Abstract
The objective of the current study was to evaluate the effects of cage density, sanitation frequency, and bedding type on animal growth and welfare. At weaning, Sprague–Dawley rats and C57BL/6 mice were allocated to treatment groups according to sex, bedding type (shredded aspen, cellulose, or a 50:50 mixture), and cage density and sanitation frequency (inhouse cage density standards and sanitation procedures measured against Guide recommendations) for an 8-wk period. Body weight, feed disappearance, cage ammonia, ATP concentrations, behavior, morbidity, and mortality were assessed weekly; fecal corticosterone, microbiology, and lung histopathology (rats only) were evaluated at the culmination of the trial. In both rats and mice, parameters indicative of animal health and welfare were not significantly affected by cage density and sanitation frequency or bedding type. Occasional effects of feed disappearance and cage ammonia concentrations due to density and sanitation guidelines were noted in rat cages, and bedding type affected cage ammonia and ATP concentrations. Periodic spikes of cage ammonia and ATP concentrations were recorded in mouse cages maintained according to inhouse compared with Guide standards and in cages containing aspen compared with cellulose or aspen–cellulose mixed bedding. Ongoing studies and historical data support the finding that deviations or exceptions from the cage density and sanitation frequency standards set forth in the Guide do not negatively affect animal health, welfare, or production parameters at our institution. These parameters appear to be credible measures of animal health and wellbeing and may be useful for evaluating performance standards for animal husbandry.
The Guide for the Care and Use of Laboratory Animals (Guide) recommends cage density and sanitation guidelines for laboratory mice and rats. These guidelines include cage density recommendations appropriate for the size of the animal, to allow for normal postural adjustments, and floor space that is unsoiled by animal waste as a location in which the animal may rest.11 Guide sanitation guidelines include sanitation once weekly for solid-bottom cages through disinfection with chemicals, hot water, or both.11 Deviations from these recommendations should be approved by the IACUC of the institution.
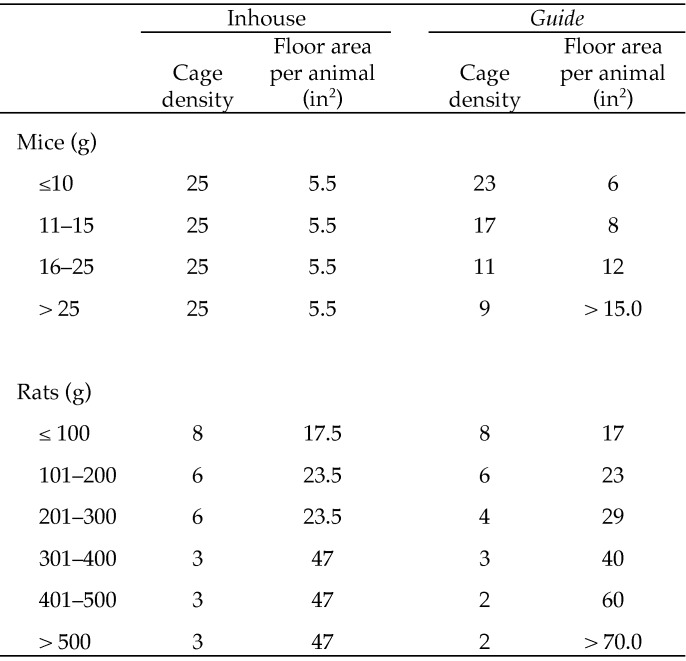
Our institution, through internal investigation26 and review of published literature,1,3,6,8,9,11,13,17,19,20,22,23 has developed density and sanitation guidelines that differ from those recommended by the Guide but still provide an environment in which animals can thrive; these amendments are IACUC-reviewed and ‑approved. Density guidelines according to both Guide recommendations and inhouse standards are provided (Figure 1). Inhouse standards have been reported not to cause negative effects on animal wellbeing.26 Approved inhouse standards include removing soiled bedding once weekly, with full cage changes every 4 wk. More frequent sanitation is performed as needed.
Figure 1.
Cage density guidelines. The number of animals per cage was determined based on cage size (9 in. × 15.75 in. × 8 in.) and space requirements per animal.
Bedding type and subsequently environmental microflora affect ammonia levels within cages.1,9,14,15,21,24 Bedding type selection also may correlate to increased husbandry costs due to increased number of cage changes needed and to potential negative effects on animal livelihood, including decrease in weight gain.3,13
We hypothesized that less frequent cage sanitation and increased cage density, in accordance with inhouse standards, would not negatively affect animal welfare and cage environment parameters. Bedding type may alter cage environment parameters and was included as a separate variable to discern the most suitable bedding type for inhouse use.
Materials and Methods
Animals.
