Abstract
Background
Smokers are often reluctant to quit because they fear long-lasting withdrawal. Yet little research prospectively examines smokers’ withdrawal longer than 1 month post-quit.
Purpose
To compare successful versus unsuccessful quitters’ withdrawal, positive affect/pleasure, and lifestyle at 1 year post-quit.
Methods
Smokers (N=572) in a cessation trial completed ecological momentary assessments four times a day for 1 week pre-quit, 1 week post-quit, and 1 week at 1 year post-quit.
Results
From pre-quit to 1 year later, only quitters reported sizeable declines in craving and restlessness, and fewer stressful events. At 1 year, quitters, on average, reported no significant craving. Continuing smokers reduced their cigarette consumption considerably from pre-quit to 1 year later.
Conclusions
Contrary to smokers’ worries, long-term quitters reported less craving and restlessness than when they smoked (perhaps because cessation eliminates the acute nicotine withdrawal smokers experience between cigarettes). This information may encourage smokers to quit and endure withdrawal.
Keywords: Smoking, Smoking cessation, Withdrawal, Craving, Ecological momentary assessment
Introduction
Smokers often worry about how their lives will change if they quit smoking. For instance, they worry that—because of either withdrawal or the loss of smoking as a coping response—quitting will cause them to experience more stress, restlessness, irritability, anxiety, negative affect, and nagging cravings for cigarettes (1–6). For many smokers sustained cessation is a mystery, and they no doubt wonder how they will feel and function—how life will change—if they are successful at quitting.
Research has shed some light on this topic. For instance, a review (7) concluded that quitters typically experience withdrawal symptoms for just 2 to 4 weeks after the quit day. The review noted, however, that some smokers experience more prolonged withdrawal, with symptoms persisting for months (8, 9). Furthermore, smokers sometimes report that in prior quit attempts they experienced cravings after months or even years of abstinence. Hughes (10) found that half of participants who were abstinent at 6 months reported experiencing some craving.
Little research has prospectively examined withdrawal symptoms over longer than a month after the quit day. One study (11) with a longer follow-up assessed self-quitters’ withdrawal symptoms at 1 week and at 1, 6, and 12 months post-quit. However this study did not collect pre-quit data on withdrawal symptoms and did not use ecological momentary assessment, which collects data from participants multiple times a day as they go about their daily lives. A second study—the Hughes study mentioned in the previous paragraph (10)—assessed pre- and post-quit withdrawal symptoms through 6 months post-quit but again did not use ecological momentary assessment. Compared to retrospective recall methods, ecological momentary assessment tends to provide more accurate reports of ongoing events and experiences (12, 13). To the best of our knowledge, the current study is the first to analyze ecological momentary assessment data among quitters and continuing smokers at 1 year after the target quit day.
In the current study, we analyzed real-time measures of smokers’ withdrawal symptoms, positive affect, and behavioral/contextual lifestyle characteristics during (a) the week prior to the quit day, (b) the week after the quit day, and (c) a week 1 year after the quit day. We first performed omnibus analyses comparing the extent to which successful quitters versus continuing smokers changed on the real-time measures from pre-quit (during on-going smoking) to 1 year post-quit. We followed up on the omnibus analyses by separately examining (a) the extent to which successful quitters changed on these measures from pre-quit to 1 year, and (b) the extent to which continuing smokers changed from pre-quit to 1 year. We then redid the entire series of analyses but instead of examining changes from pre-quit to 1 year post-quit, we examined changes from immediately post-quit (the estimated peak of withdrawal) to 1 year post-quit.
A priori, we predicted that successful quitters’ withdrawal symptoms would decrease from pre-quit to 1 year post-quit, relative to continuing smokers’ withdrawal symptoms. We reasoned that long-term successful quitters versus continuing smokers would experience less intense withdrawal symptoms such as craving because of their diminished tobacco dependence and/or because they no longer experienced the iterative bouts of craving between cigarettes that often occur during ongoing smoking (14, 15). The main goal of this work was to determine which symptoms and behaviors changed after prolonged cessation and how much they changed, relative to continued smoking. The results should help elucidate the natural history of tobacco dependence and also contribute to efforts to educate smokers about the long-term outcomes of cessation (e.g., to either reassure them or prepare them for prolonged challenges).
Method
Participants
Participants were 572 smokers (for demographics see Table 1) who took part in a placebo-controlled trial evaluating five smoking cessation pharmacotherapies (see (16)). Trial inclusion criteria included smoking more than 9 cigarettes a day and being motivated to quit. Exclusion criteria included consuming 6 or more alcoholic drinks 6 to 7 days a week or reporting a history of an eating disorder (both are contraindications for bupropion, one of the study’s pharmacotherapies). Additional exclusion criteria included reporting a history of schizophrenia, psychosis, or bipolar disorder. Participants provided written informed consent, and the University of Wisconsin Health Sciences Institutional Review Board approved the trial.
Table 1.
Baseline Sample Characteristics
| Total sample (N = 572) |
Abstinent at 1 year (n = 215; 37.6%) |
Smoking at 1 year (n = 357; 62.4%) |
|
|---|---|---|---|
| % Female | 57.5% | 52.6% | 60.5% |
| % White | 86.3% | 91.2% b | 83.3% b |
| % with a high school education or greater | 94.7% | 97.2% a | 93.2% a |
| Mean age (SD) | 45.39 (10.86) | 46.04 (11.49) | 44.99 (10.46) |
| Mean number of cigarettes per day at baseline (SD) | 21.29 (8.93) | 19.68 (7.42) c | 22.26 (9.61)c |
Note. We compared the baseline characteristics of participants who were abstinent versus smoking at 1 year after the target quit date using t-tests and chi-square tests where appropriate.
Items with the same superscript differ significantly from each other.
p < .05.
p < .01.
p < .001.
The parent trial involved 1504 smokers, but the sample for this study was 572. We lost participants because we required that they: (a) attend the 1-year follow-up visit to provide abstinence data (carbon monoxide-confirmed if claiming abstinence; 502 participants lost); (b) provide self-reports via ecological momentary assessment in the days surrounding the target quit day and again at 1 year after the quit day (393 participants lost); and (c) be verified as abstinent at both 6 months and 1 year after the target quit day, smoking at both time points, or abstinent at 6 months but smoking at 1 year (37 participants lost). We required that participants considered abstinent be verified as abstinent at both 6 months and 1 year to increase the likelihood that they had maintained abstinence for a substantial time. This conservative policy was adopted because it is unknown how long a period of abstinence is required for some of the positive effects of tobacco abstinence to occur.
Baseline and Abstinence Measures
Baseline demographics
Participants reported their age, education level, and other demographic information.
Cigarettes per day
At baseline, participants reported on average how many cigarettes they smoked per day. At 1 year after the target quit day, participants completed a follow-up phone call and reported on average in the past month how many cigarettes they smoked on days they smoked.
