Abstract
Background
Dopaminergic medications and subthalamic nucleus deep brain stimulation (STN-DBS) alleviate motor symptoms in Parkinson disease, but balance and gait are more variably affected. Balance reports are particularly inconsistent. Further, despite their prevalence in daily life, complex gait situations including backward and dual task gait are rarely studied. We aimed to assess how medications, STN-DBS, and both therapies combined affect balance and complex gait.
Methods
Twelve people with Parkinson disease were evaluated OFF medication with STN-DBS OFF and ON as well as ON medication with STN-DBS OFF and ON. Motor impairment was measured with the Movement Disorder Society Unified Parkinson Disease Rating Scale motor section (MDS-UPDRS-III). The Mini-Balance Evaluations Systems Test, timed-up-and-go, and dual task timed-up-and-go measured balance and mobility. Preferred-pace forward, fast as possible, backward, dual task forward, and dual task backward gait were also analyzed.
Results
Medication improved MDS-UPDRS-III scores, dual task timed-up-and-go, and stride length across all gait tasks. STN-DBS improved MDS-UPDRS-III scores, balance scores, dual task timed-up-and-go, and stride length and velocity across all gait tasks. Medication and STN-DBS combined did not provide additional benefits over either therapy alone.
Conclusions
Overall, dopaminergic medications and STN-DBS provided similar improvements in balance and gait tasks, although the effects of STN-DBS were stronger, potentially due to reductions in medication doses after surgery. Lack of synergistic effect of treatments may suggest both therapies improve balance and gait by influencing similar neural pathways.
Keywords: Parkinson Disease, Medication, Deep Brain Stimulation, Gait, Balance
Introduction
People with Parkinson disease (PD) experience various motor symptoms, including tremor, rigidity, bradykinesia, and postural instability. Motor symptoms are typically treated pharmacologically with dopaminergic medications, and surgical interventions including deep brain stimulation of the subthalamic nucleus (STN-DBS) are increasingly utilized in conjunction with pharmacological treatments as the disease progresses. Tremor, bradykinesia, and rigidity are generally responsive to levodopa and STN-DBS.[1,2] Postural instability and gait are more variably affected by medications and STN-DBS. Importantly, postural stability and gait impairments in PD may lead to falls, and serious injuries.[3] Few studies have measured DBS effects on balance and gait tasks both ON and OFF medication to determine the extent to which motor deficits are remedied by these therapies individually or in combination.
Conflicting reports exist on whether functional balance tests, including the timed-up-and-go (TUG), improve with STN-DBS. Despite definite improvements in motor function (UPDRS-III) and dyskinesia, TUG ON medication did not significantly improve.[4] However, OFF medication TUG did improve with STN-DBS.[5] When both ON and OFF medication conditions were evaluated, TUG improved with STN-DBS and with medication, but there were no additional improvements with both treatments together.[6]
There is also discrepancy regarding the effects of STN-DBS on various other balance measures. STN-DBS improved Berg Balance Scale Scores and postural instability (UPDRS-III pull-test) OFF medication[5,7] and static and dynamic posturography measures ON medication.[8] Conversely, evidence also suggests STN-DBS may worsen postural stability, degrade gait, and increase fall risk.[9] Center-of-mass displacement with postural perturbations was impaired with DBS ON medication.[10] Axial/PIGD UPDRS-III items did not improve with STN-DBS in older individuals ON medication, and these items were worsened by STN-DBS when OFF medication.[11] DBS and levodopa improve balance in terms of postural sway parameters during unperturbed stance,[12] while measures related to external platform and visual tilt perturbations were not affected by levodopa or DBS.[12] Given the mixed results of these studies, the effects of STN-DBS on balance remain unclear.
In addition to investigating balance, STN-DBS effects on preferred-pace forward gait have been assessed OFF and ON medication. In general, STN-DBS improves spatiotemporal parameters of preferred-pace gait, including velocity and stride length, OFF and ON medication.[13–15] Preferred-pace gait velocity and stride length improved similarly with STN-DBS or levodopa, and both therapies combined did not confer additional improvement.[6] Cadence did not change with DBS or medication.[6] In contrast, other studies report combined effects of DBS and medication on gait velocity were greater than either treatment alone.[13,14] Considering these results, it remains unclear whether STN-DBS and medication combined provide additional improvements in preferred-pace gait parameters compared to each individual treatment alone.