Male and female Sprague–Dawley rats (Hsd:SpragueDawley SD; age, 3 wk; 192 male, 192 female) and male and female C57BL/6NHsd mice (age, 3 wk; 432 male, 432 female) were produced at a commercial vendor (Harlan Laboratories, Indianapolis, IN). All animals were maintained in a maximum-security conventional production facility. Rooms were maintained on a 12:12-h light:dark cycle; average room temperature during the trial period was 22.8 ± 0.7 °C, and room humidity was 41.6% ± 6.6%. According to the health surveillance program in use, mice and rats were known to be free of common adventitious agents as published on the vendor's monthly health reports (available at www.harlan.com). Animals were maintained in open, wire-top, polypropylene shoebox-type cages (9 in. width × 15.75 in. length × 8 in. height; FAS Plastics, Hanover, IN) at various cage densities (Figure 1). Cage density differs between Guide and inhouse standards during weeks 6 through 8 for rats and weeks 1 through 8 for mice. Over the 8-wk trial, 99 cages were used for rats, with 48 cages used for mice. Animals received ad libitum cage-top access to autoclaved, pelleted diet containing 18% protein and 6% fat (diet 2018S, Harlan Teklad, Madison, WI) and chlorinated (8 to 10 ppm), acidified (pH 5.8 to 6.0), and filtered (0.2-µm) water via an automatic watering system.
The protocol was approved by the Harlan Laboratories’ IACUC and was performed in accordance with an AAALAC-accredited program.
Sanitation frequency.
Cages containing mice and rats were sanitized according to recommendations from the Guide or inhouse standards. Guide recommendations include a complete cage sanitization weekly, whereas inhouse standards include a complete removal of bedding weekly with a complete cage sanitization every 4 wk.
Cage density.
Animals were maintained either in accordance with Guide recommendations or inhouse standards, which are based on historical internal review and have been evaluated and approved by the IACUC (Figure 1). Cage density is based on individual animal weight and is evaluated weekly, with additional cages used as necessary for both inhouse and Guide treatments.
Bedding types.
Two different bedding types and a mixture containing both were used for this study. Autoclaved shredded aspen (Harlan Teklad 7093), cellulose (Harlan Teklad 7070), or a 50:50 blend of shredded aspen and cellulose were used.
Experimental design.
Parameters were evaluated during an 8-wk trial. At 3 wk of age, mice (initial body weight, 9.02 ± 1.78 g) and rats (initial body weight, 43.28 ± 9.10 g) were randomly selected and allocated into 1 of 12 treatment groups. Animals were housed by sex. At the onset of the study, 4 cages were used per rat treatment group, with each cage density and sanitation frequency treatment allotted to each bedding type. Three cages were used per mouse treatment group at the onset of the study, with each cage density and sanitation frequency treatment allotted to each bedding type (Figure 1). Approximately 1 in. (141.75 in3) of bedding was added to each cage, according to treatment.
Animals were monitored daily for any signs of morbidity or mortality; ill or deceased animals were removed and clinical signs noted. Behavior was monitored daily, with behavior also monitored weekly during bedding or cage change for approximately 5 min. Animals were monitored for normal behaviors (normal grooming, foraging or feeding, drinking, nesting, and sleeping), nonsocial behaviors (climbing, digging, gnawing, investigative, and scent-marking), social behaviors (allogrooming, huddling, parental care, and aggressive or defensive behavior), and abnormal behaviors (including but not limited to injurious, overgrooming, excessive fearfulness, and persistent attempts to escape).8,11,27
Body weight was assessed weekly by individually weighing each animal and recording the body weight. Total cage animal weights were determined by summation of individual animal weights. When necessary, animals were regrouped to accommodate weight gain and to ensure adherence to cage density guidelines for both Guide and inhouse standards. At the beginning of each week, feed was weighed and added to the cage top. Throughout the week, if necessary, additional feed was weighed and added to the top of the cage to ensure ad libitum food availability. At the end of each week, any feed remaining on the cage top was weighed. Individual animal feed disappearance was calculated as the difference in the weight of the feed added and the feed remaining, divided by the number of animals per cage.
At the end of each week, on days 7, 14, 21, 28, 35, 42, 49, and 56, prior to bedding or cage change, ammonia levels were monitored at cage level, in the center of each cage, approximately 1 in. above the level of bedding, by using an aspirating detector tube pump (AP-20S, Sensidyne, Clearwater, FL). Any sample reading greater than 30 ppm was repeated, with the range in readings calibrated between 5 and 30 ppm. ATP concentrations (SystemSURE Plus, Hygiena, Camarillo, CA) were measured by swabbing the inner longest cage wall, directly above the bedding at the same time points as for ammonia levels.
On day 56 (end of week 8), a single fecal pellet was collected from each cage; pellets were pooled according to treatment for determination of corticosterone concentration. Samples were placed in a −20 °C freezer and submitted to PreClinOmics (Indianapolis, IN) for analysis. Briefly, approximately 100 mg of fecal sample was mixed with 1 mL 80% methanol, vortexed for 2 min, and centrifuged at 2500 × g for 15 min. The supernatant was removed, and 0.1 mL of the supernatant was diluted into 0.9 mL of 80% methanol. Both samples were stored at −20 °C until analysis with the Corticosterone EIA kit (Cayman Chemical Company, Ann Arbor, MI).
