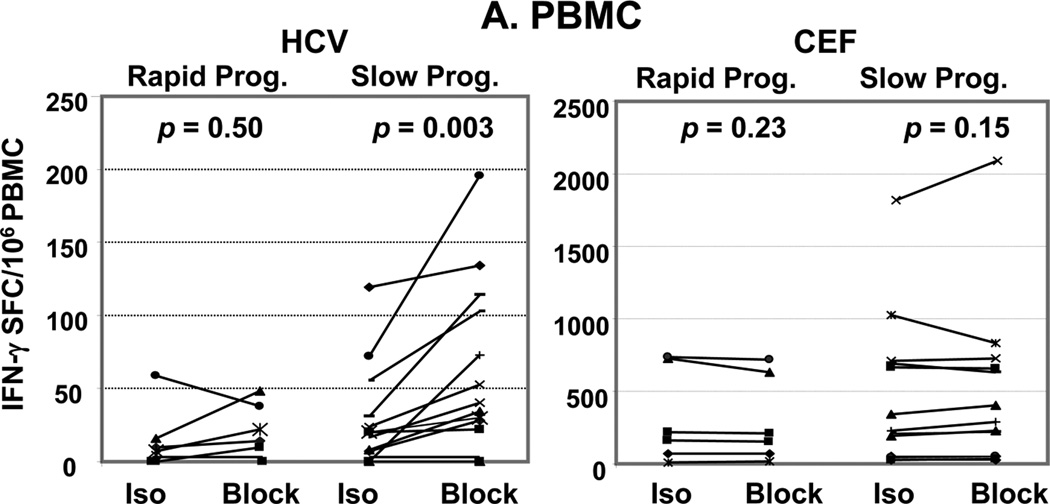
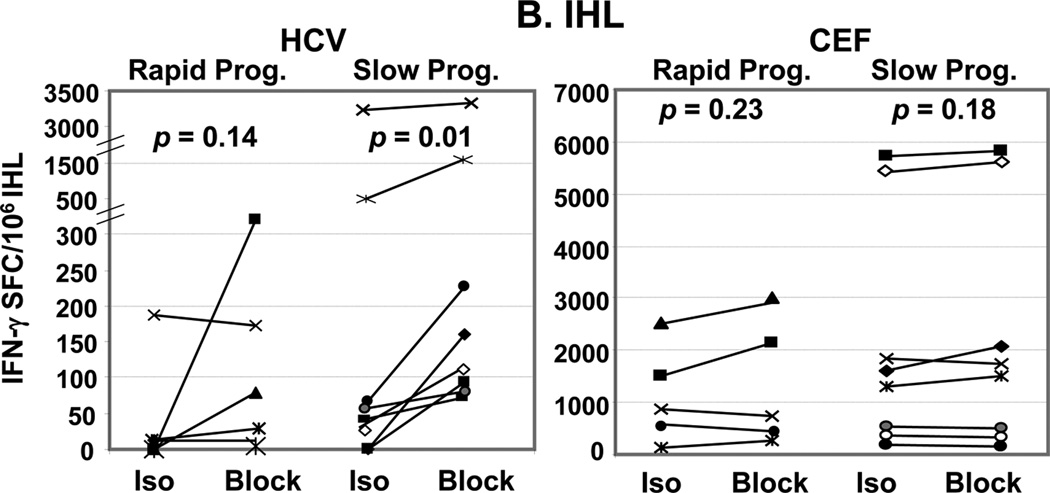
Virus-specific effector T cell responses were studied by IFNγ-Elispot with or without mAbs against the Treg-associated cytokines TGFβ and IL-10. There was no significant difference in HCV-specific effector IFNγ response between SP and RP in both PBMC and IHL when measured without blocking Abs (p=0.37). Treg-associated cytokine blockade significantly increased HCV-specific IFNγ response in SP only, in PBMC (p=0.003)(Figure 1A) but also in IHL despite their enrichment (p=0.01)(Figure 1B). No significant increase was observed in CEF-specific responses (as defined in Materials & Methods) in either compartment. The increase in HCV-specific IFNγ response upon use of Treg cytokine blocking Abs, measured as “block - isotype” was greater in SP than in RP: in PBMC (p = 0.047) and in IHL (p = 0.08). Of note, undetectable PBMC HCV-specific IFNγ responses of healthy donors were not increased upon Treg-associated cytokine blockade (25). In addition, IHL and PBMC IFNγ responses revealed upon Treg-associated cytokine blockade significantly correlated in their response to HCV peptides (R=0.6, p=0.038)(Figure 2A). Interestingly, in response to HCV peptides, PBMC IFNγ responses revealed upon Treg blockade strongly correlated with IHL IFNγ responses assayed without blockade (R=0.8, p=0.006)(Figure 2B). Again, there was no such correlations in response to control CEF (R<0.23, p>0.36). These findings imply similar regulatory T cell populations suppressing HCV-specific effector T cell responses in both periphery and liver, and suggest that suppression of effector HCV-specific T cell responses via the Treg-associated cytokine system might be associated with slower HCV related liver disease progression.
Figure 1. Greater suppression of HCV-specific effector T cell responses in slow progressors.
PBMC (A) and IHL (B) IFNγ ELISpot assays for HCV-Core and CEF peptides in presence of isotype controls (Iso.) or anti-Treg-associated cytokine Abs (TGFβ and IL-10)(Block) of 6 rapid progressors (>0.1 Metavir units/year) and 13 slow progressors. Each line corresponds to one subject. Spot forming cells (SFC)/106 PBMC over background (unstimulated cells) were expressed for HCV as sum of 3 pools of HCV-Core peptides. There was no difference between media alone and isotype (data not shown). In both compartments, addition of blocking Abs significantly enhanced median HCV-specific T-cell responses in the slow progressor group only. No enhancement of median T-cell responses to non HCV-antigens CEF was observed (Wilcoxon sign rank test). The change from Iso to block in HCV-specific T cell responses is significantly greater in SP vs RP in PBMC (p = 0.047) with a trend to significance in intrahepatic lymphocytes (p = 0.08)(Wilcoxon rank sum test)(not shown).