Further, little is known about STN-DBS and medication effects on more complex gait tasks, including fast gait, backward gait, and dual task gait, despite the fact that complex gait tasks are encountered daily. STN-DBS effects on gait velocity and step length were evaluated both OFF and ON medication during slow gait, preferred-pace gait, and fast as possible gait.[15] Gait velocity improved similarly across slow, fast, and preferred-pace gait tasks with either treatment alone and both combined.[15] A similar pattern was present for slow and fast step length.[15] Preferred-pace step length improved similarly with either treatment alone, and combining the treatments produced additional benefit.[15] Evidence suggests people with PD have particular difficulty with backward and dual task gait.[16,17] As a result, it is important to better understand how current therapies affect more complex gait. To our knowledge, no studies have examined the individual and combined effects of STN-DBS and medication on backward walking, dual task forward or dual task backward walking.
With inconsistent reports, it is unclear how bilateral STN-DBS affects balance and complex gait in PD. Potential impairments in gait and balance with STN-DBS may increase the likelihood of falls and serious injuries in these individuals. It is also possible that the potential for increased fall risk with DBS may occur not as a result of impaired balance, but as a result of increased mobility with treatment that prompts patients to engage in more challenging activities.[13] Clarifying the effects of STN-DBS on balance and complex gait, particularly in the ON medication state, will improve our understanding of factors that may contribute to falls in this population. This study aimed to assess how balance and complex gait were influenced by medications, STN-DBS, and both therapies combined in people with PD. We hypothesized that clinical balance tests and preferred-pace gait would improve substantially with medication and with DBS alone, but smaller magnitude improvements would occur in more complex gait tasks including backward walking and dual task walking. We also expected larger improvements across tasks with both treatments combined as compared to either treatment alone.
Methods
Participants
Sixteen individuals with idiopathic PD (age 65±8 years, mean±SD, 12 males, PD duration 16±6 years) were recruited from the Movement Disorders Center at Washington University School of Medicine. Criteria for inclusion were: currently taking levodopa for PD, previously implanted bilateral STN DBS electrodes, ambulatory, MMSE score ≥24, no prior brain surgery other than STN-DBS, and no recent surgeries or injuries affecting locomotion. All participants had DBS for at least three months prior to participation (3.0±1.8 years since surgery) and were taking carbidopa/levodopa medications (LEDD: 840.8±345.4). Additionally, 8 were taking amantadine, 3 pramipexole, 1 ropinirole, and 4 entacapone. The Washington University Human Research Protection Office approved this study, and all participants provided written informed consent.
Experimental Design
Participants were tested on two separate days within one week. On one day, participants took all medications as prescribed (ON state). The other day, participants were tested in the practically defined OFF state after overnight withdrawal from anti-Parkinson medications. Within OFF and ON medication days, individuals were assessed in two sessions: one with bilateral STN-DBS operating at the clinically determined settings (ONMeds+DBS, OFFMeds+DBS) and one with bilateral STN-DBS turned completely off (ONMeds-DBS, OFFMeds-DBS). Clinical STN-DBS settings for each participant are listed in Table 1.
Table 1.
Bilateral STN-DBS Stimulation Parameters
| Left Side STN-DBS | Right Side STN-DBS | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Subject No. | Voltage(V) | Pulse Width(μs) | Frequency(Hz) | Voltage(V) | Pulse Width(μs) | Frequency(Hz) |
| 01 | 3.6 | 90 | 180 | 2.0 | 90 | 180 |
| 02 | 2.3 | 60 | 185 | 3.5 | 60 | 185 |
| 03 | 2.8 | 60 | 185 | 2.8 | 60 | 185 |
| 04 | 3.0 | 60 | 185 | 3.2 | 60 | 185 |
| 05 | 3.3 | 60 | 185 | 3.6 | 60 | 185 |
| 06 | 3.3 | 60 | 185 | 3.2 | 60 | 185 |
| 07 | 3.0 | 60 | 185 | 3.0 | 60 | 185 |
| 08 | 2.8 | 60 | 185 | 2.0 | 60 | 185 |
| 09 | 2.9 | 60 | 185 | 3.0 | 60 | 185 |
| 10 | 3.2 | 60 | 185 | 3.2 | 60 | 185 |
| 11 | 3.1 | 90 | 185 | 3.1 | 90 | 185 |
| 12 | 2.4 | 60 | 185 | 2.8 | 60 | 185 |
The orders of medication day assignments and DBS sessions were counterbalanced across participants. Investigators were blind to medication and DBS status, and participants were blind to DBS status. Prior to each session, DBS settings were checked or changed, and testing began at least 42 minutes after any change in settings.