On day 56 (end of week 8), one animal per cage was submitted to the Research Animal Diagnostic Laboratory (Columbia, MO) for necropsy and nasopharyngeal (Corynebacterium kutscheri, Pasteurella pneumotropica, Staphylococcus aureus, Streptococcus pneumoniae, and Streptococcus spp. Group B β) and cecal (Citrobacter rodentium, Klebsiella oxytoca, K. pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, and Salmonella spp.) microbiologic culture as well as microscopic evaluation of lungs (rats only) for presence of lung lesions.
Statistical analysis.
Data were tested for normality by using the Univariate procedures of SAS (version 9.1, SAS Institute, Cary, NC). Body weight, feed disappearance, cage ammonia concentration, cage ATP concentration, incidence of mortality and morbidity, and incidence of microbiologic organism presence and lung histopathology underwent ANOVA (general linear model procedure). The cage served as the experimental unit and measures were averaged for body weight and feed disappearance within the cage. Parameters were analyzed according to cage sanitation frequency and density standards (Guide or inhouse) and bedding type in 2 separate analyses. The model included the effect of bedding type or sanitation frequency and density and all possible interactions. Means were separated by using least square differences when the P value was less than 0.05. Differences were considered significant at P values of 0.05 or less.
Results
Sprague–Dawley Rats.
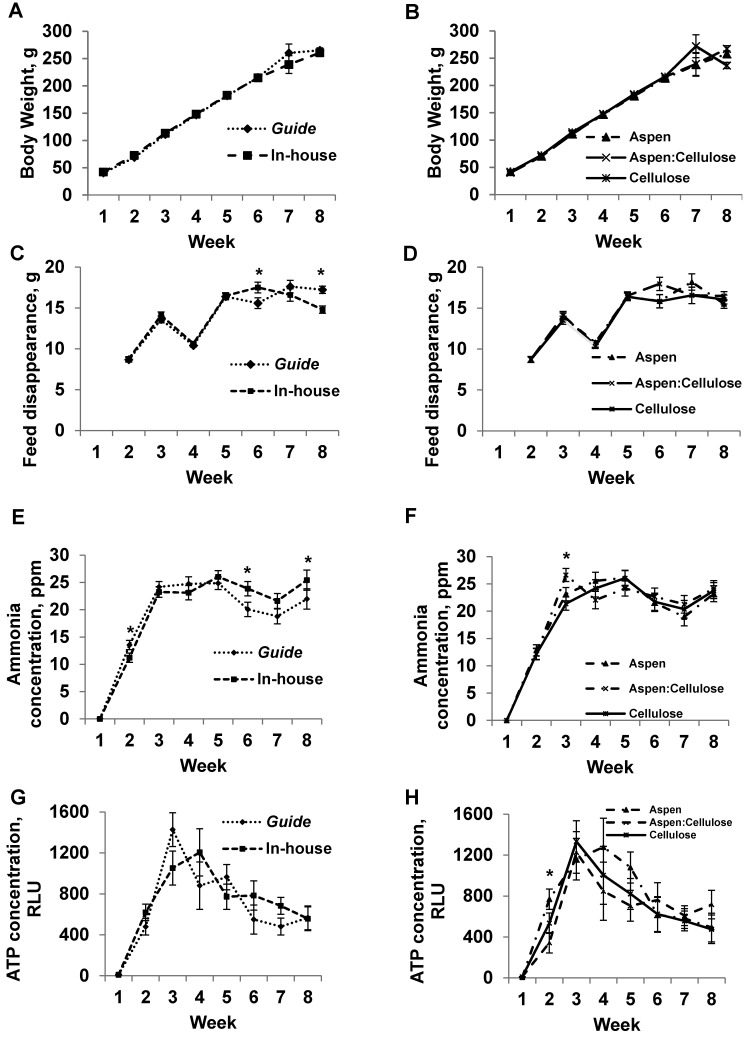
All rats, regardless of treatment, gained approximately 200 g over the 8-wk trial period (Figure 2 A and B). By week 6, rats maintained according to inhouse standards consumed more (P = 0.04) food than did rats maintained by Guide standards during week 6, whereas by week 8, rats maintained by Guide standards consumed more (P = 0.001) food than did rats maintained by inhouse standards during week 8 (Figure 2 C and D). Bedding type had no effect on feed disappearance. Cage ammonia concentration (Figure 2 E and F) was higher (P = 0.05) during week 2 in Guide-maintained rats compared with those maintained according to inhouse standards and was higher by weeks 6 (P = 0.04) and 8 (P = 0.04) in rats maintained according to inhouse standards compared with Guide standards during week 2 and 6, respectively. Cages containing rats maintained on aspen:cellulose bedding had a higher ammonia concentration during week 3 (P = 0.01) compared with that of those containing aspen or cellulose bedding alone during week 3. Cage density and sanitation guidelines had no effect on cage ATP concentration (Figure 2 G and H); however, during week 2, cages containing rats maintained on aspen:cellulose had lower cage ATP concentrations than did cages with rats maintained on aspen or cellulose alone (P = 0.02).