Figure 2. Correlations between peripheral and intra-hepatic HCV-specific IFNγ.
Peripheral IFNγ response to HCV-Core peptides revealed by ELISpot upon blockade of TGFβ and IL-10 significantly correlated (Spearman rank test) to (A) intrahepatic IFNγ response to HCV-Core peptides revealed upon use of blocking Abs, and (B) to directly assayed intrahepatic IFNγ (without Treg cytokines blockade).
Predominant involvement of TGFβ in the regulatory/suppressive process
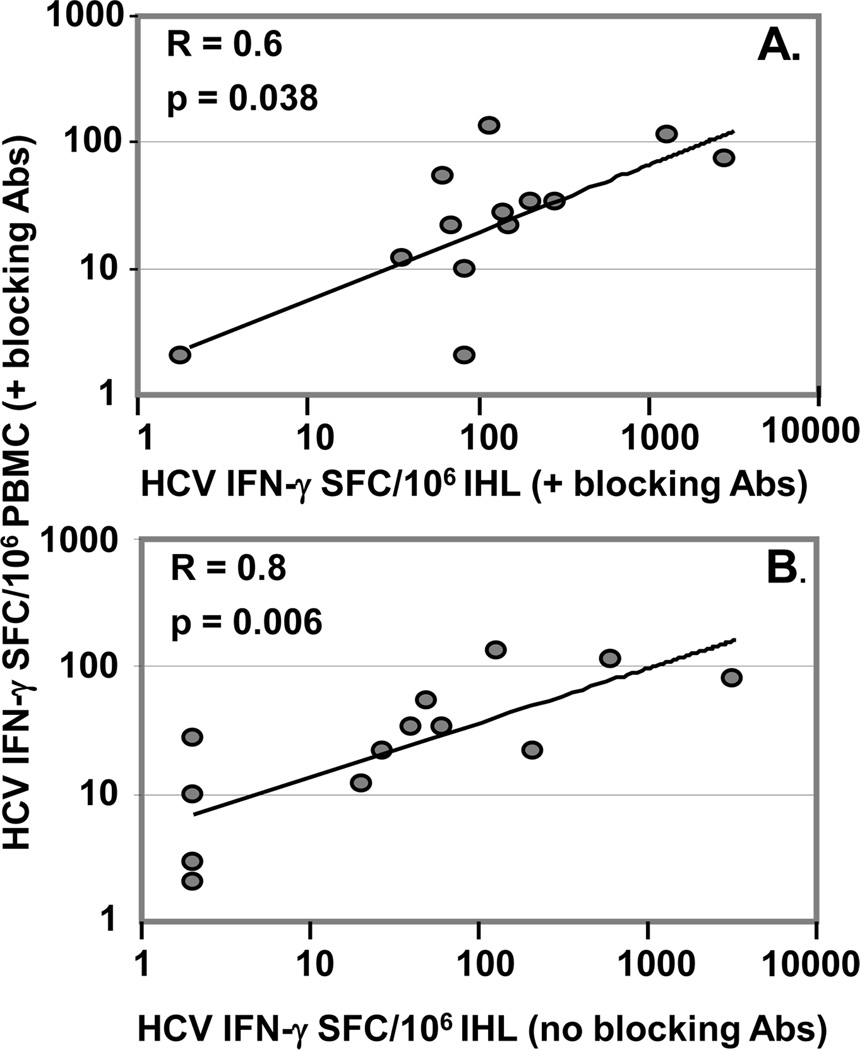
Correlations of Th1, Th2 and Treg-associated cytokines secreted by PBMC in response to HCV-Core peptides with peripheral IFNγ response, as revealed by ELISpot upon use of Treg-associated TGFβ and IL-10 blocking Abs, were studied. Total TGFβ secreted by T cells in response to HCV peptides without blocking Treg cytokines significantly correlated with HCV-specific T cell IFNγ, as revealed by Treg cytokine blockade (R=0.84; p=0.0003)(Figure 3A). There was a trend toward correlation between HCV-specific IL-10 secretion without Treg blockade and HCV-specific T cell IFNγ response, as revealed upon Treg blockade(R=0.43; p=0.08) (Figure 3B). These results suggest that the predominant cytokine involved in regulatory/suppressive activity is Treg-associated TGFβ, although IL-10 might also participate.
Figure 3. Involvement of TGFβ in suppression.
Peripheral IFNγ response to HCV-Core peptides revealed upon blockade of TGFβ and IL-10 (ELISpot) significantly correlated (Spearman rank test) with (A) TGFβ, but not (B) IL-10 secreted by PBMC in response to HCV-Core peptides (ELISA). As for ELISpot, ELISA results are expressed as total responses to HCV-Core peptides (summed for 3 pools).