Smoking status
Participants were coded as achieving extended biochemically confirmed 7-day point-prevalence abstinence at 1 year post-quit if (a) their report of abstinence over the previous 7 days was confirmed by an expired carbon monoxide rating of less than 10 parts per million, and (b) they had also achieved carbon monoxide-confirmed 7-day point-prevalence abstinence at 6 months post-quit. Participants were coded as continuing smokers if they were either (a) smoking at both 6 months and 1 year post-quit (n = 294; 82.4% of those coded as continuing smokers), or (b) abstinent at 6 months but smoking at 1 year post-quit (n = 63; 17.6% of those coded as continuing smokers). (Please note that the terms “pre-quit” and “post-quit” mean, respectively, the time period before and after a participant’s target quit day.)
Ecological Momentary Assessments
Personal digital assistants prompted participants to answer ecological momentary assessment questions four times a day (after waking, before bed, and at two other random times during the day; all prompts were separated by an hour or more). Participants received daily ecological momentary assessment prompts for up to 2 weeks prior to their target quit day, for 2 weeks after their target quit day, and for a week 1 year after their target quit day. As in our previous research (17) that examined the ecological momentary assessment data surrounding the target quit day (but that did not examine the ecological momentary assessment data gathered 1 year after the target quit day), we analyzed responses from (a) the 10 days prior to the target quit day, and (b) the target quit day plus the following 10 days. As we did previously, responses to the first and last 2 days of prompting were eliminated from analyses because they were not representative of the other responses (in terms of both completion rates and mean values) and because not all participants completed the full assessment period based on appointment scheduling.
Each ecological momentary assessment prompt asked participants to respond to questions based on how they felt in the last 15 minutes. As in our previous research (18, 19), we included items selected from validated questionnaires (typically scale salients) that would provide face valid information on a broad array of constructs while minimizing participant burden. The questions included: (a) two positive affect items (excited and enthusiastic) from the Positive and Negative Affect Scale (20); (b) ten items from the Wisconsin Smoking Withdrawal Scale (21) assessing five withdrawal dimensions (anger/irritability, anxiety, sadness, difficulty concentrating, and hunger); each withdrawal dimension consisted of the two strongest loading items in the scale derivation factor analysis (21); (c) two craving items—“I have had an urge to smoke” (22) and the craving item from the Wisconsin Smoking Withdrawal Scale (“I have been bothered by the desire to smoke a cigarette”); and (d) an item assessing restlessness. The mean of the items for each domain (e.g., positive affect, craving) was used for each analysis (see the analytic plan for more details). The positive items from the Positive and Negative Affect Scale used a 5-point scale ranging from 1 (not at all) to 5 (extremely), and the remaining items described above used a slider (i.e., a visual analogue scale) on the personal digital assistant ranging from “disagree!!” (coded as 0) to “agree!!” (coded as 10). The Wisconsin Smoking Withdrawal Scale has a 0 to 4 response scale, but, as in previous ecological momentary assessment research (18), we used a 0 to 10 response scale to increase response sensitivity.
Participants also reported whether smoking was permitted at the location where they were in the last 15 minutes, the number of cigarettes they smoked since their last ecological momentary assessment prompt, and how recently they last smoked. In the evening assessment, participants additionally reported: (a) whether any stressful events happened since their evening report the previous night, (b) how many alcoholic drinks they had that day, and (c) how much pleasure they got that day from three domains—contact with others, performance (work, school, or chores), and recreation. The three pleasure items used a slider that ranged from “no pleasure” (coded as 0) to “extreme pleasure” (coded as 10). There were also some ecological momentary assessment questions that were either redundant or not relevant for the current study (e.g., smoking cessation medication use).
Procedure
Qualified participants gave written, informed consent and completed baseline assessments including a carbon monoxide breath test and demographic and smoking history questionnaires. Participants were then randomized to one of six treatment conditions: (1) sustained-release bupropion (for 1 week prior to the quit day plus 8 weeks starting on the quit day); (2) nicotine lozenge (for 12 weeks starting on the quit day); (3) nicotine patch (for 8 weeks starting on the quit day); (4) nicotine patch + nicotine lozenge; (5) sustained-release bupropion + nicotine lozenge; or (6) one of five placebo conditions matched to the five active conditions. All participants also received six brief (10–20 minute) individual counseling sessions (see (16) for details).
Analytic Plan
Craving (the mean of the two craving items described earlier) and pleasure (the mean of the three pleasure items described earlier) composite measures were grouped with the other ecological momentary assessment items into three substantively related domains: (a) withdrawal symptoms (7 tests: craving, restlessness, and the five withdrawal dimensions assessed by the Wisconsin Smoking Withdrawal Scale); (b) positive affect and pleasure (2 tests: the positive items from the Positive and Negative Affect Scale and the composite pleasure measure); and (c) behavioral/contextual features (3 tests— number of alcoholic drinks that day, occurrence of stressful events, and whether smoking was permitted at their current location). There were also two additional tests for analyses involving only participants smoking at 1 year: how recently they last smoked, and cigarettes smoked since the last prompt.
We analyzed participants’ responses on each ecological momentary assessment measure (e.g., the composite craving measure) using analysis of variance (ANOVA). We reported two effect sizes—generalized eta squared (23) and partial eta squared (conservative and liberal effect size estimates, respectively)—to provide a reasonable range of effect sizes. For generalized eta squared, .02, .13, and .26 can be considered small, medium, and large effects, respectively (24). For partial eta squared, .02, .15, and .35 can be considered small, medium, and large effects, respectively (25). We conducted two omnibus analyses comparing the extent to which successful quitters versus continuing smokers changed on each ecological momentary assessment measure (from pre-quit to 1 year post-quit, and then from immediately post-quit to 1 year post-quit). These two omnibus analyses included a between-subjects factor of Abstinence Status (abstinent vs. smoking at 1 year post-quit) and a within-subjects repeated measures factor of Time (either pre-quit vs. 1 year post-quit or immediately post-quit vs. 1 year post-quit). We used these two omnibus analyses to examine effects within the three ecological momentary assessment measure domains (withdrawal symptoms, positive affect/pleasure, and behavioral/contextual features); thus, there were six families of analyses, and we used the Holm-Bonferroni method to correct for conducting multiple comparisons within families.
We followed up any significant interactions between Abstinence Status and Time with focused tests of simple effects to determine if the Time effect was significant within each group (i.e., in successful quitters alone and in continuing smokers alone) using a one-way repeated measures ANOVA with a within-subjects repeated measures factor of Time. We also followed up any significant main effects of Time (in the absence of an Abstinence Status by Time interaction) to determine if the Time effect was significant within each group and suggested there was change over time. Thus, we performed the first omnibus analysis (comparing the extent to which successful quitters versus continuing smokers changed from pre-quit to 1 year) and then followed-up with analyses separately assessing (a) the extent to which successful quitters changed on each ecological momentary assessment measure from pre-quit to 1 year and (b) the extent to which continuing smokers changed from pre-quit to 1 year. We subsequently performed the second omnibus analysis (comparing the extent to which successful quitters versus continuing smokers changed from immediately post-quit to 1 year) and then followed-up with analyses separately assessing (a) the extent to which successful quitters changed on each ecological momentary assessment measure from post-quit to 1 year and (b) the extent to which continuing smokers changed from post-quit to 1 year. We believed it was clinically important to identify significant changes within each group because information on long-term changes from the precessation or withdrawal periods could be helpful in educating and counseling smokers.