Figure 2.
(A, B) body weight (g), (C, D) feed disappearance (g), (E, F) cage ammonia concentration (ppm), and (G, H) cage ATP concentration (relative light units, RLU) of Hsd:SpragueDawley® SD® rats, according to either cage density and sanitation frequency recommendations (Guide versus inhouse [panels A, C, E, and G]) or bedding type (aspen, cellulose, 50:50 aspen:cellulose [panels B, D, F, and H]). For week 1 (trial initiation), body weight, cage ammonia and ATP concentrations were measured prior to placing animals into cages. For weeks 2 through 8, body weight, feed disappearance, cage ammonia concentration, and ATP concentration values represent data measured at the end of the week. *, Significant (P < 0.05) difference between treatments.
Behavior.
In all cages, rats exhibited normal grooming, feeding, drinking, nesting, sleeping, climbing, digging, and investigative behavior. Huddling was reported also. During the 8-wk trial, no morbidity or mortality was reported.
Pathology.
The lungs from a single rat per cage (Guide, 55; inhouse, 44; aspen, 33; cellulose, 34; aspen:cellulose, 32) were assessed grossly and microscopically. The presence of peribronchiolar lymphoid hyperplasia was reported in 99% of rats submitted. Perivascular eosinophilic infiltrates (Guide, cellulose bedding), osseous metaplasia (inhouse, cellulose bedding), multifocal foreign body bronchopneumonia (Guide, aspen:cellulose bedding), and focal interstitial pneumonia at the lung tip (Guide, cellulose bedding) were noted in 4 rats (incidence, 1.0%) and were not treatment related (P > 0.05).
Microbiology.
P. mirabilis was isolated from the cecum of 58% of rats submitted overall, and incidence did not differ among treatments (Guide, 30%; inhouse, 28%; aspen, 21%; cellulose, 18%; aspen:cellulose, 17%; P > 0.05). In addition, Proteus overgrowth was present in 51% of all nasopharyngeal cultures, but incidence was not treatment-related (Guide, 25%; inhouse, 26%; aspen, 15%; cellulose, 20%; aspen:cellulose, 16%; P > 0.05).
Clinical chemistry markers.
Fecal corticosterone concentration did not differ due to treatment (P > 0.05; Table 1).
Table 1.
Fecal corticosterone concentrations in Sprague–Dawley rats
| Mean fecal corticosterone (ng/g feces) | SE | P | |
| Cage density and sanitation frequency guidelines | |||
| Inhouse | 28.23 | 5.15a | 0.918a |
| Guide | 27.09 | ||
| Bedding type | |||
| Aspen | 29.71 | 5.15b | 0.932b |
| Cellulose | 28.56 | ||
| 50% aspen : 50% cellulose | 24.71 | ||
Fecal corticosterone was measured through collection of a single fecal pellet per cage on day 56 of the study.
SE and P value correspond to statistical analysis of inhouse compared with Guide treatment.
SE and P value correspond to statistical analysis of 3 bedding treatments.
C57BL/6NHsd.
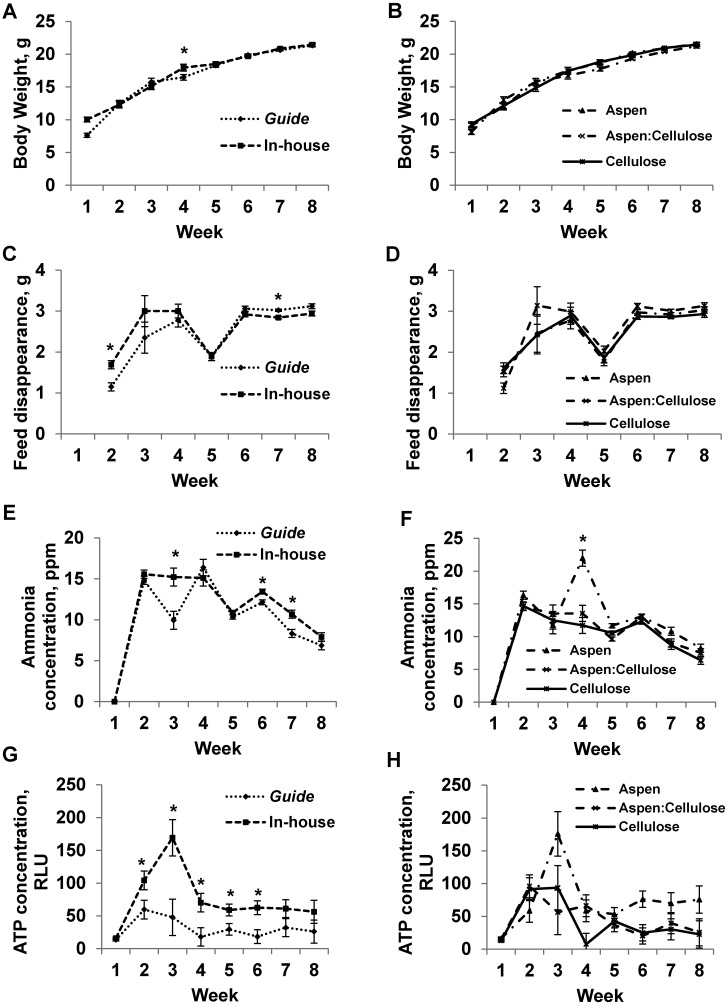
Initial body weight (week 1) was significantly (P < 0.05) different among treatments; therefore, week 1 body weight was included as a covariate in the analyses of data all subsequent weeks, given that this difference is not due to treatment but to natural interanimal variation at the time of allocation (Figure 3 A and B). During week 4, mice maintained according to inhouse standards were significantly (P = 0.02) heavier than were mice maintained by using Guide standards. By week 2, mice maintained by using inhouse protocols consumed more (P = 0.001) feed than did mice according to Guide standards (Figure 3 C and D). By week 7, Guide-maintained mice consumed more (P = 0.001) feed than did mice maintained according to inhouse standards. Cage ammonia concentration was higher during weeks 3 (P = 0.002), 6 (P = 0.003), and 7 (P = 0.001) in mice maintained according to inhouse standards compared with Guide recommendations (Figure 3 E and F). Cage ammonia levels peaked during week 4 in cages containing aspen