Identical evaluations were conducted in each session on both days. Overall motor impairment and postural stability were assessed by the same physical therapist across all sessions using the Movement Disorder Society Unified Parkinson Disease Rating Scale motor section (MDS-UPDRS-III)[18] and the Mini-Balance Evaluations Systems Test of dynamic balance (mini-BESTest),[19] respectively. The MDS-UPDRS-III was divided into subsections assessing tremor, rigidity, bradykinesia, and postural instability and gait (PIGD) as described previously.[20] We extracted Timed-Up-and-Go (TUG) and dual task TUG (DT-TUG, dual task: random number listing) from the mini-BESTest to measure functional mobility with and without a dual task. RM-ANOVAs (factors: medication, DBS) were used to compare mini-BESTest scores, MDS-UPDRS-III subsections, TUG, and DT-TUG across conditions.
A 4.8m GAITRite instrumented walkway (CIR Systems, Havertown, PA) was used for gait evaluation. Participants completed 3 trials each of preferred-pace forward (Fwd), fast as possible forward (Fast), preferred-pace backward (Bkd), and preferred-pace dual task forward (DT-Fwd) and dual task backward (DT-Bkd) gait. For each participant, gait task order was randomized within each session. In dual task trials, participants walked forward or backward at a comfortable pace while listing words that belonged to specified categories (semantic listing task). Different categories were given for each trial (examples include: shapes, cities, and fruit). Correct words and errors were recorded. Categories were grouped based on difficulty so each group of three categories was similarly difficult. Category groups were randomly assigned for dual tasks across all sessions for each participant. The category task was also assessed while seated in three 10 second trials. For each participant, three trials for each task were averaged for each session. Repeated measures ANOVA (factors: medication, DBS, Gait task) was used to evaluate gait variables across the five gait tasks, with follow-up tests as warranted. Greenhouse-Geisser corrections were used for violations of sphericity.
Results
Of the sixteen participants recruited, 4 did not tolerate DBS OFF and withdrew from the study. Twelve participants with PD were included in analyses. One participant was unable to complete Bkd and DT-Bkd gait in the OFFMeds-DBS condition and was excluded from gait analysis.
Functional Measures and Balance
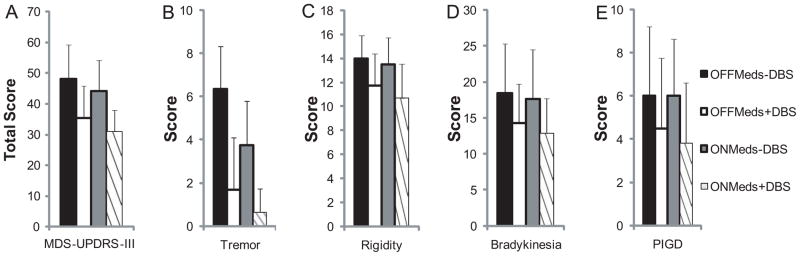
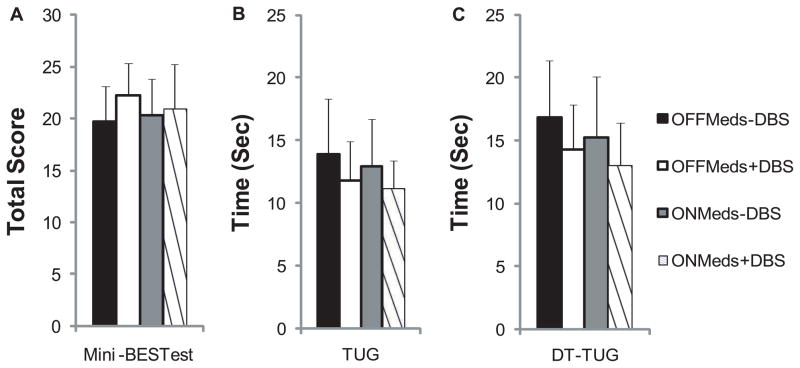