Results
Successful quitters’ and continuing smokers’ mean ecological momentary assessment responses at pre-quit, immediately post-quit, and 1 year post-quit are reported in Table 2. For all omnibus analyses reported below, results that were significant with the Holm-Bonferroni correction remained significant (analyses not shown) after controlling for the following covariates (chosen because those who were abstinent versus continuing to smoke at 1 year post-quit differed on these variables at baseline): education (less than high school vs. high school education or greater), race (white vs. not white), and cigarettes per day.
Table 2.
Means (and standard deviations in parentheses) on ecological momentary assessment measures at three time points among quitters versus continuing smokers.
| Pre-quit | Post-quit | 1 year post-quit | ||||
|---|---|---|---|---|---|---|
| Measure | Abstinent at 1 year |
Smoking at 1 year |
Abstinent at 1 year |
Smoking at 1 year |
Abstinent at 1 year |
Smoking at 1 year |
| Craving†‡ | 3.96a (2.13) |
4.42b (2.14) |
3.51c (2.48) |
4.61d (2.48) |
0.60ac (1.15) |
4.17bd (2.41) |
| Restlessness† | 1.26a (1.31) |
1.70 (1.72) |
1.79c (1.86) |
2.32d (2.24) |
0.81ac (1.04) |
1.64d (1.73) |
| Anger/ Irritability†* |
1.05a (1.16) |
1.30 (1.33) |
1.26c (1.47) |
1.66d (1.66) |
0.89ac (1.01) |
1.40d (1.43) |
| Anxiety | 1.26 (1.12) |
1.64 (1.53) |
1.62c (1.53) |
1.99d (1.81) |
1.10c (1.11) |
1.66d (1.50) |
| Sadness | 0.73 (1.02) |
1.02 (1.43) |
.75 (1.15) |
1.11 (1.50) |
0.69 (1.12) |
1.13 (1.46) |
| Difficulty concentrating |
0.85 (1.02) |
1.14 (1.46) |
1.12c (1.51) |
1.40d (1.66) |
0.74c (0.98) |
1.16d (1.44) |
| Hunger | 2.07 (1.58) |
2.11 (1.60) |
2.51c (1.80) |
2.46d (1.88) |
2.19c (1.79) |
2.19d (1.60) |
| Positive affect | 2.04 (0.75) |
1.95b (0.72) |
2.06 (0.81) |
1.93d (0.79) |
2.11 (0.76) |
2.02bd (.71) |
| Pleasure1 | 5.86a (1.44) |
5.59b (1.88) |
5.73c (1.67) |
5.42d (2.04) |
5.52ac (1.76) |
5.15bd (1.80) |
| % days with a stressful event1 |
18.83a (22.33) |
22.15 (24.87) |
20.37c (24.48) |
22.04 (25.73) |
14.29ac (21.33) |
19.54 (25.38) |
| Alcoholic drinks that day1 |
1.05 (1.54) |
0.75 (1.12) |
0.76c (1.70) |
0.66d (1.42) |
0.98c (2.20) |
0.95d (1.99) |
| % of prompts smoking was permitted†‡ |
84.01a (21.07) |
84.25 (21.41) |
78.27c (30.02) |
80.15d (28.34) |
70.85ac (35.25) |
85.33d (23.96) |
| Cigarettes smoked since last prompt2 |
4.71 (2.93) |
5.30b (3.13) |
-- | -- | -- | 3.77b (2.97) |
| % time smoked <15 min. ago2 |
24.62 (18.11) |
29.46b (20.87) |
-- | -- | -- | 21.77b (21.94) |
Note. Within each row, numbers with the same superscript lowercase letter differ significantly.
denotes a measure that showed a significant interaction effect between Time and Abstinence Status such that quitters versus continuing smokers differed in how much they changed on this measure from pre-quit to 1 year post-quit, while
denotes a measure that showed a significant interaction effect from immediately post-quit to 1 year.
The interaction effect between Time (pre-quit vs. 1 year post-quit) and Abstinence Status for anger/irritability had a p value of .012 (just shy of the p < .01 needed for the effect to be significant with the Holm-Bonferroni correction). N = 572 (215 abstinent and 357 smoking at 1 year) except for measure names followed by numerical superscripts.
n = 517 (202 abstinent and 315 smoking at 1 year).
n = 534 (215 abstinent and 319 smoking at 1 year).
Changes from Pre-quit to 1 Year Post-quit
Omnibus results for changes among quitters versus continuing smokers from pre-quit to 1 year post-quit
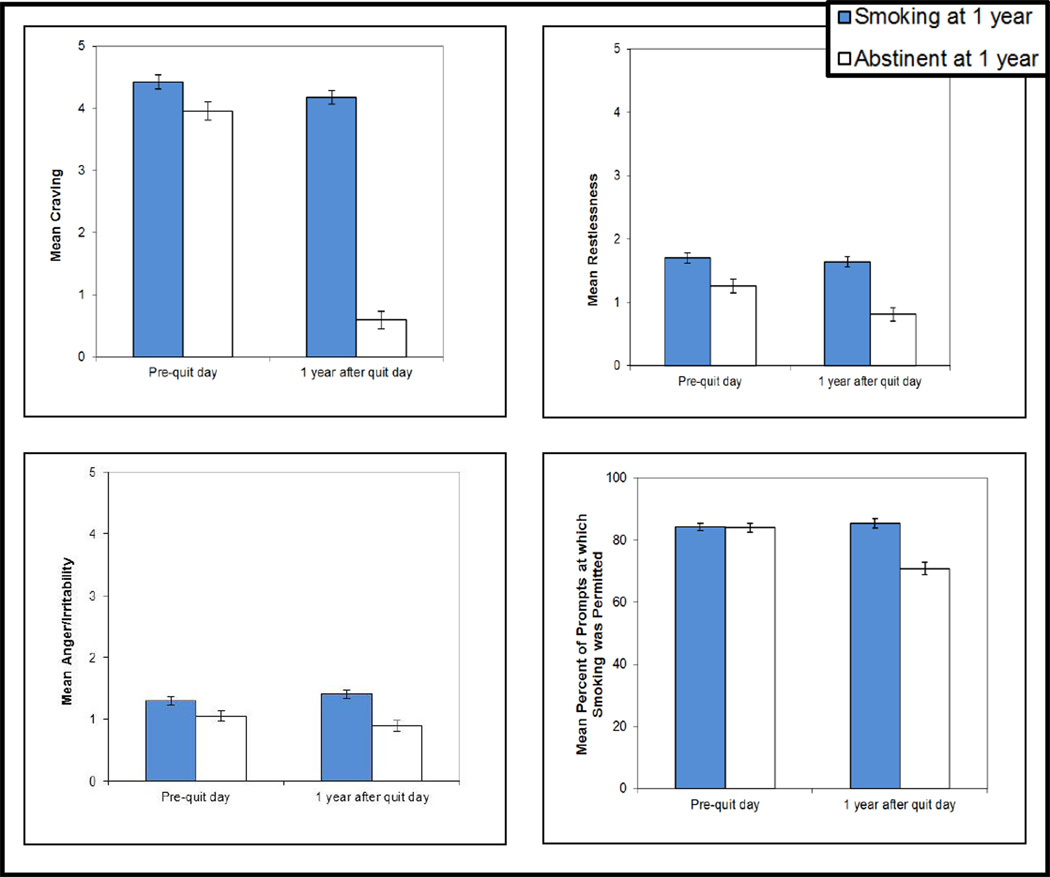
There was a significant interaction effect between Time (pre-quit vs. 1 year post-quit) and Abstinence Status (abstinent vs. smoking at 1 year post-quit) for four of the ecological momentary assessment measures: craving, restlessness, anger/irritability, and the percentage of prompts at which smoking was permitted at their location (see Figure 1; the effect for anger/irritability, however, had a p value of .012 which was just shy of the p < .01 needed for the effect to be significant with the Holm-Bonferroni correction). Additionally, there was a main effect of Time for positive affect, pleasure, and the percentage of days with a stressful event.