bedding only (P < 0.001), and ammonia levels in cages containing aspen bedding were numerically higher during weeks 2, 4, 5, 7, and 8 than were those for mice maintained on aspen:cellulose or cellulose bedding. Compared with cages containing mice maintained by Guide standards, those containing mice maintained according to inhouse standards had higher (P < 0.05) ATP concentrations during weeks 2, 3, 5, and 6 (Figure 3 G and H). Interaction between cage density and sanitation frequency occurred during week 6, when cages containing mice maintained on aspen bedding according to inhouse standards yielded higher ATP concentrations (134.47 relative light units) than did those maintained by Guide standards on aspen bedding (17.93 relative light units; P < 0.001)
Figure 3.
(A, B) body weight (g), (C, D) feed disappearance (g), (E, F) cage ammonia concentration (ppm), and (G, H) cage ATP concentration (relative light units, RLU) of C57BL/6NHsd mice, according to either cage density and sanitation frequency recommendations (Guide versus inhouse [panels A, C, E, and G]) or bedding type (aspen, cellulose, 50:50 aspen:cellulose [panels B, D, F, and H]). For week 1 (trial initiation), body weight, cage ammonia and ATP concentrations were measured prior to placing animals into cages. For weeks 2 through 8, body weight, feed disappearance, cage ammonia concentration, and ATP concentration values represent data measured at the end of the week. *, Significant (P < 0.05) difference between treatments.
Behavior.
Mice in 78% of cages exhibited normal grooming, feeding, drinking, nesting, sleeping, climbing, digging, and investigative behavior. In addition, huddling occurred in all cages during the 8-wk period. Aggressive–defensive behavior occurred during week 4 in a single cage, which contained female mice maintained on aspen bedding according to inhouse standards. Overgrooming and allogrooming occurred in 11 cages during weeks 5 through 8. Cage density and sanitation frequency did not significantly affect mouse behavior (P > 0.05); however, during weeks 5 and 6, mice maintained on aspen:cellulose bedding displayed overgrooming or allogrooming behaviors (28% of cages during week 5; 22% during week 6) compared with 0% occurrence during weeks 5 and 6 in cages housing mice on either aspen (P < 0.001) or cellulose (P = 0.002). During the 8-wk trial, the overall mortality rate in mice was 21% (Guide, 15%; inhouse, 6%; P < 0.001). Overall mortality in mice maintained on aspen bedding was 3%, on cellulose bedding was 4%, and on aspen:cellulose bedding was 14% (P < 0.001). Eight mice were removed from the study due to malocclusion (inhouse, 5; Guide, 3; aspen, 2; cellulose, 4; aspen:cellulose, 2; P > 0.05), with 2 additional mice removed due to vaginal (inhouse; aspen:cellulose) or head (inhouse; cellulose) swelling, which did not appear to be treatment related.
Microbiology.
S. aureus was isolated from 46% of mice submitted overall for testing (Guide, 31%; inhouse, 15%; aspen, 17%; cellulose, 19%; aspen:cellulose, 10%), and Proteus overgrowth was reported in 2% of mice submitted for testing (Guide, 0%; inhouse, 2%; aspen, 2%; cellulose, 0%; aspen:cellulose, 0%). However, there were no statistical differences among treatments (P > 0.05).
Clinical chemical markers.
Fecal corticosterone concentration did not differ due to treatment (P > 0.05; Table 2).
Table 2.
Fecal corticosterone concentration in C57BL/6NHsd mice
| Mean fecal corticosterone (ng/g feces) | SE | P | |
| Cage density and sanitation frequency guidelines | |||
| Inhouse | 0.330 | 0.04a | 0.421a |
| Guide | 0.396 | ||
| Bedding type | |||
| Aspen | 0.397 | 0.04b | 0.752b |
| Cellulose | 0.321 | ||
| 50% aspen : 50% cellulose | 0.372 | ||
Fecal corticosterone was measured through collection of a single fecal pellet per cage on day 56 of the study.