T cells are the source of HCV-specific Treg-associated cytokine production
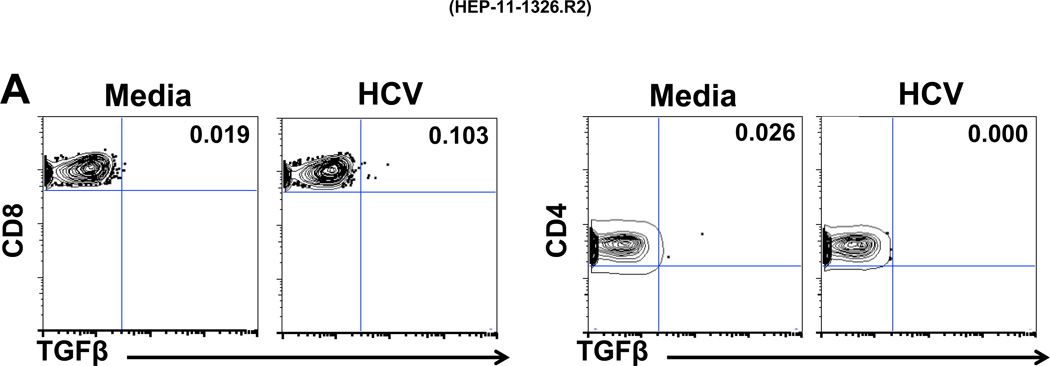
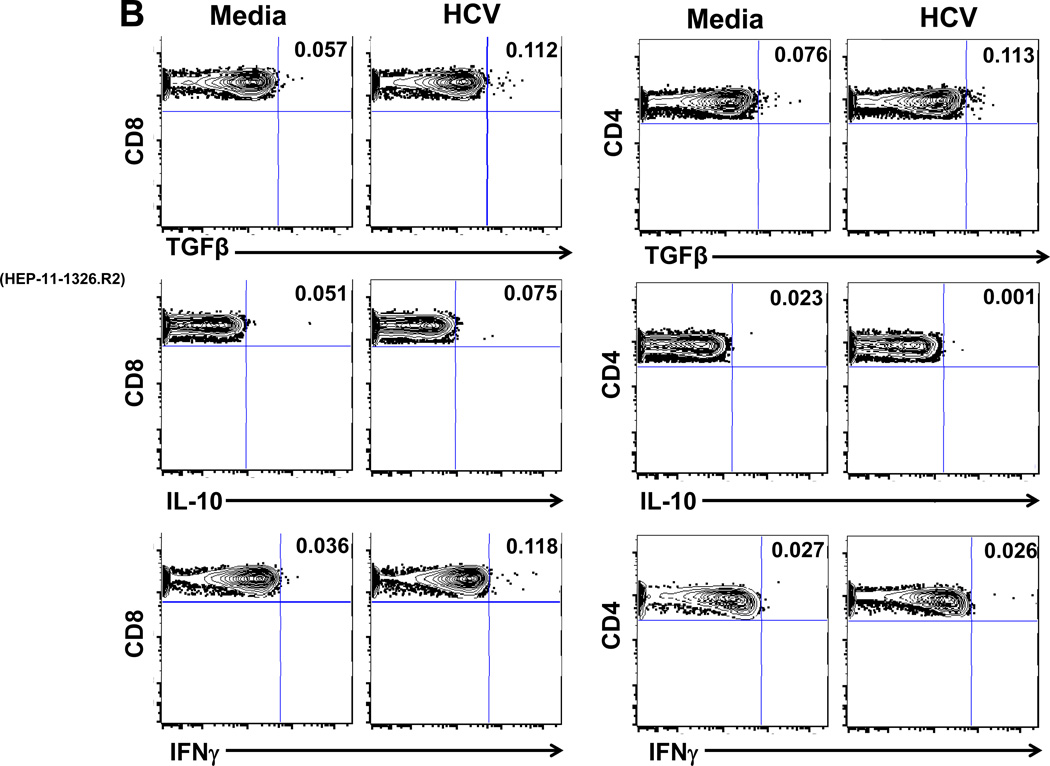
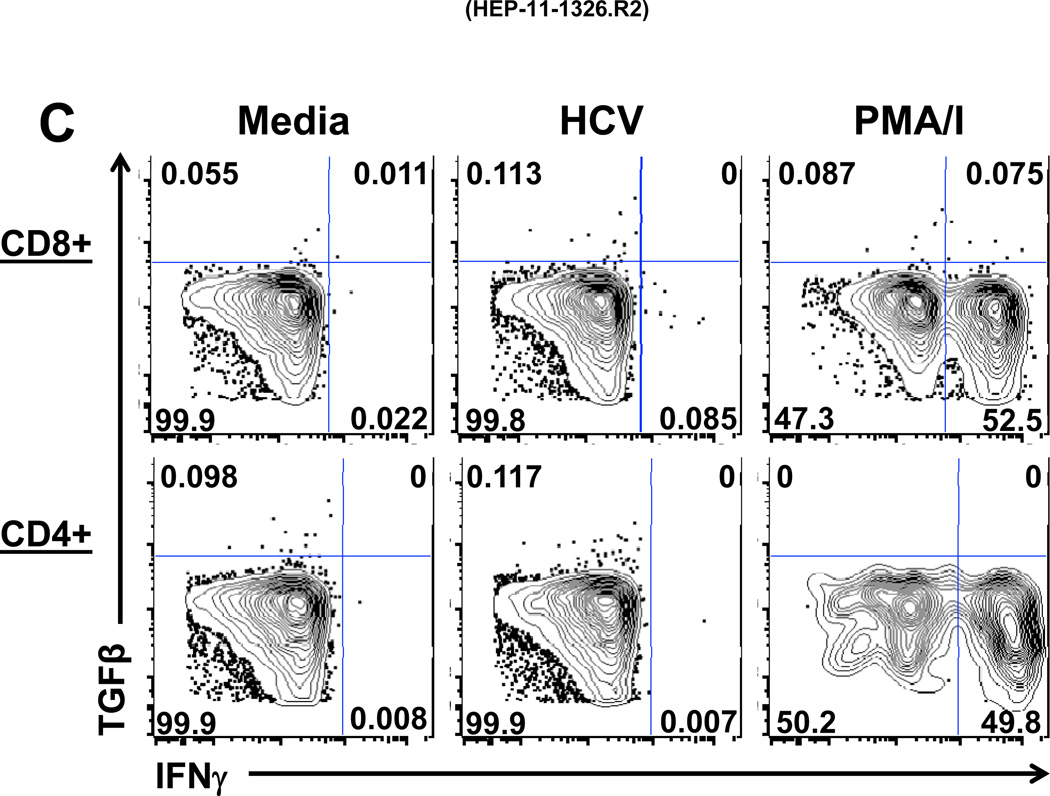
The type of PBMC involved in HCV-specific production of Treg-associated cytokines was analyzed by multicolor FACS (Figure 4) in 2 patients (A and B) with whom Treg cytokine blockade increased PBMC IFNγ by ELISpot (Figure 1A): 35 to 120 (patient A) and 55 to 105 SFC/106 PBMC (patient B). FACS analysis showed that in response to HCV stimulation, TGFβ was produced by CD8 T cells of patient A (Figure 4A), and by both CD8 and CD4 T cells as well as IFNγ by CD8, but minimal IL-10 (rare CD8 cells only) from patient B (Figure 4B). Interestingly, the T cell population producing TGFβ was distinct from the IFNγ producing population (Figure 4C). Control data from other subpopulations of PBMC were also analyzed: no HCV-specific TGFβ responses were observed in CD4/CD8 double negative or double positive T cells, nor, significantly, in CD3-negative populations (not shown). T cells were also the likely source of the studied effect of intrahepatic HCV-specific Treg-associated cytokines (Figure 1B), as only IHL and irradiated EBV-transformed B cells were present. These observations are in accord with our previously published results demonstrating that HCV-Core specific T cells can secrete TGFβ (25).