Total MDS-UPDRS-III and subsection scores are shown in Figure 1, and mini-BESTest, TUG, and DT-TUG are shown in Figure 2. There were medication effects on total MDS-UPDRS-III score (F(1,11)=5.1, p=0.03, Figure 1A), the MDS-UPDRS-III rigidity subsection (F(1,11)= 5.4, p=0.04, Figure 1C), and DT-TUG (F(1,11)=6.4, p=0.03, Figure 2C). There was a trend toward a medication effect on the MDS-UPDRS-III tremor subsection (F(1,11)= 4.3, p=0.06, Figure 1B). DBS effects were present for total MDS-UPDRS-III score (F(1,11)=29.8, p<0.001, Figure 1A), as well as MDS-UPDRS-III tremor (F(1,11)=14.0, p=0.003, Figure 1B), rigidity (F(1,11)=20.4, p=0.001, Figure 1C), bradykinesia (F(1,11)=17.7, p=0.001, Figure 1D), and PIGD subsections (F(1,11)=8.4, p=0.02, Figure 1E). Further, DBS effects were detected in mini-BESTest score (F(1,11)=6.4, p=0.03, Figure 2A) and DT-TUG (F(1,11)=11.7, p=0.006, Figure 2C). In all cases, performance improved ON medication and ON DBS. There was a trend toward TUG improvement with DBS (F(1,11)=3.4, p=0.09, Figure 2B). No medications x DBS interactions were present for the above tasks.
Figure 1.
Total MDS-UPDRS-III (A), as well as tremor (B), rigidity (C), bradykinesia (D), and PIGD (E) subsections across STN-DBS and medication conditions. Medication improved total MDS-UPDRS-III and rigidity scores. STN-DBS improved total MDS-UPDRS-III and all subsection scores. Values are means+SDs.
Figure 2.
Balance was quantified using the mini-BESTest (A), as well as timed-up-and-go (TUG, B) and dual task timed-up-and-go tests (DT-TUG, C) across STN-DBS and medication conditions. Mini-BESTest score improved with STN-DBS. DT-TUG improved with medication and STN-DBS. Values are means+SDs.
Gait
Cognitive task performance (correct word generation rates) while seated and during DT-Bkd gait was consistent across all sessions (p>0.05). Medication improved cognitive task performance during DT-Fwd gait (F(1,10)=5.6, p=0.04), and there was a trend toward improvement with DBS (F(1,10)=4.3, p=0.07) during DT-Fwd gait.
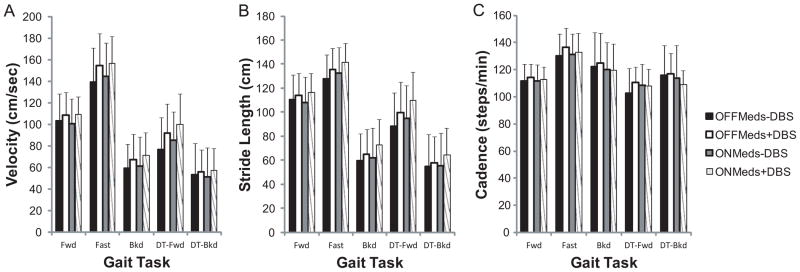
There were DBS (F(1,10)=15.90, p=0.003) and task effects (F(2.2,21.6)=90.4, p<0.001) on gait velocity (Figure 3A). Gait velocity increased with STN-DBS. Velocity differed across all gait tasks and was highest in Fast, followed by Fwd, DT-Fwd, Bkd, and DT-Bkd gait. No medication effects or medication x DBS, medication x gait task, or DBS x gait task interactions were present.
Figure 3.
Velocity (A), stride length (B), and cadence (C) were assessed across conditions during Fwd, Fast, Bkd, DT-Fwd, and DT-Bkd gait. Velocity improved with STN-DBS. Stride length improved with medication or STN-DBS. Improvements were similar across gait tasks. Values are means+SDs.