Figure 1.
Interaction effects showing that those abstinent versus smoking at 1 year post-quit differed in the extent to which they changed from pre-quit to 1 year post-quit in terms of their mean craving, restlessness, anger/irritability, and percentage of prompts at which smoking was permitted at their location. The interaction effect for anger/irritability (p = .012) was no longer significant after correcting for multiple comparisons. Additional analyses (not graphed here) found the interaction effect for the percentage of prompts at which smoking was permitted differed significantly as a function of treatment condition. Error bars represent SEs.
In terms of the interaction effects, quitters showed a steeper decrease than continuing smokers in craving from pre-quit to 1 year post-quit, F(1, 570) = 277.24, p < .001, ηG2 = .097, ηp2 = .33. Similarly, quitters showed a decrease in restlessness while continuing smokers did not, F(1, 570) = 11.77, p < .001, ηG2 = .004, ηp2 = .02. Quitters decreased in anger/irritability while continuing smokers increased slightly, although, as mentioned above, this interaction was not significant with the correction for multiple comparisons, F(1, 570) = 6.28, p = .012, ηG2 = .002, ηp2 = .01. Finally, there was a decrease in the percentage of prompts at which quitters reported that smoking was permitted at their location (from 84 to 71% of the time), while continuing smokers reported a slight increase (from 84 to 85%), F(1, 570) = 35.05, p < .001, ηG2 = .018, ηp2 = .06.
We were interested in whether any of the significant relationships we found between Time and Abstinence Status differed as a function of treatment condition (interacted with medication condition). To examine this we coded treatment based on Cohen and colleagues’ recommendations for 2 × 2 designs (26) and created two treatment variables (one for the bupropion effect and one for the nicotine replacement therapy effect). We then entered both variables simultaneously as between subjects factors in the ANOVA analyses. We chose this coding because (a) bupropion may have unique effects on symptoms, (b) the coding allowed us to examine the effect of the combination of bupropion and nicotine lozenge (through the interaction of the bupropion effect and the nicotine replacement therapy effect), and (c) it maintains orthogonality of effects. For the bupropion effect (pharmacotherapy involving bupropion), participants were assigned a +.5 if they were randomized to bupropion or bupropion + lozenge and a −.5 if they were randomized to placebo, patch, lozenge, or patch + lozenge. For the nicotine replacement therapy effect (pharmacotherapy involving at least some nicotine replacement therapy), participants were assigned a +.5 if they were randomized to patch, lozenge, patch + lozenge, or bupropion + lozenge and a −.5 if they were randomized to placebo or bupropion.
Only one of the significant interactions between Time and Abstinence Status described above—involving the percentage of prompts at which smoking was permitted—was subsumed by a 3-way interaction that comprised treatment condition. For this analysis, adding the two treatment variables (the bupropion effect and the nicotine replacement therapy effect) simultaneously as between subjects factors in the ANOVA resulted in a Time × Abstinence Status × Bupropion Treatment interaction, F(1, 564) = 6.60, p = .01, ηG2 = .003, ηp2 = .01. To unpack this interaction, we examined the unadjusted means in a separate analysis that coded treatment as a categorical variable with three levels: (a) placebo, (b) some bupropion (i.e., bupropion or bupropion + lozenge), and (c) nicotine replacement therapy only (i.e., nicotine patch, lozenge, or patch + lozenge). This analysis revealed that successful quitters randomized to placebo reported a large decrease from pre-quit to 1 year post-quit in the percentage of prompts at which smoking was permitted at their location (from 84 to 52% of the time), while quitters randomized to at least some bupropion reported a small decrease (from 83 to 76%), and quitters randomized to receive only nicotine replacement therapy reported an intermediate decrease (from 85 to 71%). Continuing smokers—regardless of treatment condition—reported smoking was permitted at their location approximately 85% of the time at both pre-quit and 1 year post-quit. (Because including the two treatment variables resulted in a significant interaction, we also included the treatment variables in the analysis below examining the extent to which successful quitters reported a change from pre-quit to 1 year in the percentage of prompts at which smoking was permitted at their location.)
Changes among successful quitters from pre-quit to 1 year post-quit
Successful quitters showed significant decreases from pre-quit to 1 year post-quit on six ecological momentary assessment measures: (a) craving, F(1, 214) = 599.63, p < .001, ηG2 = .493, ηp2 = .74; (b) restlessness, F(1, 214) = 40.37, p < .001, ηG2 = .035, ηp2 = .16; (c) anger/irritability, F(1, 214) = 5.08, p = .03, ηG2 = .005, ηp2 = .02; (d) pleasure, F(1, 201) = 10.91, p = .001, ηG2 = .011, ηp2 = .05; (e) the percentage of time a stressful event occurred since the last evening report (from 19 to 14%), F(1, 201) = 8.36, p = .004, ηG2 = .011, ηp2 = .04; and (f) the percentage of prompts at which smoking was permitted at their location, F(1, 211) = 30.46, p < .001, ηG2 = .050, ηp2 = .13. This final analysis included the two treatment variables (for reasons described above), and the main effect of Time was subsumed by a significant Time × Bupropion Treatment interaction, F(1, 211) = 4.92, p = .03, ηG2 = .012, ηp2 = .02. To unpack this interaction, we examined the unadjusted means in a separate analysis described earlier. This analysis revealed that successful quitters randomized to placebo reported a large decrease from pre-quit to 1 year post-quit in the percentage of prompts at which smoking was permitted at their location, while quitters randomized to the bupropion treatments reported a small decrease and quitters randomized to receive only nicotine replacement therapy reported an intermediate decrease.