Figure 4. Frequencies of T cells producing TGFβ, IFNγ and IL-10 in response to HCV.
ICS multicolor FACS analysis was performed on PBMC from two subjects A (4A) and B (4B and 4C). 150,000 events were acquired, of which 50,000 to 100,000 were gated viable cells. The staining rates for all isotype controls were low (<0.02%) (not shown). Gating on CD8+CD3+ lymphocytes and CD4+CD3+ lymphocytes, in response to HCV stimulation, showed production of TGFβ by CD8+ T cells in patient A (4A) and by both CD8+ and CD4+ T cells, IFNγ from by CD8+ T cells and little IL-10 from patient B (4B). The T cell population producing TGFβ was distinct from the T cell population producing IFNγ (C). Stimulation with PMA/I (phorbol myristate acetate / ionomycin) was used as technical control.
Relation of HCV-specific, cytokines produced by PBMC to liver histology
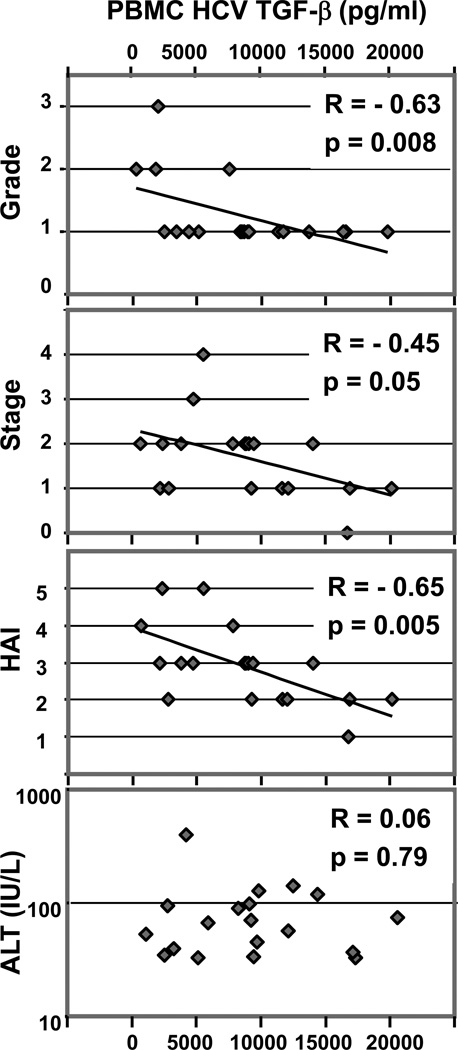
Cytokines produced by PBMC in response to HCV and control CEF peptide pools were studied in relation to liver histology of matched liver biopsies. No direct association was found between any cytokines produced in response to HCV and liver fibrosis progression rate (p>0.13)(not shown). However, there was significant inverse correlation between HCV-specific TGFβ and liver inflammation grade (R=-0.63; p=0.008) (Figure 5). Interestingly, HCV-specific TGFβ also significantly inversely correlated with liver fibrosis stage (R=-0.46; p=0.05)(Figure 5), although correlation with inflammation was more significant. Because grading and staging scores for liver biopsies are not continuous variables, we also analyzed relation to liver histology using TGFβ median values, considering high grade and stage as >1 (not shown). In accordance with inverse correlation results above, median HCV-specific TGFβ was significantly higher in subjects with lower grade and stage (p=0.009 and p=0.05, respectively). Furthermore, the index combining inflammation and fibrosis scores, HAI, strongly correlated with HCV-specific TGFβ (R=-0.65; p=0.006)(Figure 5). Of note, HCV-specific TGFβ did not correlate with peripheral ALT elevations (R=0.06; p=0.79)(Figure 5). Intriguingly, HCV-specific IL-17 secretion was also inversely correlated to liver fibrosis stage (R=-0.55; p=0.02), but not to liver inflammation grade (not shown). HCV-specific IL-17 also significantly inversely correlated to HAI (R=-0.62; p=0.01)(not shown). No such relations were observed in response to CEF control (not shown) (p>0.12). Similarly, no significant correlation was observed between liver histology and other studied cytokines, including HCV-specific IL-10 (p>0.2). T cells from both slow and rapid progressors secreted high levels of IL-6 (median, range: 155 pg/ml, 4–1113) and IL-1β (1569 pg/ml, 42–11373) in response to HCV peptides (not shown).
Figure 5. HCV-specific TGFβ correlates with histological liver disease severity.
TGFβ secreted by PBMC in response to HCV-Core peptides significantly inversely correlated with liver inflammation grade, fibrosis stage and histological activity index (HAI = stage + grade), but not with serum ALT. TGFβ results are expressed as total responses to HCV-Core peptides (summed for 3 pools) (Spearman rank test).