SE and P value correspond to statistical analysis of inhouse and Guide treatments.
SE and P value correspond to statistical analysis of bedding treatments.
Discussion
As a commercial vendor, our institution (Harlan Laboratories) produces animals for the research community and routinely evaluates appropriate housing and husbandry conditions to effectively and economically maintain laboratory rodents with regard to animal welfare. The Guide recommends housing and husbandry parameters for use when maintaining group-housed laboratory animals, and through evaluation of these recommendations and subsequent approval from the IACUC, exceptions from Guide recommendations that are suitable for appropriate animal growth and wellbeing are being used in our production facilities. Routine evaluation of these approved exceptions occurs, and determining whether such exceptions affect animal production parameters and animal wellbeing is the purpose of the current study. At the time this study was performed, the Guide did not include recommendations for female rats or mice with litters; therefore, this parameter was not evaluated. Evaluation of novel bedding types was included to provide recommendations to our production facilities with regard to potential alterations in bedding type; however, the study design and statistical analysis accounted for the use of bedding type as a separate variable in the study.
As indicators of animal production and animal wellbeing, body weight, feed disappearance, mortality, morbidity, and animal behavior were assessed. The body weight of our Sprague Dawley rats over an 8-wk period was not affected by cage density or sanitation frequency, and growth was comparable to inhouse growth curves. During the 8-wk trial, cage density differed only when rats were heavier than 200 g, corresponding to a maximal difference of 2 rats per cage. Mouse cage density differed by as few as 2 to as many as 14 mice during the trial, depending on body weight (Figure 1). Although cage density differed, cage area per animal met or exceeded Guide recommendations for rats, but mice had less cage space than Guide recommendations. A previous study reported a lack of body weight difference due to higher cage density compared with Guide recommendations in Long–Evans outbred rats, but singly housed F344 inbred rats gained more weight than did group-housed inbred F344 rats.13 Other studies reported similar lack of body weight changes due to differing cage densities in mice.6,22 Bedding type had no effect on body weight or gain in rats or mice. A previous study reported differences in body weight, with rats maintained on aspen bedding weighing more than rats maintained on other bedding types, potentially due to ingestion of the aspen bedding,3 but these results were not reproduced in the current study.
Differences in feed disappearance occurred during weeks 6 and 8 in rats and weeks 2 and 7 in mice. However, these differences did not represent overall trends, nor did they correlate to increased growth rates in rats and mice. The overall decrease in feed disappearance by week 5 may be due to the parallel increase in ammonia concentrations, but animals consumed similar amounts by week 6.
Behavioral assessment has been suggested to be a good measure of animal wellbeing.8 Caging density and sanitation frequency had no effect on animal behavior; however, mice maintained on aspen:cellulose bedding did display abnormal grooming behaviors during 2 wk of the trial. Brief visual assessment of animal behavior was performed during the photoperiod, and future studies should involve assessment of animals during the dark cycle, given that this normally is the active time for rodents, as well as for a prolonged duration.19
Week 1 mortality, observed in mice but not rats, accounted for 91% of overall mouse mortality within the trial, given that 6 cages experienced greater than 50% mortality during this time period. This event was an anomalous and unexpected finding that we do not believe is related to the method of husbandry. Cage density differed by 2 animals per cage (comparing Guide and inhouse standards), and husbandry was comparable among all cages. Therefore, these losses may have been due to poor acclimation to the weaned environment or to difficulty in acclimating to the sipper tube. These difficulties occasionally occur in individual cages but are not typical of mortality rates within our production environment. There was no evidence of colony health problems in our production facility during this time period.