Changes among continuing smokers from pre-quit to 1 year post-quit
Continuing smokers showed decreases from pre-quit to 1 year post-quit on two ecological momentary assessment measures: (a) craving, F(1, 356) = 4.38, p = .037, ηG2 = .003, ηp2 = .01; and (b) pleasure, F(1, 314) = 25.97, p < .001, ηG2 = .014, ηp2 = .08. They also showed a small increase in positive affect, F(1, 356) = 4.48, p = .035, ηG2 = .002, ηp2 = .01.
We then performed several additional analyses that were separate from the omnibus analyses because only continuing smokers’ answers to these questions were meaningful at 1 year post-quit. All these analyses included the two treatment variables (bupropion and nicotine replacement therapy) as between-subjects factors. Continuing smokers showed decreases from prequit to 1 year in the number of cigarettes they smoked since the last report, F(1, 315) = 74.41, p < .001, ηG2 = .060, ηp2 = .19; this effect, however, was subsumed by a significant interaction between Time and Treatment with nicotine replacement therapy, F(1, 315) = 6.35, p = .01, ηG2 = .005, ηp2 = .02. To make sense of this interaction, we examined the unadjusted means in a separate analysis that again coded treatment as a categorical variable with three levels: (a) placebo, (b) some bupropion, and (c) nicotine replacement therapy only. This analysis revealed that continuing smokers randomized to placebo reported only a slight decrease from pre-quit to 1 year post-quit in cigarettes smoked since the last report (from 4.79 to 4.21 cigarettes). In contrast, continuing smokers randomized to the bupropion treatments reported a decrease from 5.25 to 3.65 cigarettes, and continuing smokers randomized to receive only nicotine replacement therapy reported a decrease from 5.44 to 3.75.
Continuing smokers also showed decreases in the percentage of the time they smoked their most recent cigarette less than 15 minutes before the prompt from 29% of the time (SE = 1.4) pre-quit to 22% of the time (SE = 1.4) at 1 year post-quit, F(1, 315) = 35.53, p < .001, ηG2 = .032, ηp2 = .10; there were no significant interactions with treatment. In addition to these analyses of the ecological momentary assessment measures, we examined whether continuing smokers changed the number of cigarettes they smoked per day from pre-quit (assessed via questionnaire) to month 12 after the target quit day (assessed via a follow-up call). We found continuing smokers decreased their mean cigarettes per day from 22.35 (SE = .64) pre-quit to 12.57 (SE = .58) at 1 year, F(1, 317) = 324.84, p < .001, ηG2 = .215, ηp2 = .51; there were no significant interactions with treatment.
Changes from Immediately Post-quit to 1 Year Post-quit
Omnibus results for changes among quitters versus continuing smokers from immediately post-quit to 1 year post-quit
There was a significant interaction effect between Time (immediately post-quit vs. 1 year post-quit) and Abstinence Status (abstinent vs. smoking at 1 year post-quit) for two of the ecological momentary assessment measures: craving and the percentage of prompts at which smoking was permitted at their location. Additionally, there was a main effect of Time for: restlessness, anger/irritability, anxiety, difficulty concentrating, hunger, positive affect, pleasure, the percentage of days with a stressful event, and the number of alcoholic drinks consumed that day.
In terms of the interaction effects, quitters showed a relatively steep decrease in craving while continuing smokers showed a slight decrease from immediately post-quit to 1 year post-quit, F(1, 570) = 130.95, p < .001, ηG2 = .054, ηp2 = .19. Additionally, among quitters there was a decrease in the percentage of prompts at which they reported smoking was permitted at their location (from 78 to 71% of the time), while among continuing smokers there was an increase (from 80 to 85%), F(1, 570) = 28.14, p < .001, ηG2 = .011, ηp2 = .05. Finally, quitters showed a steeper decrease in restlessness than continuing smokers, but this interaction effect was not significant after correcting for multiple comparisons, F(1, 570) = 4.09, p = .04, ηG2 = .0015, ηp2 = .01. None of the significant relationships we found between Time and Abstinence Status differed as a function of treatment condition (coded using two variables as described above).
Changes among successful quitters from immediately post-quit to 1 year post-quit
Successful quitters showed decreases from immediately post-quit to 1 year post-quit on nine ecological momentary assessment measures: (a) craving, F(1, 214) = 347.03, p < .001, ηG2 = .364, ηp2 = .62; (b) restlessness, F(1, 214) = 100.43, p < .001, ηG2 = .097, ηp2 = .32; (c) anger/irritability, F(1, 214) = 19.11, p < .001, ηG2 = .021, ηp2 = .08; (d) anxiety, F(1, 214) = 33.71, p < .001, ηG2 = .037, ηp2 = .14; (e) difficulty concentrating, F(1, 214) = 19.67, p < .001, ηG2 = .023, ηp2 = .08; (f) hunger, F(1, 214) = 8.08, p = .005, ηG2 = .008, ηp2 = .04; (g) pleasure, F(1, 201) = 4.05, p = .046, ηG2 = .004, ηp2 = .02; (h) the percentage of time a stressful event occurred since the last evening report (from 20 to 14%), F(1, 201) = 11.32, p = .001, ηG2 = .017, ηp2 = .05; and (i) the percentage of prompts at which smoking was permitted at their location, F(1, 214) = 11.86, p = .001, ηG2 = .013, ηp2 = .05. Quitters also showed a slight increase in the number of alcoholic drinks they reported drinking on a given day, F(1, 201) = 4.61, p = .03, ηG2 = .003, ηp2 = .02.
Changes among continuing smokers from immediately post-quit to 1 year post-quit
Continuing smokers showed decreases from immediately post-quit to 1 year post-quit on seven ecological momentary assessment measures: (a) craving, F(1, 356) = 9.54, p = .002, ηG2 = .008, ηp2 = .03; (b) restlessness, F(1, 356) = 47.19, p < .001, ηG2 = .028, ηp2 = .12; (c) anger/irritability, F(1, 356) = 11.82, p = .001, ηG2 = .007, ηp2 = .03; (d) anxiety, F(1, 356) = 16.62, p < .001, ηG2 = .010, ηp2 = .05; (e) difficulty concentrating, F(1, 356) = 12.81, p < .001, ηG2 = .006, ηp2 = .04; (f) hunger, F(1, 356) = 10.43, p = .001, ηG2 = .006, ηp2 = .03; and (g) pleasure, F(1, 314) = 8.60, p = .004, ηG2 = .005, ηp2 = .03. Continuing smokers also showed increases from immediately post-quit to 1 year post-quit on three ecological momentary assessment measures: (a) the number of alcoholic drinks they consumed that day (from .66 to .95 drinks), F(1, 314) = 6.89, p = .009, ηG2 = .004, ηp2 = .02; (b) the percentage of prompts at which smoking was permitted at their location, F(1, 356) = 15.64, p < .001, ηG2 = .010, ηp2 = .04; and (c) positive affect, F(1, 356) = 8.15, p = .005, ηG2 = .004, ηp2 = .02.