Stride length (Figure 3B) was affected by medication (F(1,10)=4.7, p=0.05), DBS (F(1,10)=15.1, p=0.003), and gait task (F(2.3,23.2)=119.4, p<0.001). Strides were longer with medication and with DBS. Stride length was greatest in Fast, followed by Fwd, DT-Fwd, Bkd, and DT-Bkd gait. Stride length differed between all gait conditions, except between Bkd and DT-Bkd gait (p=0.2). As above, there were no interaction effects.
Cadence (Figure 3C) was highest in Fast, followed by Bkd, DT-Bkd, Fwd, and DT-Fwd gait. Cadence differed (F(1.7,17.3)=9.1, p=0.003, Figure 3C) in Fast gait compared to Fwd (p<0.001), DT-Fwd (p<0.001), and DT-Bkd (p=0.04) gait. There were no medication, DBS, or interaction effects.
Discussion
We evaluated the effects of medication, STN-DBS, and both therapies combined on balance and complex gait tasks in PD. Medication alone or STN-DBS alone both conferred improvements in motor symptoms, balance and gait. The lack of interaction between medication and STN-DBS across all measures suggests the combination of therapies did not confer additional benefit over either therapy alone.
Functional Measures and Balance
As expected, MDS-UPDRS-III scores improved substantially with medication[21] and STN-DBS.[1] Medication tended to reduce rigidity and tremor as assessed by MDS-UPDRS-III subsections, while STN-DBS improved a broader range of symptoms, including rigidity, tremor, bradykinesia, and PIGD.[1]
Non-significant[4] and significant[5,6] improvements in TUG with medication or STN-DBS have been reported.[5,6] In the present study, TUG improved substantially but not significantly with medication (1.0±2.7 sec) or STN-DBS (2.1±3.1 sec). There was no added effect of both therapies together on TUG.[6] DT-TUG improved with medication or STN-DBS, but again there were no added benefits with both therapies combined.
In prior studies, both Berg Balance Scale[22] and mini-BESTest scores improved with medication, but ceiling effects were less of a concern with the mini-BESTest (Earhart lab, unpublished results). Mini-BESTest scores did not improve significantly with medication in the present study. However, prescribed medication doses are typically reduced following STN-DBS surgery, with reductions of approximately 50% reported.[2,7] Lower medication doses likely contributed to smaller medication effects in our sample, since current LEDD was 26% lower than pre-surgery LEDD for our participants (1135.2±428.0, data not available for one participant).
This is the first study to evaluate STN-DBS effects using clinical balance scales both OFF and ON medication. STN-DBS improved mini-BESTest scores in the present study and improved Berg Balance Scale scores OFF medication in prior reports,[5,7] suggesting STN-DBS enhances functional balance. STN-DBS effects on posturographic balance and stability measures are more variable, with reports of improvements,[8,23,24] no effect, or even detrimental effects of STN-DBS.[5,10,12,24] Posturographic balance and stability measures are generally not affected or detrimentally affected by medication.[23,24] Further, for some balance parameters, there were synergistic effects with medication and STN-DBS combined,[12] while for others, there were no additional benefits with combined therapies.[12,23] Use of different parameters to quantify balance likely contributes to conflicting results across studies, as STN-DBS may have differential effects on particular aspects of balance, and synergistic effects of medication and DBS may occur for some parameters.