Effect of supernatants from HCV-stimulated IHL on fibrogenic activation of hepatic stellate cells
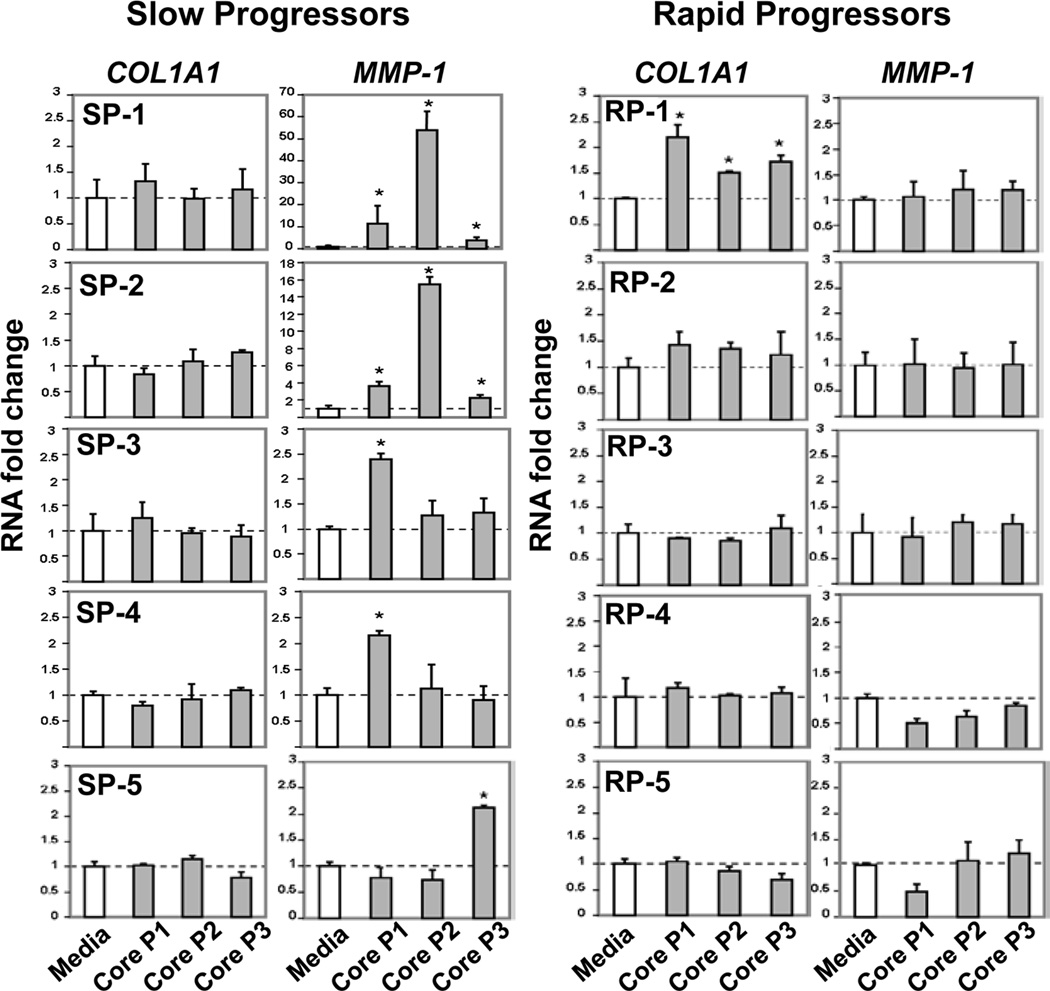
To further explore potential effects of IHL regulatory activity, we tested effects of IHL stimulation in response to HCV peptides on human HSC by transfer of conditioned supernatants. This was tested with IHL from 5 SP and 5 RP. In Elispot assays, blocking TGFβ increased the intra-hepatic HCV-specific IFNγ response in all tested SP, but not in any RP. HSC significantly increased expression of putatively fibrolytic transcript for MMP-1 upon culture with HCV-stimulated IHL supernatants from slow, but not rapid progressors (Figure 6). However, pro-fibrotic COL1A1 gene expression significantly increased in response to IHL supernatant from one rapid progressor (RP-1) and remained unaffected by remaining IHL supernatants.
Figure 6. Effect of supernatants from HCV-stimulated IHL on fibrogenic activation of HSC.
Supernatants from IHL stimulated with media (control), HCV-Core pool-1, pool-2 and pool-3 peptides (1:20 dilutions of conditioned media) were incubated with cultures of human HSC (LX-2 cell line) for 24 h. The HSC fibrogenic RNA response was assessed by qRT-PCR for COL1A1 and MMP-1 normalized to B2M, shown as relative fold change to media control. In comparison to media, HCV-stimulated supernatants from slow progressors (SP), but not rapid progressors (RP) induced expression of putatively fibrolytic MMP-1, but not of pro-fibrogenic COL1A1. Stars indicate significant difference (p<0.05).
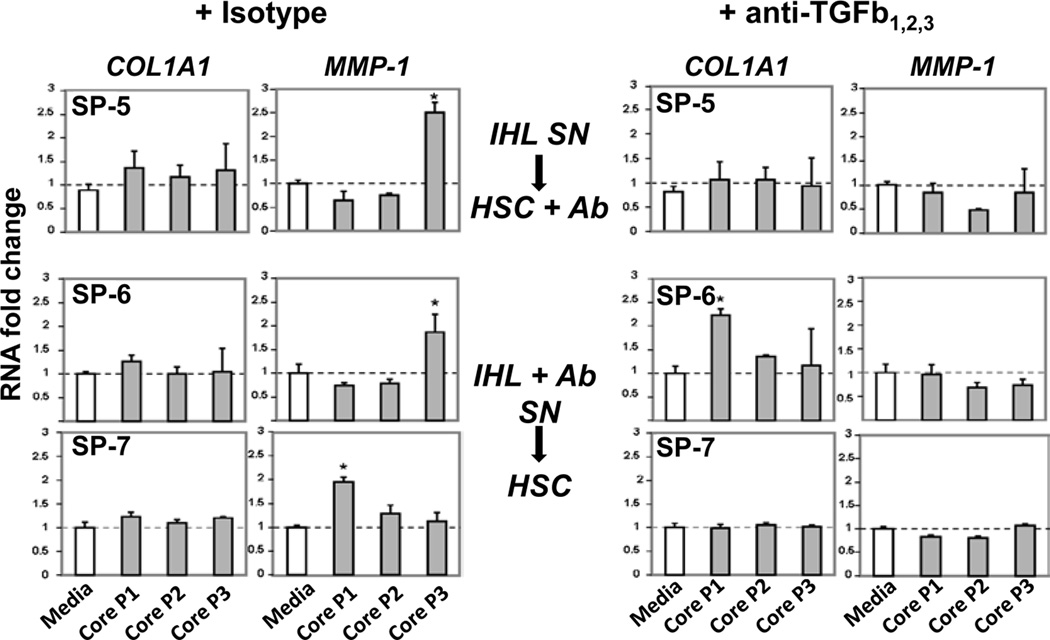
Interestingly, treatment of supernatants above (SP-5) or IHL cultures (SP-6 and SP-7) with blocking anti-TGFβ mAb abrogated HSC MMP-1 expression (Figure 7).