Changes in Cravings to Smoke
To understand more fully the changes in craving experienced by quitters and continuing smokers, we calculated the percentage in each group whose ecological momentary assessment scores indicated they had experienced significant craving—i.e., they had a mean score of 6 or higher on the craving scale which ranged from “disagree!!” (coded as 0) to “agree!!” (coded as 10). The percentage of quitters whose mean score indicated they had experienced significant craving at pre-quit, post-quit, and 1 year respectively was 16.3%, 17.2%, and 0.5%. Among continuing smokers, the corresponding percentages were 23.2%, 28.6%, and 23.0%.
Discussion
Many smokers worry that if they quit smoking they will feel worse psychologically than they felt before they quit. For instance, they worry that they will miss the pleasure of smoking or that their lives will be more stressful if they cannot smoke as a coping response (3, 4, 6). The present study found, however, that smokers who quit—and were still abstinent 1 year after their target quit day—actually improved from pre-quit to 1 year on several symptomatic and contextual measures. Specifically, from pre-quit to 1 year, quitters showed sizeable decreases in craving—including a near extinction of significant cravings—and they showed decreases in restlessness, stressful event frequency, and the percentage of time they spent in a location permitting smoking. Unexpectedly, quitters showed a decrease in pleasure from pre-quit to 1-year, but so did continuing smokers. Moreover, the effect in each group was of small magnitude, and it was correlated with age in the combined sample, r(515) = .10, p = .02, suggesting a general decline in pleasure with aging.
That these findings arise from real-time assessment of symptom and behavioral ratings gives credence to the results. It lends further credence to the results that they agree with Hughes’ (10) findings showing continuous quitters’ withdrawal symptoms (except hunger and weight gain) returned to pre-quit levels by 30 days post-quit and remained at pre-quit levels or decreased further at the 6 month follow-up (this research did not use ecological momentary assessment, however). The finding that quitters showed a decrease in stressful event frequency from pre-quit to 1 year post-quit is also consistent with other studies that have found quitting smoking decreases stress (for a review, see (15)). Chassin et al. (27), in particular, found that quitters reported less stress (but not less negative affect) than when they were smoking 6 years earlier, and those findings are consistent with the current study’s findings.
It is important to note that in the current study stress was assessed by asking about the occurrence of stressful events (“Have any stressful events happened since your evening report last night?”), rather than feelings of stress. It is possible, though, that participants responded based on their perceived stress level rather than based on the occurrence of stressful events, in which case the quitters were really reporting at 1 year post-quit that they felt that life seemed less stressful. Indeed, it is possible that smoking is motivated, in part, by a vicious cycle; smokers believe that smoking alleviates their stress, but smoking actually changes their evaluations of events so the events seem more stressful. It is also possible that the quitters actually did encounter fewer stressors because smoking itself had constituted a major stressor for them (e.g., due to withdrawal symptoms, the expense, or conflicts with family members over smoking), or because quitting smoking entailed major lifestyle changes that reduced their exposure to stressors.
Although we predicted that quitters’ anxiety and sadness would decrease from pre-quit to 1 year post-quit relative to continuing smokers, omnibus analyses found that neither quitters nor continuing smokers showed a substantial change in anxiety or sadness over this period. It is possible that quitters’ anxiety and sadness did not improve with abstinence because these symptoms reflect person factors that are comorbid with smoking (e.g., neuroticism-related negative affect). This account is consistent with the observation that withdrawal symptoms involving negative affect such as anxiety and sadness tend to be less affected by nicotine abstinence than is craving (e.g., (17)). It may also be that anxiety and sadness are more tightly homeostatically regulated than is craving (28). Thus, perhaps anxiety and sadness did not improve with time because they are less affected by abstinence in the first place.
The omnibus analyses of changes from pre-quit to 1 year also revealed that quitters experienced a steep decline in craving while continuing smokers’ craving levels only decreased slightly. The omnibus analyses further revealed that quitters experienced declines in restlessness and anger/irritability from pre-quit to 1 year while continuing smokers’ levels either remained stable or increased. (The anger/irritability interaction was not significant, however, with the correction for multiple comparisons.) In general, the data show that on-going smoking is not effective at sustained craving control (or at controlling certain other withdrawal symptoms).
Parrott (15) has theorized that regular smokers experience acute nicotine withdrawal and associated mood fluctuations during the time between cigarettes, and laboratory research has shown that withdrawal symptoms can begin as soon as 30 minutes after a cigarette (14). Parrott (29) has also shown that smokers typically experience fluctuating stress with greater stress prior to smoking (probably due to nicotine withdrawal) followed by a brief decrease in stress after smoking. The current study’s main findings can be explained by Parrott’s model (30) that smokers’ acute nicotine withdrawal between cigarettes causes them to experience ongoing distress and withdrawal symptoms (such as craving, restlessness, and anger/irritability), whereas if they quit smoking (for long enough to make it past the immediate effects of withdrawal), they experience declines in these symptoms.
Counselors can use the current study’s findings to underscore the message that only successful cessation can produce long-term elimination of craving (just 0.5% of quitters had mean scores indicating they had experienced significant craving at 1-year post-quit; also cf. (11, 31)). Counselors can also use these findings to educate smokers that—contrary to what many smokers may fear—evidence shows that from pre-quit to 1 year post-quit, successful quitters report experiencing (a) large reductions in cravings for cigarettes, (b) less restlessness, and (c) fewer stressful events than when they smoked. In other words, not only do their withdrawal symptoms get better after quitting and staying quit, by 1 year post-quit their withdrawal symptoms tend to be even better than when they were smoking.
Continuing smokers (regardless of their treatment condition) reported approximately 85% of the time at both prequit and 1 year post-quit that smoking was permitted at their location. (These data were collected before Wisconsin instituted a state-wide indoor smoking ban.) Quitters, by contrast, reported decreased time spent in places where smoking was permitted. (Counselors encouraged all participants in the current study to limit their exposure to environments permitting smoking.) Quitters randomized to placebo showed the largest decrease (from 84 to 52% of the time; a 32 percentage point drop) while quitters randomized to the nicotine replacement therapy-only and bupropion conditions showed 14 and 7 percentage point drops respectively. This pattern may have occurred because only those participants with few contextual prompts to smoke during the follow-up period were able to quit with placebo, while active medication enabled participants to better withstand such contextual challenges while remaining abstinent.
The current study also examined quitters’ changes from the early days post-quit (i.e., during acute withdrawal) to 1 year post-quit and found that quitters showed decreases on almost all the withdrawal symptoms assessed (i.e., they showed decreases in craving, restlessness, anger, anxiety, difficulty concentrating, and hunger), and they reported fewer stressful events. That these withdrawal symptoms decreased for quitters from post-quit to 1 year is not surprising given that withdrawal symptoms typically peak in the first week post-quit and last, on average, 2 to 4 weeks (7). Nevertheless, smokers may find it reassuring to learn that acute withdrawal symptoms, including craving, largely dissipate for those who remain abstinent through 1 year post-quit and may even fall below the levels they experienced while actively smoking.
Of course, returning to smoking after a quit attempt also reduces withdrawal symptoms (32) from levels experienced during the peak of withdrawal; continuing smokers showed decreases from post-quit to 1 year after the quit day for almost all the withdrawal symptoms assessed. However these effects were consistently smaller than the decreases reported by successful quitters (although there was only a significant interaction between time and abstinence status for craving and not for any of the other withdrawal symptoms assessed).