Regardless of overall effects, previous studies allude to inter-individual variability in balance changes with STN-DBS. Some individuals improved TUG[4] or center-of-mass displacement after backward perturbation[10] with STN-DBS, while others performed similarly or worse. Consistent with prior reports of inter-individual variability, changes with STN-DBS alone were variable in the present study. For both mini-BESTest scores and TUG time, ten out of twelve individuals in our sample improved with STN-DBS while two performed the same or worse. Further, DT-TUG improved with STN-DBS in eight individuals with DBS alone, but worsened in four individuals. These ratios are similar to previous reports of balance improvements with STN-DBS in approximately 70% of participants.[7,10]
Gait
Our results confirm that similar improvements in preferred-pace stride length and velocity occur with medication or STN-DBS alone.[13–15] Cadence was not systematically affected by medication or STN-DBS.[15] Reports of synergistic effects of medication and STN-DBS in combination are inconsistent. Some studies report no additional improvements in preferred-pace gait velocity[6,15] and stride length[6] with STN-DBS ON medication, while others report preferred-pace gait improvements with combined therapies, compared to either therapy alone.[14,15]
Considerable inter-individual variability in gait changes with STN-DBS has been reported ON medication, with some individuals improving, while others perform worse.[4,14] In our sample, variability in preferred-pace gait velocity and stride length changes with STN-DBS occurred OFF and ON medication, with approximately two thirds of individuals improving with STN-DBS in either medication condition, while remaining participants performed worse. Certain subject characteristics may influence balance and gait responses to STN-DBS and account for variability in the literature. However, age, DBS voltage, disease duration, time since DBS surgery, OFFMeds-DBS MDS-UPDRS-III total and PIGD scores, self-reported annual fall frequency, and pre-surgery and current LEDD were not correlated with changes in mini-BESTest score, Fwd gait velocity, or Fwd stride length with STN-DBS alone. Future studies should further evaluate participant characteristics, including electrode contact location, which may contribute to favorable balance and gait responses to STN-DBS.
We expected substantial improvements in Fwd and Fast gait with medication or STN-DBS, as Fwd and Fast gait velocity and stride length were previously reported to be similarly affected by medication, STN-DBS, or both treatments in combination.[15] No previous studies have evaluated the effects of medication, STN-DBS, and combined therapies on Bkd, DT-Fwd, and DT-Bkd gait in PD, but we expected less pronounced improvements in these complex gait tasks which are potentially controlled by neural systems distinct from those for preferred-pace forward walking.[25] Contrary to our initial hypothesis, gait velocity and stride length improved similarly with medication or STN-DBS across gait tasks, regardless of task complexity. Further, there were no synergistic effects of medication and STN-DBS, suggesting both therapies may influence similar control mechanisms for various gait types.
Though STN-DBS effects on dual task gait had not been evaluated previously, other non-locomotor dual task paradigms have been studied. STN-DBS did not affect performance of simultaneous finger tapping and peg board tasks ON medication,[26] but negatively affected simultaneous performance of the n-back test with isometric grip force tracking OFF medication.[27] In the present study, STN-DBS enhanced velocity and stride length during DT-Fwd and DT-Bkd gait. Further, word generation rates were not negatively affected, and tended to improve with STN-DBS. Our results may differ from previous upper extremity studies due to the dual task used or differential effects of STN-DBS on upper extremity control versus locomotor control. Additional research on STN-DBS effects on various dual task paradigms is warranted.
Limitations
The relatively small sample size may limit generalization of the results. Further, STN-DBS effects on balance and gait were more pronounced than medication effects. Overall motor function (MDS-UPDRS-III) improved 7.4% with medication alone and 26.5% with STN-DBS alone, suggesting current medication doses may not provide sufficient motor benefit in the absence of STN-DBS. Supraclinical medication doses may have increased improvements with medication alone. However, despite the small sample size and lower medication doses, significant medication and STN-DBS effects were detected for balance and gait variables.
Conclusions
Overall, dopaminergic medications and STN-DBS had similar beneficial effects on balance and spatiotemporal parameters of different gait tasks in people with PD. Our results do not support a synergistic effect of medication and STN-DBS combined. Similar improvements in balance and gait with STN-DBS or medication may occur through improvements in abnormal cortical activity, including interactions between the SMA and basal ganglia which are important for movement planning and preparation.[6,28,29] Dopaminergic medications improve motor symptoms by increasing striatal dopamine levels and increasing thalamocortical output. Though the exact mechanism of STN-DBS is still unknown, DBS may improve abnormal STN firing patterns or hyperactivity,[29,30] normalizing thalamocortical output. However, inter-individual variability in this and previous studies suggests there may be sub-populations who respond favorably to STN-DBS or who experience minimal or negative effects with STN-DBS. Further research to distinguish characteristics of potential STN-DBS responders and non-responders may be beneficial for developing post-surgical treatment strategies or refining initial surgical selection criteria.