Figure 7. Effect of treating IHL supernatants or IHL cultures with anti-TGFβ on HSC.
Response of LX-2 human hepatic stellate cells to T cell supernatnats (SN) from 3 slow progressors in whom blocking TGFβ in IHL Elispot assay increased HCV-specific IFNγ response. Either SN were harvested from IHL culture ±HCV stimulation then treated with anti-TGFβ mAb prior to co-culture with LX-2 (SP-5) or IHL ±HCV were cultured with blocking Abs, then SN were harvested for co-culture with LX-2 (SP-6 and SP-7). Treatment with anti-TGFβ Ab (in comparison with Isotype control) abrogated induction of MMP-1 expression by HSC and also up-regulated COL1A1 (SP-7). Stars indicate significant difference (p<0.05).
As several reports indicated that TGFβ produced by Treg could provide an effective mechanism of control of fibrosis progression in association with IL-10 (32), IHL HCV-specific IL-10 production was measured in remaining available IHL supernatants (8 patients) by ELISA. Significant amounts of IL-10 were observed in response to HCV-core stimulation (median, range: 365 pg/ml; 0–2788).
DISCUSSION
Treg roles in HCV disease progression are not yet clearly established. Reasons could be that Treg are heterogeneous populations and unambiguous Treg markers remain elusive. Consequently, potential Treg subsets are certainly missed, since most Treg studies use phenotypic markers to identify Treg, even if identified cells are then studied functionally. In peripheral blood of subjects with chronic HCV infection, we previously detected TGFβ mediated suppressive activity against HCV-specific effector function and identified a novel population of non-classical human Tregs responsive to HCV that produced the Treg-associated cytokine TGFβ (25). In this report, we defined the relation of hepatic and peripheral HCV-specific T cell-produced TGFβ to HCV related liver disease.
Blocking Treg-associated cytokines increased effector HCV-specific T cell responses in slow progressing subjects with chronic HCV infection. This suppressive function was detected in both peripheral and liver compartments, suggesting presence of similar T regulatory activity in peripheral blood and liver, at least for certain types of Treg populations. Presence of various hepatic Treg populations have been suggested: CD4+CD25+Foxp3+ (by liver histological co-staining assays)(17) and CD8+IL-10+ Treg cells (systematic random cloning)(23). However, it is not clear whether there are differences or similarities in Treg content and function between periphery and liver. Our finding of a strong correlation between HCV-specific PBMC and IHL IFNγ responses revealed upon Treg cytokine blockade support similarities in cytokine mediated Treg activity between these compartments. In addition, revealed PBMC HCV-specific effector responses actually correlated with IHL HCV-specific IFNγ responses assayed without Treg cytokine blockade. It would be ideal if these peripheral responses revealed upon use of Treg cytokine blockade reflect, at least in part, what is occurring in liver, since this would provide a robust surrogate, enabling follow-up longitudinal studies of T cell immunity to HCV.
Importantly, increases of both PBMC and IHL HCV-specific IFNγ responses resulting from Treg-associated cytokine blockade were significantly greater in patients with slow liver fibrosis progression compared with subjects with rapid liver fibrosis progression, suggesting presence of effective suppressive Treg-associated cytokine activity in slow, but not rapid progressors, and therefore involvement of this regulatory activity in protection from destructive inflammation and subsequent liver disease progression. Alternatively, it is possible that Treg-associated cytokine blockade in rapid progressors did not increase effector HCV-specific T cell response because T cells became anergic upon long term immunosuppression. However, no differences in effector HCV-specific T cell responses were observed between the groups without Treg cytokine blockade or in response to mitogen. The immunosuppressive effect appeared to be predominantly mediated by HCV-specific TGFβ rather than IL-10. This is consistent with our previous results with a different cohort of HCV subjects, where blocking TGFβ significantly increased IFNγ response to HCV while IL-10 blockade did not have significant effect (25), confirming predominant involvement of TGFβ in this suppressive activity, rather than IL-10 .
T cell secretion of TGFβ in response to HCV has been described for CD4+CD25+ (22) and CD8+CD25-Foxp3- (25) Tregs in subjects with CHC and an anti-inflammatory role for TGFβ during chronic HCV infection has been suggested (22). Interestingly, in HCV-HIV co-infection, high levels of plasma TGFβ, but not CD4+Foxp3+ cells, is associated with low levels of liver fibrosis (33). Here, we provide a plausible mechanistic explanation for this observation, since we studied HCV-specific TGFβ T cell production in relation to liver inflammation and fibrosis. Not only do we confirm an inverse correlation of HCV-specific TGFβ (not IL-10) with histological liver inflammation, but we also found significant inverse correlation with liver fibrosis stage and progression. Together, these findings support the hypothesis that locally HCV-specific T cell-produced TGFβ, may play a role in controlling the chronic inflammatory response, and consequently may even have an anti-fibrotic role, thereby attenuating hepatic scarring in chronic HCV infection. Intriguingly our data also suggest involvement of IL-17 as anti-fibrotic in this setting, since we found a strong inverse correlation of liver fibrosis stage with HCV-specific IL-17. Other cytokines which we found at substantial amounts in HCV-stimulated supernatants, IL-1β and IL-6, might be concurrently expressed in vivo and influence T cell lineage commitment. Together, these observations underline complexity of the system, since IL-17 is a pro-inflammatory cytokine and TGFβ, generally assumed to be anti-inflammatory, can become pro-inflammatory in combination with other cytokines such as IL-1 and IL-6 (26, 34). In this context ability of TGFβ-producing Treg to readily loose Foxp3 and acquire IL-17 expression in Th17-polarizing conditions has been described (34, 35).