In terms of alcohol intake, both quitters and continuing smokers reported a small increase in the number of alcoholic drinks they consumed on a given day from post-quit to 1 year after the quit day, such that by 1 year they had returned to drinking at approximately their pre-quit levels. This temporary post-quit decrease in both quitters’ and continuing smokers’ alcohol consumption may reflect the counseling they received encouraging them to reduce their alcohol intake during the quit attempt.
Continuing smokers reduced their cigarette consumption considerably from a mean of 22 cigarettes per day pre-quit to 13 at 1 year. Thus—assuming, as is likely, that continuing smokers were not using cessation medication at 1 year—treatment (counseling plus, for most participants, medication) appears to have had the unanticipated long-term effect of causing continuing smokers to smoke fewer cigarettes a day. Moreover, there was a significant interaction effect in the ecological momentary assessment data suggesting that continuing smokers who had received cessation medication may have decreased the number of cigarettes they smoked more than continuing smokers who had received placebo treatment a year earlier. These data encourage further investigation of the long-term effects of cessation treatment on smoking heaviness among those who fail to quit successfully. It would also be important to determine the extent to which these effects occur because of cessation treatment versus making a quit attempt per se. Cutting back does not confer the dramatic health benefits and mortality reduction that quitting does (33–35), but sustained smoking reduction may have public health consequences because of reduced toxicant exposure (although the effect on disease risk is unclear) and because reduced smoking increases the likelihood a smoker will quit permanently in the future (36, 37). It is not clear what mechanisms account for the effect of treatment on smoking heaviness in continuing smokers, but perhaps cessation medication helps smokers eliminate smoking in key situations, resulting in sustained reduced smoking after relapse.
Four study limitations should be noted. First, this study’s sample was much smaller than the parent trial’s sample, and this reduced sample size decreases power to detect effects. Furthermore, dropout may have been systematic, meaning that particular types of smokers may have been more likely not to complete the ecological momentary assessment data and not to attend the 1-year follow-up, and such differential dropout limits the results’ generalizability. A second limitation is that smokers were excluded from the trial for some forms of psychiatric comorbidity (very heavy drinking, psychosis) and this also limits the results’ generalizability. However, the sample nevertheless had high levels of nonexcluded psychopathology as measured by the World Mental Health Survey Initiative version of the Composite International Diagnostic Interview (38, 39). Of participants in the current sample who completed the interview at baseline, 18.3% had a lifetime history of major depressive disorder, 38.1% had a lifetime history of anxiety (panic attacks, social anxiety, or generalized anxiety disorder), and 50.9% had a lifetime history of alcohol abuse. A third limitation is that the ecological momentary assessment of craving and other withdrawal symptoms may have caused some reactivity—especially among the quitters who had been abstinent for a year—but if this is the case, it would make the findings a more conservative test of the relationship between long-term abstinence and withdrawal. A fourth limitation is that participants self-selected into groups (continuing smokers vs. successful quitters), and therefore we cannot necessarily use the data from successful quitters to prognosticate what life would have been like for the continuing smokers, had they succeeded in quitting.
To conclude, in this study, when quitters were compared with continuing smokers, the quitters showed greater positive changes from pre-quit to 1 year in terms of decreased craving and restlessness. In follow-up analyses, quitters showed the following improvements from pre-quit to 1 year: sizeable reductions in craving, less restlessness and anger/irritability, and fewer days with stressful events. By contrast, continuing smokers showed no noteworthy improvements at 1 year except that they smoked fewer cigarettes a day.
These findings expand the arsenal of encouraging messages that clinicians can share regarding patients who successfully quit smoking for a year. The withdrawal symptoms these patients experience immediately after the quit attempt abate. Furthermore, by 1 year, compared to how they were feeling while still smoking: (a) their cravings for cigarettes have essentially disappeared, (b) they feel less restless, and (c) their lives are less stressful. These messages may help alleviate the concerns that prevent many smokers from trying to quit and may help smokers to cope with the immediate effects of withdrawal during a quit attempt.
Acknowledgments
We would like to thank the staff at the Center for Tobacco Research and Intervention in the UW School of Medicine and Public Health for their help with this research. We are particularly grateful to Stevens S. Smith for statistical advice and to Linda Kurowski and Wendy Theobald for their technical assistance.
Over the last 3 years, Dr. Michael C. Fiore has served as an investigator on a research study at the University of Wisconsin that was funded in part by Nabi Pharmacueticals. From 1997 to 2010, Dr. Fiore held a University of Wisconsin named Chair for the Study of Tobacco Dependence, made possible by a gift to University of Wisconsin from GlaxoWellcome.
This research was conducted at the University of Wisconsin-Madison and was supported by grant P50 DA019706 from the National Institutes of Health/National Institute on Drug Abuse (NIH/NIDA); by grant M01 RR03186 from the General Clinical Research Centers Program of the National Center for Research Resources, NIH; by grants 1K05CA139871 and K08DA021311 from NIH; and by the Wisconsin Partnership Program. Medication was provided to participants at no cost under a research agreement with GlaxoSmithKline; no part of this manuscript was written or edited by anyone employed by GlaxoSmithKline. The authors are solely responsible for the analyses, content, and writing of this article. The authors have full control of all primary data, and they agree to allow the journal to review the data if requested.
We declare that this research complies with the current laws of the United States of America. All participants gave written informed consent prior to entering the study, and the study was approved by the University of Wisconsin Health Sciences Institutional Review Board. Clinical trial registration: NCT00332644 (www.ClinicalTrails.gov).
Footnotes
Conflict of Interest Statement
Tanya R. Schlam, Megan E. Piper, Jessica W. Cook, and Timothy B. Baker have no potential conflicts of interest to disclose.