Acknowledgments
Thank you to Ryan Duncan and Corey Lohnes for data collection assistance. Research support was provided by NIN/NINDS F31 NS071639, NIH/NCMRR R01 HD056015, the Parkinson’s Disease Foundation, the American Parkinson Disease Association (APDA) Advanced Center for PD Research at Washington University School of Medicine, and the Greater Saint Louis Chapter of the APDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kumar R, Lozano AM, Kim YJ, Hutchison WD, Sime E, Halket E, Lang AE. Double-blind evaluation of subthalamic nucleus deep brain stimulation in advanced Parkinson’s disease. Neurology. 1998;51(3):850–5. doi: 10.1212/wnl.51.3.850. [DOI] [PubMed] [Google Scholar]
- 2.Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 1998;339(16):1105–11. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- 3.Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, Hausdorff JM. Gait dynamics in Parkinson’s disease: relationship to Parkinsonian features, falls and response to levodopa. J Neurol Sci. 2003;212(1–2):47–53. doi: 10.1016/s0022-510x(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 4.Kelly VE, Israel SM, Samii A, Slimp JC, Goodkin R, Shumway-Cook A. Assessing the effects of subthalamic nucleus stimulation on gait and mobility in people with Parkinson disease. Disabil Rehabil. 2010;32(11):929–36. doi: 10.3109/09638280903374139. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson MH, Fransson PA, Jarnlo GB, Magnusson M, Rehncrona S. The effects of high frequency subthalamic stimulation on balance performance and fear of falling in patients with Parkinson’s disease. J Neuroeng Rehabil. 2009;6:13. doi: 10.1186/1743-0003-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie J, Krack P, Benabid AL, Pollak P. Effect of bilateral subthalamic nucleus stimulation on parkinsonian gait. J Neurol. 2001;248(12):1068–72. doi: 10.1007/s004150170027. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson MH, Tornqvist AL, Rehncrona S. Deep-brain stimulation in the subthalamic nuclei improves balance performance in patients with Parkinson’s disease, when tested without anti-parkinsonian medication. Acta Neurol Scand. 2005;111(5):301–8. doi: 10.1111/j.1600-0404.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- 8.Colnat-Coulbois S, Gauchard GC, Maillard L, Barroche G, Vespignani H, Auque J, Perrin PP. Bilateral subthalamic nucleus stimulation improves balance control in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2005;76(6):780–7. doi: 10.1136/jnnp.2004.047829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hariz MI, Rehncrona S, Quinn NP, Speelman JD, Wensing C. Multicenter study on deep brain stimulation in Parkinson’s disease: an independent assessment of reported adverse events at 4 years. Mov Disord. 2008;23(3):416–21. doi: 10.1002/mds.21888. [DOI] [PubMed] [Google Scholar]
- 10.Visser JE, Allum JH, Carpenter MG, Esselink RA, Speelman JD, Borm GF, Bloem BR. Subthalamic nucleus stimulation and levodopa-resistant postural instability in Parkinson’s disease. J Neurol. 2008;255(2):205–10. doi: 10.1007/s00415-008-0636-x. [DOI] [PubMed] [Google Scholar]
- 11.Russmann H, Ghika J, Villemure JG, Robert B, Bogousslavsky J, Burkhard PR, Vingerhoets FJ. Subthalamic nucleus deep brain stimulation in Parkinson disease patients over age 70 years. Neurology. 2004;63(10):1952–4. doi: 10.1212/01.wnl.0000144198.26309.d8. [DOI] [PubMed] [Google Scholar]
- 12.Maurer C, Mergner T, Xie J, Faist M, Pollak P, Lucking CH. Effect of chronic bilateral subthalamic nucleus (STN) stimulation on postural control in Parkinson’s disease. Brain. 2003;126(Pt 5):1146–63. doi: 10.1093/brain/awg100. [DOI] [PubMed] [Google Scholar]
- 13.Hausdorff JM, Gruendlinger L, Scollins L, O’Herron S, Tarsy D. Deep brain stimulation effects on gait variability in Parkinson’s disease. Mov Disord. 2009;24(11):1688–92. doi: 10.1002/mds.22554. [DOI] [PubMed] [Google Scholar]