Ammonia levels were increased during week 2 in cages containing rats maintained according to Guide standards and by weeks 6 and 8 in cages containing rats maintained according to inhouse standards. The increased ammonia levels during weeks 6 and 8 (and perhaps week 7) may be due to the increased cage density as compared with that of cages containing rats maintained according to Guide standards, but because all ranges were within acceptable limits (at or below 25 ppm1,3,12,17,23), the increased cage density does not negatively affect the cage environment. Other colleagues reported similar values for ammonia levels at different cage densities.23 As expected, ammonia concentrations were lower in cages housing mice compared with those housing rats, given a lower biomass within the cage.16,18 Cages containing mice maintained according to inhouse standards had a higher ammonia concentrations during weeks 3, 6, and 7 than did cages containing mice maintained according to Guide standards. These differences may be attributable to the higher cage density at these time points, but all ammonia levels were below acceptable limits. One previous study reported similar ammonia values for cages containing C57BL/6 mice,24 and another reported differences in cage ammonia levels due to differing bedding types.16 For cages containing rats, aspen:cellulose bedding was related to a higher ammonia concentration during week 3 and potentially during weeks 6 through 8. In-cage ammonia concentrations were numerically but not statistically lowest in cages that maintained mice on cellulose bedding for most time points. Cages containing mice showed no discernible effects of bedding type on cage ammonia concentration. However, cages with aspen bedding had a dramatic peak in ammonia levels during week 4. Ammonia levels in cages with aspen bedding were numerically higher than those for cellulose bedding at most time points, reflecting the decreased absorbency of aspen bedding.2,14
Monitoring cages via an ATP-based system has been reported to be an effective measure of sanitation effectiveness.25 We noted only sporadic effects of cage density and sanitation and bedding type on ATP cage concentrations in cages containing rats. These data revealed that even with a monthly cage change, ATP concentrations are not affected, allowing the use of fewer whole-cage changes without a negative effect on the effectiveness of sanitation. The decrease from week 4 to 8 in cages maintained according to inhouse standards was unexpected. We anticipated an increase in ATP cage concentrations in cages maintained by using inhouse standards, because of the fewer whole-cage changes compared with Guide recommendations; however, the data did not support this theory. One previous study reported no significant differences in ATP levels on cage accessories that had been held 14 to 90 d without sanitization, although values did increase numerically.20 Compared with mice maintained in cages according to Guide standards, mice housed according to inhouse standards had higher ATP concentrations during weeks 2 to 6, possibly due to increased cage density. However, ATP levels appeared to reach a threshold level by week 4 in both test groups.
No statistically significant differences were detected in culture results from the nasopharynges or ceca of rats housed according to any conditions of the study. Proteus spp. is a ubiquitous organism identified in conventional animal facilities, and our facility historically is positive for P. mirabilis. Neither cage density and sanitation frequency nor bedding type affected the incidence of P. mirabilis, which averaged 28% in rats. S. aureus is another common organism found in our facility. However, the incidence in the mice on study was increased compared with that reported for mice maintained within this facility.
At the time this study was performed, lung histology was performed to explore possible presence of lung lesions, which have been correlated with high in-cage ammonia levels.4 However, in-cage ammonia levels were below recommended maximal levels, and lungs did not appear to be negatively affected.19 A previous study reported differences in lung pathology based on differing bedding types;3 however, this effect was not noted in the current study. Neither aspen nor cellulose bedding significantly affected lung pathology.
Corticosterone levels are routinely monitored by using feces5,19 and were measured here at a single time point as an indicator of chronic animal stress. Inappropriate cage densities and environmental conditions are well-known stressors for rodents.7,10 Fecal corticosterone concentrations were not affected by cage density, sanitation frequency, or bedding type in rats or mice in this study, but appeared to be low in this study compared with other trials.19 A previous study reported no difference in plasma corticosterone levels in groups of F344 and Long–Evans rats maintained at various cage densities.13 For future studies, fecal samples must be collected to obtain baseline values for corticosterone analysis, in addition to periodic sampling of feces.10
In conclusion, modest deviations from the cage density and sanitation frequency performance standard recommendations set forth in the Guide do not negatively affect animal health, welfare, or production parameters at our institution. Although some statistically significant differences did arise, these did not appear to be biologically significant, nor did they represent overall trends or interactions that might affect animal welfare, as confirmed by the lack of differences in production indices and environmental conditions monitored in the current study. These parameters appear to be credible measures of animal health and wellbeing; may be useful for evaluating performance standards for animal husbandry; and are consistent with the use of various performance criteria outlined in the Guide.11 Continued research in this area will help elucidate the best practices for rodent housing and wellbeing.
Acknowledgments
We thank Penny Zielinski and Diane Lester for their assistance in performing the animal trial and Drs Joseph Curlee and Thomas Davis for their technical support.
References
- 1.Allmann-Iselin I.2000. Husbandry, p 45–55. In: Krinke GJ, editor. The laboratory rat. London (UK): Academic Press.
- 2.Burn CC, Mason GJ. 2005. Absorbencies of 6 different rodent beddings: commercially advertised absorbencies are potentially misleading. Lab Anim 39:68–74 [DOI] [PubMed] [Google Scholar]
- 3.Burn CC, Peters A, Day MJ, Mason GJ. 2006. Long-term effects of cage-cleaning frequency and bedding type on laboratory rat health, welfare, and handleability: a cross-laboratory study. Lab Anim 40:353–370 [DOI] [PubMed] [Google Scholar]
- 4.Broderson JR, Lindsey JR, Crawford JE. 1976. The role of environmental ammonia in respiratory mycoplasmosis of rats. Am J Pathol 85:115–130 [PMC free article] [PubMed] [Google Scholar]
- 5.Cavigelli SA, Monfort SL, Whitney TK, Mechref YS, Novotny M, McClintock MK. 2005. Frequent serial fecal corticoid measures from rats reflect circadian and ovarian corticosterone rhythms. J Endocrinol 184:153–163 [DOI] [PubMed] [Google Scholar]
- 6.Davidson LP, Chedester AL, Cole MN. 2007. Effects of cage density on behavior in young adult mice. Comp Med 57: 355–359 [PubMed] [Google Scholar]
- 7.Eriksson E, Royo F, Lyberg K, Carlsson HE, Hau J. 2004. Effect of metabolic cage housing on immunoglobulin A and corticosterone excretion in faeces and urine of young male rats. Exp Physiol 89:427–433 [DOI] [PubMed] [Google Scholar]
- 8.Foltz C, Carbone L, DeLong D, Rollin BE, Van Loo P, Whitaker J, Wolff A. 2007. Considerations for determining optimal mouse caging density. Lab Anim (NY) 36:40–49 [DOI] [PubMed] [Google Scholar]
- 9.Gonder JC, Laber K. 2007. A renewed look at laboratory rodent housing and management. ILAR J 48:29–36 [DOI] [PubMed] [Google Scholar]
- 10.Harper JM, Austad SN. 2000. Fecal glucocorticoids: a noninvasive method of measuring adrenal activity in wild and captive rodents. Physiol Biochem Zool 73:12–22 [DOI] [PubMed] [Google Scholar]
- 11.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press.