References
- 1.Asher MK, Martin RA, Rohsenow DJ, MacKinnon SV, Traficante R, Monti PM. Perceived barriers to quitting smoking among alcohol dependent patients in treatment. J Subst Abuse Treat. 2003;24(2):169–174. doi: 10.1016/s0740-5472(02)00354-9. [DOI] [PubMed] [Google Scholar]
- 2.Hendricks PS, Wood SB, Baker MR, Delucchi KL, Hall SM. The Smoking Abstinence Questionnaire: Measurement of smokers' abstinence-related expectancies. Addiction. 2011;106(4):716–728. doi: 10.1111/j.1360-0443.2010.03338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKee SA, O'Malley SS, Salovey P, Krishnan-Sarin S, Mazure CM. Perceived risks and benefits of smoking cessation: Gender-specific predictors of motivation and treatment outcome. Addict Behav. 2005;30(3):423–435. doi: 10.1016/j.addbeh.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Orleans CT, Rimer BK, Cristinzio S, Keintz MK, Fleisher L. A national survey of older smokers: Treatment needs of a growing population. Health Psychol. 1991;10(5):343–351. doi: 10.1037//0278-6133.10.5.343. [DOI] [PubMed] [Google Scholar]
- 5.Solway ES. The lived experiences of tobacco use, dependence, and cessation: Insights and perspectives of people with mental illness. Health Soc Work. 2011;36(1):19–32. doi: 10.1093/hsw/36.1.19. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger AH, Mazure CM, McKee SA. Perceived risks and benefits of quitting smoking in non-treatment smokers. Addiction Res Theory. 2010;18(4):456–463. doi: 10.3109/16066350903145072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine Tob Res. 2007;9(3):315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- 8.Piasecki TM, Fiore MC, Baker TB. Profiles in discouragement: Two studies of variability in the time course of smoking withdrawal symptoms. J Abnorm Psychol. 1998;107(2):238–251. doi: 10.1037//0021-843x.107.2.238. [DOI] [PubMed] [Google Scholar]
- 9.Piasecki TM, Niaura R, Shadel WG, Abrams D, Goldstein M, Fiore MC, et al. Smoking withdrawal dynamics in unaided quitters. J Abnorm Psychol. 2000;109(1):74–86. doi: 10.1037//0021-843x.109.1.74. [DOI] [PubMed] [Google Scholar]
- 10.Hughes JR. Tobacco withdrawal in self-quitters. J Consult Clin Psychol. 1992;60(5):689–697. doi: 10.1037//0022-006x.60.5.689. [DOI] [PubMed] [Google Scholar]
- 11.Gritz ER, Carr CR, Marcus AC. The tobacco withdrawal syndrome in unaided quitters. Br J Addict. 1991;86(1):57–69. doi: 10.1111/j.1360-0443.1991.tb02629.x. [DOI] [PubMed] [Google Scholar]
- 12.Shiffman S. How many cigarettes did you smoke? Assessing cigarette consumption by global report, Time-Line Follow-Back, and ecological momentary assessment. Health Psychol. 2009;28(5):519–526. doi: 10.1037/a0015197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu Rev Clin Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- 14.Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology (Berl) 2006;187(3):385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- 15.Parrott AC. Does cigarette smoking cause stress? Am Psychol. 1999;54(10):817–820. doi: 10.1037//0003-066x.54.10.817. [DOI] [PubMed] [Google Scholar]
- 16.Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, et al. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry. 2009;66(11):1253–1262. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piper ME, Schlam TR, Cook JW, Sheffer MA, Smith SS, Loh WY, et al. Tobacco withdrawal components and their relations with cessation success. Psychopharmacology (Berl) 2011;216(4):569–578. doi: 10.1007/s00213-011-2250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarthy DE, Piasecki TM, Fiore MC, Baker TB. Life before and after quitting smoking: An electronic diary study. J Abnorm Psychol. 2006;115(3):454–466. doi: 10.1037/0021-843X.115.3.454. [DOI] [PubMed] [Google Scholar]
- 19.Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, et al. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. J Abnorm Psychol. 2008;117(1):94–105. doi: 10.1037/0021-843X.117.1.94. [DOI] [PubMed] [Google Scholar]
- 20.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 21.Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7(4):354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- 22.Shiffman S, Khayrallah M, Nowak R. Efficacy of the nicotine patch for relief of craving and withdrawal 7–10 weeks after cessation. Nicotine Tob Res. 2000;2(4):371–378. doi: 10.1080/713688158. [DOI] [PubMed] [Google Scholar]
- 23.Olejnik S, Algina J. Generalized Eta and Omega squared statistics: Measures of effect size for some common research designs. Psychol Methods. 2003;8(4):434–447. doi: 10.1037/1082-989X.8.4.434. [DOI] [PubMed] [Google Scholar]
- 24.Bakeman R. Recommended effect size statistics for repeated measures designs. Behav Res Methods. 2005;37(3):379–384. doi: 10.3758/bf03192707. [DOI] [PubMed] [Google Scholar]
- 25.Cohen J. A power primer. Psychol Bull. 1992 Jul;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 26.Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd ed. Mahwah, NJ: Lawrence Erlbaum; 2003. pp. 354–357. [Google Scholar]
- 27.Chassin L, Presson CC, Sherman SJ, Kim K. Long-term psychological sequelae of smoking cessation and relapse. Health Psychol. 2002;21(5):438–443. doi: 10.1037//0278-6133.21.5.438. [DOI] [PubMed] [Google Scholar]
- 28.Solomon RL, Corbit JD. An opponent-process theory of motivation. II. Cigarette addiction. J Abnorm Psychol. 1973;81(2):158–171. doi: 10.1037/h0034534. [DOI] [PubMed] [Google Scholar]
- 29.Parrott AC. Acute pharmacodynamic tolerance to the subjective effects of cigarette smoking. Psychopharmacology (Berl) 1994;116(1):93–97. doi: 10.1007/BF02244877. [DOI] [PubMed] [Google Scholar]
- 30.Parrott AC. Nicotine psychobiology: How chronic-dose prospective studies can illuminate some of the theoretical issues from acute-dose research. Psychopharmacology (Berl) 2006;184(3–4):567–576. doi: 10.1007/s00213-005-0294-y. [DOI] [PubMed] [Google Scholar]
- 31.Hughes JR. Craving among long-abstinent smokers: An Internet survey. Nicotine Tob Res. 2010;12(4):459–462. doi: 10.1093/ntr/ntq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB. Smoking withdrawal dynamics: II. Improved tests of withdrawal-relapse relations. J Abnorm Psychol. 2003;112(1):14–27. [PubMed] [Google Scholar]
- 33.Fiel SB. Chronic obstructive pulmonary disease. Mortality and mortality reduction. Drugs. 1996;52(Suppl 2):55–61. doi: 10.2165/00003495-199600522-00012. [DOI] [PubMed] [Google Scholar]
- 34.Godtfredsen NS, Holst C, Prescott E, Vestbo J, Osler M. Smoking reduction, smoking cessation, and mortality: A 16-year follow-up of 19,732 men and women from The Copenhagen Centre for Prospective Population Studies. Am J Epidemiol. 2002;156(11):994–1001. doi: 10.1093/aje/kwf150. 2002. [DOI] [PubMed] [Google Scholar]
- 35.Godtfredsen NS, Vestbo J, Osler M, Prescott E. Risk of hospital admission for COPD following smoking cessation and reduction: A Danish population study. Thorax. 2002;57(11):967–972. doi: 10.1136/thorax.57.11.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes JR. Reduced smoking: An introduction and review of the evidence. Addiction. 2000;95(Suppl 1):S3–S7. doi: 10.1080/09652140032008. [DOI] [PubMed] [Google Scholar]
- 37.Hughes JR, Carpenter MJ. Does smoking reduction increase future cessation and decrease disease risk? A qualitative review. Nicotine Tob Res. 2006;8(6):739–749. doi: 10.1080/14622200600789726. [DOI] [PubMed] [Google Scholar]
- 38.Kessler RC, Ustun TB. The World Mental Health (WMH) Survey Iniative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int J Methods Psychiatr Res. 2004;13:93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization. Geneva, Switzerland: World Health Organization; 1990. Composite International Diagnostic Interview. [Google Scholar]