Because of lack of definitive surface marker(s) for TGFβ producing Treg, and therefore, currently an absence of methods allowing their depletion, direct demonstration of effect of their elimination was not possible. Since establishing pure co-cultures of the 'Treg' of interest with human HSC is not currently practical, we tested the effect of IHL supernatants in response to HCV peptides on human HSC. Interestingly, factor(s) produced in supernatants in response to HCV from slow progressors, in whom TGFβ blockade increased IHL effector IFNγ response, had an anti-fibrotic effect on human HSC and treating these supernatants with anti-TGFβ antibodies abrogated fibrolytic gene expression by HSC. Conversely, no such effect was observed with rapid progressors for whom TGFβ blockade had no such enhancing effect. Such apparently paradoxical observations for TGFβ, most often considered a profibrogenic cytokine, could be explained since TGFβ is a multifunctional cytokine that modulates its function depending on cell type producing it and other factors present with it or induced by it. In this regard, regulatory IL-10 might also be involved. In a fibrosis mouse model, for example, TGFβ gene therapy lead to appearance of cells producing IL-10 (32). TGFβ ’gene therapy’ ameliorated fibrosis in wild type, but not IL-10 deficient mice and induced Smad4, which then binds to and activates the IL-10 promoter. In our study, we observed significant production of IL-10 by IHL in response to HCV and peripheral HCV-specific IL-10 data did not exclude its participation in suppressive activity. Although too preliminary to formally conclude, it is possible that when TGFβ is locally produced by HCV-specific Treg, it induces substantial amounts of other cytokines, including IL-10, that participate to counterbalance profibrogenic effect of TGFβ produced by other surrounding hepatic cells. Whether IL-10 and/or other factors contribute to explain the anti-fibrotic effect of Treg TGFβ will be the object of our future studies.
In conclusion, these results suggest that TGFβ produced locally by Tregs suppresses, rather than enhances, hepatic fibrogenesis. The data also suggest that suppression is in part in concert with other regulatory factor(s) secreted by intrahepatic lymphocytes in response to HCV. Treg have been associated with HCV persistence in chronic HCV infection (13, 14, 36). However, they may play a more beneficial anti-inflammatory role by locally protecting against surrounding tissue damage. Failure to develop appropriate effector and regulatory HCV-specific T cell responses presumably serves to drive HCV-related liver fibrosis. A better understanding of opposing effects and roles of different T cell subsets could provide novel tools allowing maintenance of such a beneficial balance, suggesting novel therapeutic approaches to prevent HCV-mediated liver disease progression.
Acknowledgments
Financial support: U19 AI066313 to NA, ME and DS, R01 DK066917 and R21 CA143748 to ME, China Scholarship Council to SL and U01 AI068636 to NA.
Abbreviations
- HSC
hepatic stellate cells
- IHL
intrahepatic lymphocytes
- TGFβ
transforming growth factor beta
- IFN
interferon
- IL
interleukin
- CHC
chronic hepatitis C
- CTL
cytotoxic T lymphocyte
- Treg
regulatory T cell(s)
- Foxp3
forkhead box p3 transcription factor
- HAI
histological activity index
- PBMC
peripheral blood mononuclear cells
- mAbs
monoclonal antibodies
- EBV
Epstein-Barr virus
- CMV
Cytomegalovirus
- CEF
pool of peptides derived from CMV, EBV, and influenza-virus
- DMSO
Dimethyl sulfoxide solvent
- SFC
spot-forming cell
- TNF
tumor necrosis factor
- MMP-1
matrix metalloproteinase-1
- RP
rapid liver disease progressor(s)
- SP
slow liver disease progressor(s)
Footnotes
The protocol was reviewed and approved by the Investigational Review Board of Beth Israel Deaconess Medical Center and all subjects gave informed consent for the collection of specimens.
The authors have no conflicts of interest to declare
Authors’ involvement in the manuscript:
Data acquisition, analysis: SL, LV, IAN, YP
Providing samples, clinical consulting: NHA, DS, MJK
Funding/material support: MJK, MAE, NA.
Data interpretation, MS revision: NA, DS, MAE
Study concept, design, MS draft: NA
References
- 1.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 138:513–521. 521 e511–521 e516. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 3.McHutchison JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhal NH, Heathcote EJ, et al. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 362:1292–1303. doi: 10.1056/NEJMoa0908014. [DOI] [PubMed] [Google Scholar]
- 4.Missale G, Bertoni R, Lamonaca V, Valli A, Massari M, Mori C, Rumi MG, et al. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J Clin Invest. 1996;98:706–714. doi: 10.1172/JCI118842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shoukry NH, Cawthon AG, Walker CM. Cell-mediated immunity and the outcome of hepatitis C virus infection. Annu Rev Microbiol. 2004;58:391–424. doi: 10.1146/annurev.micro.58.030603.123836. [DOI] [PubMed] [Google Scholar]
- 6.Wedemeyer H, He XS, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, Liang TJ, et al. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 7.Grabowska AM, Lechner F, Klenerman P, Tighe PJ, Ryder S, Ball JK, Thomson BJ, et al. Direct ex vivo comparison of the breadth and specificity of the T cells in the liver and peripheral blood of patients with chronic HCV infection. Eur J Immunol. 2001;31:2388–2394. doi: 10.1002/1521-4141(200108)31:8<2388::aid-immu2388>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 8.He XS, Rehermann B, Lopez-Labrador FX, Boisvert J, Cheung R, Mumm J, Wedemeyer H, et al. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc Natl Acad Sci U S A. 1999;96:5692–5697. doi: 10.1073/pnas.96.10.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 10.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 11.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 12.Hartigan-O'Connor DJ, Poon C, Sinclair E, McCune JM. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods. 2007;319:41–52. doi: 10.1016/j.jim.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Gowans EJ, Chougnet C, Plebanski M, Dittmer U. Natural regulatory T cells and persistent viral infection. J Virol. 