- 12.Lipman NS. 1999. Isolator rodent caging systems (state of the art): a critical view. Contemp Top Lab Anim Sci 38:9–17 [PubMed] [Google Scholar]
- 13.Nemelka KW, Bean K, Sturdivant R, Hacker SO, Rico PJ. 2008. Effects of high-density housing on behavioral and physiologic parameters in F344 rats and Long–Evans rats (Rattus norvegicus). Online J Vet Res 12:28–40 [Google Scholar]
- 14.Perkins SE, Lipman NS. 1995. Characterization and quantification of microenvironmental contaminants in isolator cages with a variety of contact beddings. Contemp Top Lab Anim Sci 34:93–98 [PubMed] [Google Scholar]
- 15.Potgieter FJ, Wilke PI. 1996. The dust content, dust generation, ammonia production, and absorption properties of 3 different rodent bedding types. Lab Anim 30:79–87 [DOI] [PubMed] [Google Scholar]
- 16.Reeb C, Jones RB, Bearg DW, Bedigian H, Myers DD, Paigen B. 1998. Microenvironment in ventilated animal cages with differing ventilation rates, mice populations, and frequency of bedding changes. Contemp Top Lab Anim Sci 37:43–49 [PubMed] [Google Scholar]
- 17.Reeb-Whitaker CK, Paigen B, Beamer WG, Bronson T, Churchill GA, Schweitzer IB, Myers DD. 2001. The impact of reduced frequency of cage changes on the health of mice housed in ventilated cages. Lab Anim 35:58–73 [DOI] [PubMed] [Google Scholar]
- 18.Riskowski GL, Harrison PC, Memarzadeh F. 2006. Mass generation rates of ammonia, moisture, and heat production in mouse cages with 2 bedding types, 2 mouse strains, and 2 room relative humidities. ASHRAE Transactions 112:134–144 [Google Scholar]
- 19.Rosenbaum MD, VandeWoude S, Johnson TE. 2009. Effects of cage-change frequency and bedding volume on mice and their microenvironment. J Am Assoc Lab Anim Sci 48:763–773 [PMC free article] [PubMed] [Google Scholar]
- 20.Schondelmeyer CW, Dillehay DL, Webb SK, Huerkamp MJ, Mook DM, Pullium JK. 2006. Investigation of appropriate sanitization frequency for rodent caging accessories: evidence supporting less-frequent cleaning. J Am Assoc Lab Anim Sci 45:40–43 [PubMed] [Google Scholar]
- 21.Silverman J, Bays DW, Cooper SF, Baker SP. 2009. Ammonia and carbon dioxide concentrations in disposable and reusable static mouse cages. Lab Anim (NY) 38:16–23 [DOI] [PubMed] [Google Scholar]
- 22.Smith AL, Mabus SL, Muir C, Woo Y. 2005. Effects of housing density and cage floor space on 3 strains of young adult inbred mice. Comp Med 55:368–376 [PubMed] [Google Scholar]
- 23.Smith AL, Mabus SL, Stockwell JD, Muir C. 2004. Effects of housing density and cage floor space on C57BL/6J mice. Comp Med 54:656–663 [PubMed] [Google Scholar]
- 24.Smith E, Stockwell JD, Schweitzer I, Langley SH, Smith AL. 2004. Evaluation of cage microenvironment of mice housed on various types of bedding materials. Contemp Top Lab Anim Sci 43:12–17 [PubMed] [Google Scholar]
- 25.Turner DE, Daugherity EK, Altier C, Maurer KJ. 2010. Efficacy and limitations of an ATP-based monitoring system. J Am Assoc Lab Anim Sci 49:190–195 [PMC free article] [PubMed] [Google Scholar]
- 26.Williams SV, Johnson DK, Bostrom LA, Cooper DM. 2009. Evaluation of housing and sanitation practices as compared to Guide recommendations using laboratory animal performance indices. J Am Assoc Lab Anim Sci 48:606 [Google Scholar]
- 27.Wimer RE, Fuller JL.1966. Patterns of behavior, p 629–653. In: Green EL, editor. Biology of the laboratory mouse. New York (NY): Drover.