- 14.Ferrarin M, Rizzone M, Bergamasco B, Lanotte M, Recalcati M, Pedotti A, Lopiano L. Effects of bilateral subthalamic stimulation on gait kinematics and kinetics in Parkinson’s disease. Exp Brain Res. 2005;160(4):517–27. doi: 10.1007/s00221-004-2036-5. [DOI] [PubMed] [Google Scholar]
- 15.Cantiniaux S, Vaugoyeau M, Robert D, Horrelou-Pitek C, Mancini J, Witjas T, Azulay JP. Comparative analysis of gait and speech in Parkinson’s disease: hypokinetic or dysrhythmic disorders? J Neurol Neurosurg Psychiatry. 2010;81(2):177–84. doi: 10.1136/jnnp.2009.174375. [DOI] [PubMed] [Google Scholar]
- 16.Hackney ME, Earhart GM. Backward walking in Parkinson’s disease. Mov Disord. 2009;24(2):218–23. doi: 10.1002/mds.22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hackney ME, Earhart GM. The Effects of a Secondary Task on Forward and Backward Walking in Parkinson’s Disease. Neurorehabil Neural Repair. 2009 doi: 10.1177/1545968309341061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–70. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 19.Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. J Rehabil Med. 2010;42(4):323–31. doi: 10.2340/16501977-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duncan RP, Earhart GM. Randomized Controlled Trial of Community-Based Dancing to Modify Disease Progression in Parkinson Disease. Neurorehabil Neural Repair. 2011 doi: 10.1177/1545968311421614. [DOI] [PubMed] [Google Scholar]
- 21.Fahn S. Levodopa in the treatment of Parkinson’s disease. J Neural Transm Suppl. 2006;(71):1–15. doi: 10.1007/978-3-211-33328-0_1. [DOI] [PubMed] [Google Scholar]
- 22.Nova IC, Perracini MR, Ferraz HB. Levodopa effect upon functional balance of Parkinson’s disease patients. Parkinsonism Relat Disord. 2004;10(7):411–5. doi: 10.1016/j.parkreldis.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Rocchi L, Chiari L, Horak FB. Effects of deep brain stimulation and levodopa on postural sway in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2002;73(3):267–74. doi: 10.1136/jnnp.73.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shivitz N, Koop MM, Fahimi J, Heit G, Bronte-Stewart HM. Bilateral subthalamic nucleus deep brain stimulation improves certain aspects of postural control in Parkinson’s disease, whereas medication does not. Mov Disord. 2006;21(8):1088–97. doi: 10.1002/mds.20905. [DOI] [PubMed] [Google Scholar]
- 25.Choi JT, Bastian AJ. Adaptation reveals independent control networks for human walking. Nat Neurosci. 2007;10(8):1055–62. doi: 10.1038/nn1930. [DOI] [PubMed] [Google Scholar]
- 26.Page D, Jahanshahi M. Deep brain stimulation of the subthalamic nucleus improves set shifting but does not affect dual task performance in Parkinson’s disease. IEEE Trans Neural Syst Rehabil Eng. 2007;15(2):198–206. doi: 10.1109/TNSRE.2007.897074. [DOI] [PubMed] [Google Scholar]
- 27.Alberts JL, Voelcker-Rehage C, Hallahan K, Vitek M, Bamzai R, Vitek JL. Bilateral subthalamic stimulation impairs cognitive-motor performance in Parkinson’s disease patients. Brain. 2008;131(Pt 12):3348–60. doi: 10.1093/brain/awn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanakawa T, Katsumi Y, Fukuyama H, Honda M, Hayashi T, Kimura J, Shibasaki H. Mechanisms underlying gait disturbance in Parkinson’s disease: a single photon emission computed tomography study. Brain. 1999;122 (Pt 7):1271–82. doi: 10.1093/brain/122.7.1271. [DOI] [PubMed] [Google Scholar]
- 29.Limousin P, Greene J, Pollak P, Rothwell J, Benabid AL, Frackowiak R. Changes in cerebral activity pattern due to subthalamic nucleus or internal pallidum stimulation in Parkinson’s disease. Ann Neurol. 1997;42(3):283–91. doi: 10.1002/ana.410420303. [DOI] [PubMed] [Google Scholar]
- 30.Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006;29:229– 57. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]