2008;82:21–30. doi: 10.1128/JVI.01768-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Billerbeck E, Bottler T, Thimme R. Regulatory T cells in viral hepatitis. World J Gastroenterol. 2007;13:4858–4864. doi: 10.3748/wjg.v13.i36.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boettler T, Spangenberg HC, Neumann-Haefelin C, Panther E, Urbani S, Ferrari C, Blum HE, et al. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol. 2005;79:7860–7867. doi: 10.1128/JVI.79.12.7860-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rushbrook SM, Ward SM, Unitt E, Vowler SL, Lucas M, Klenerman P, Alexander GJ. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol. 2005;79:7852–7859. doi: 10.1128/JVI.79.12.7852-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward SM, Fox BC, Brown PJ, Worthington J, Fox SB, Chapman RW, Fleming KA, et al. Quantification and localisation of FOXP3+ T lymphocytes and relation to hepatic inflammation during chronic HCV infection. J Hepatol. 2007;47:316–324. doi: 10.1016/j.jhep.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Claassen MA, de Knegt RJ, Tilanus HW, Janssen HL, Boonstra A. Abundant numbers of regulatory T cells localize to the liver of chronic hepatitis C infected patients and limit the extent of fibrosis. J Hepatol. 52:315–321. doi: 10.1016/j.jhep.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Ebinuma H, Nakamoto N, Li Y, Price DA, Gostick E, Levine BL, Tobias J, et al. Identification and in vitro expansion of functional antigen-specific CD25+ FoxP3+ regulatory T cells in hepatitis C virus infection. J Virol. 2008;82:5043–5053. doi: 10.1128/JVI.01548-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacDonald AJ, Duffy M, Brady MT, McKiernan S, Hall W, Hegarty J, Curry M, et al. CD4 T helper type 1 and regulatory T cells induced against the same epitopes on the core protein in hepatitis C virusinfected persons. J Infect Dis. 2002;185:720–727. doi: 10.1086/339340. [DOI] [PubMed] [Google Scholar]
- 21.Nelson DR, Tu Z, Soldevila-Pico C, Abdelmalek M, Zhu H, Xu YL, Cabrera R, et al. Long-term interleukin 10 therapy in chronic hepatitis C patients has a proviral and anti-inflammatory effect. Hepatology. 2003;38:859–868. doi: 10.1053/jhep.2003.50427. [DOI] [PubMed] [Google Scholar]
- 22.Bolacchi F, Sinistro A, Ciaprini C, Demin F, Capozzi M, Carducci FC, Drapeau CM, et al. Increased hepatitis C virus (HCV)-specific CD4+CD25+ regulatory T lymphocytes and reduced HCV-specific CD4+ T cell response in HCV-infected patients with normal versus abnormal alanine aminotransferase levels. Clin Exp Immunol. 2006;144:188–196. doi: 10.1111/j.1365-2249.2006.03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Accapezzato D, Francavilla V, Paroli M, Casciaro M, Chircu LV, Cividini A, Abrignani S, et al. Hepatic expansion of a virus-specific regulatory CD8(+) T cell population in chronic hepatitis C virus infection. J Clin Invest. 2004;113:963–972. doi: 10.1172/JCI20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abel M, Sene D, Pol S, Bourliere M, Poynard T, Charlotte F, Cacoub P, et al. Intrahepatic virusspecific IL-10-producing CD8 T cells prevent liver damage during chronic hepatitis C virus infection. Hepatology. 2006;44:1607–1616. doi: 10.1002/hep.21438. [DOI] [PubMed] [Google Scholar]
- 25.Alatrakchi N, Graham CS, van der Vliet HJ, Sherman KE, Exley MA, Koziel MJ. Hepatitis C virus (HCV)-specific CD8+ cells produce transforming growth factor beta that can suppress HCV-specific T-cell responses. J Virol. 2007;81:5882–5892. doi: 10.1128/JVI.02202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahl SM. Transforming growth factor-beta: innately bipolar. Curr Opin Immunol. 2007;19:55–62. doi: 10.1016/j.coi.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Schuppan D, Krebs A, Bauer M, Hahn EG. Hepatitis C and liver fibrosis. Cell Death Differ. 2003;10(Suppl 1):S59–S67. doi: 10.1038/sj.cdd.4401163. [DOI] [PubMed] [Google Scholar]
- 28.Alatrakchi N, Graham CS, He Q, Sherman KE, Koziel MJ. CD8+ cell responses to hepatitis C virus (HCV) in the liver of persons with HCV-HIV coinfection versus HCV monoinfection. J Infect Dis. 2005;191:702–709. doi: 10.1086/427778. [DOI] [PubMed] [Google Scholar]
- 29.Yao ZQ, King E, Prayther D, Yin D, Moorman J. T cell dysfunction by hepatitis C virus core protein involves PD-1/PDL-1 signaling. Viral Immunol. 2007;20:276–287. doi: 10.1089/vim.2006.0096. [DOI] [PubMed] [Google Scholar]
- 30.Xu L, Hui AY, Albanis E, Arthur MJ, O'Byrne SM, Blaner WS, Mukherjee P, et al. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–151. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulze-Krebs A, Preimel D, Popov Y, Bartenschlager R, Lohmann V, Pinzani M, Schuppan D. Hepatitis C virus-replicating hepatocytes induce fibrogenic activation of hepatic stellate cells. Gastroenterology. 2005;129:246–258. doi: 10.1053/j.gastro.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 32.Kitani A, Fuss I, Nakamura K, Kumaki F, Usui T, Strober W. Transforming growth factor (TGF)-beta1-producing regulatory T cells induce Smad-mediated interleukin 10 secretion that facilitates coordinated immunoregulatory activity and amelioration of TGF-beta1-mediated fibrosis. J Exp Med. 2003;198:1179–1188. doi: 10.1084/jem.20030917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rallon NI, Barreiro P, Soriano V, Garcia-Samaniego J, Lopez M, Benito JM. Elevated TGF-beta1 levels might protect HCV/ HIV-coinfected patients from liver fibrosis. Eur J Clin Invest. doi: 10.1111/j.1365-2362.2010.02381.x. [DOI] [PubMed] [Google Scholar]
- 34.Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C, Hafler DA. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–4249. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alatrakchi N, Koziel M. Regulatory T cells and viral liver disease. J Viral Hepat. 2009;16:223–229. doi: 10.1111/j.1365-2893.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- 37.Harfouch S, Guiguet M, Valantin MA, Samri A, Ouazene Z, Slama L, Dominguez S, et al. Lack of TGF-β production by hepatitis C virus-specific T cells during HCV acute phase is associated with HCV clearance in HIV coinfection. J Hepatol. 2012 Feb 9; doi: 10.1016/j.jhep.2012.01.021. [DOI] [PubMed] [Google Scholar]