Abstract
Detection of mouse parvovirus (MPV) and other murine pathogens in research colonies is dependent on the transmissibility of the agents and the sensitivity of sentinels to those agents. Transmissibility is based on several agent-dependent properties including mode of transmission, infectivity, and environmental stability, whereas host susceptibility can vary according to mouse age, strain, and sex. In this study, 4-wk-old, 12-wk-old, and aged Swiss Webster female sentinel mice were compared for their ability to detect infectious agents by using a standardized health surveillance program, to determine whether sentinels should be replaced more frequently to improve the efficiency of detection of infectious agents within a murine colony. Both experimentally and naturally infected mice were used to transmit MPV and other infectious agents from index mice to sentinels. First, Swiss Webster mice were inoculated with MPV, and transmission to 4-, 12-, and 24-wk-old contact and soiled-bedding sentinels was determined. Second, mice naturally infected with 9 infectious agents were obtained from 2 local pet stores, and transmission to 4-wk-old contact sentinels and 4-, 12-, and 44-wk-old soiled-bedding sentinels was determined. For agents that were transmitted via soiled bedding (MPV, mouse hepatitis virus, murine norovirus, Theiler murine encephalomyelitis virus, and pinworms), transmission did not differ in regard to the age of the sentinels. In conclusion, susceptibility to several infectious agents did not differ according to sentinel age in a health-surveillance protocol that used mice older than 12 wk.
Abbreviations: MAV, murine adenovirus; MHV, mouse hepatitis virus; MNV, murine norovirus; MPV, mouse parvovirus; MVM, minute virus of mice; TMEV, Theiler encephalomyelitis virus
Successful rodent health surveillance programs must perform 2 primary functions: rapid detection of excluded pathogens within established colonies and evaluation of the microbiologic status of mice within a colony and imported from outside institutions.18 Although the need for a microbiologic surveillance program is generally accepted, there is a diverse range of opinions on the design of individual programs.14 Each institution must develop a microbiologic surveillance program tailored to meet the specific needs of that institution. To develop an effective and practical program, a number of factors must be taken into consideration. Some key features that affect program design include the scientific objectives of the individual research program, the specific infectious agents to be detected, and test procedures used to detect these agents.19
The first factor to address is the selection of the specific infectious agents for which to screen. Most SPF colonies exclude ectoparasites, metazoan endoparasites, pathogenic bacteria, and most exogenous viruses, regardless of pathogenicity. Others may selectively exclude additional agents, such as opportunistic bacteria and Helicobacter spp., based on the immune status of the mice or the nature of the research for which they are used.1,10,14,19,23 The ability to detect those agents selected for exclusion within a colony by using sentinels is dependent on transmission of the agent(s) to sentinel mice resulting in active infection, including shedding of agents or seroconversion. Transmission is dependent on a number of agent-related factors, including route of infection, the infectious agent load, duration of shedding, environmental stability, and frequency of exposure.6,29
Once agents to be excluded from a research colony have been selected, the next priority is determining a method to effectively and reliably detect the excluded agents within the colony. The primary purpose of health surveillance is to detect infection in even a single mouse within a population rather than to determine the prevalence of infection or disease.14 Direct sampling of all mice is unfeasible, because doing so requires substantial amounts of time and money and the euthanasia of valuable research animals. Direct testing of a relatively small, randomly selected subset of animals within a colony has been used to monitor the microbial status of a colony,19 but this method is not applicable to mice housed in filter-top cages. Instead, direct contact or soiled-bedding sentinels are used to screen for infection. Contact sentinels offer a number of advantages (for example, increased probability of disease transmission and subsequent detection) and are preferable when assessing the health status of small groups of mice but are impractical for the surveillance of large colonies. Currently, exposure of sentinels to soiled bedding is the most common method used to monitor the microbiologic status of large rodent colonies18 and has been shown to be reliable for agents transmitted by the fecal–oral route.29 A key disadvantage of using soiled bedding sentinels is the lack of effective detection of infectious agents that are not transmitted by the fecal–oral route, such as Sendai virus and fur mites.6,17 However, the majority of agents for which most contemporary colonies are screened are transmitted fecal–orally. For those that are not, alternative methods of detection must be used.
Sentinel mice should be representative of the microbiologic status of the colony as a whole.19,24 For example, specific inbred mouse lines (for example, DBA) are more likely to develop clinical disease when infected with certain pathogens and may be preferred in instances in which increased sensitivity is desired.9 Conversely, outbred mice have the advantages of low cost, increased immune vigor, and generally low predilection for noninfectious diseases, and they tend to mount a robust immune response and respond to a larger pool of antigens. In addition to strain, other variables to consider when choosing sentinels include origin, sex, and age of the mice.19
Compared with other factors that are considered when designing a health surveillance program, the optimal age for sentinel submission is less clear. Very young mice should not be used, because maternal antibodies may interfere with a serologic response.9 Conversely, aged mice may be immunologically less responsive or may have been exposed to crossreacting antigens, resulting in false positives.18 Furthermore, the ideal age at which seroconversion or persistent infection occurs after exposure may vary according to the agent of interest.4,11
Studies over recent decades have indicated an increased susceptibility of younger mice to various infectious agents.3,4,33,35 In this regard, many institutions have developed murine microbiologic surveillance programs based on the belief that younger sentinels are better at detecting certain infectious agents, such as mouse parvovirus (MPV). However, these studies were conducted under controlled experimental conditions and do not necessarily give an accurate representation of natural infection and transmission within a research colony using soiled bedding sentinels. In addition, these studies compared mice younger than 12 wk, whereas many sentinels are not exposed to infectious agents until older than 12 wk.
The current heath surveillance program at our institution is based on the previously mentioned factors and is constructed as follows: SPF 4- to 6-wk-old Swiss Webster female mice are obtained from commercial vendors for use as sentinels. These sentinels are placed on each side of every rack in all rooms with mice. Each sentinel cage is exposed systematically (every 2 wk) to soiled bedding from occupied cages on that side of the rack and tested for infectious agents at 3 and 6 mo after arrival (at approximately 16 and 28 wk of age). At 3 mo, antemortem blood samples from soiled-bedding sentinels are tested serologically for mouse hepatitis virus (MHV), MPV, and rotavirus. Endoparasites (for example, pinworms) are screened for by anal tape test and fecal flotation. At 6 mo, sentinels are sent to necropsy and screened serologically for MHV, MPV, rotavirus, Sendai virus, pneumonia virus of mice, lymphocytic choriomeningitis virus, ectromelia virus, Theiler murine encephalomyelitis virus (TMEV), minute virus of mice (MVM), and Mycoplasma pulmonis. Microbiologic culture is performed on nasopharyngeal wash and cecocolonic samples for specific pathogenic bacteria. Direct examination of cecal and colonic contents is performed to screen for helminthes (primarily pinworms) and protozoa. Finally, the pelt is removed, allowed to cool, and examined for ectoparasites.
The purpose of the current study was to evaluate the efficacy of our current health surveillance program by investigating differences in the transmission of infectious agents to sentinels of different ages to determine whether younger sentinels were more effective at detecting infectious agents.
Materials and Methods
Mice.
For the experiments in part 1, 96 female Swiss Webster mice were obtained from Charles River (Stone Ridge, NY). For the experiments in part 2, 33 female Swiss Webster mice were obtained from Taconic (Hudson, NY). Vendor reports indicated that the mice were seronegative for ectromelia virus, lymphocytic choriomeningitis virus, MHV, MVM, MPV, mouse adenoviruses (MAV1 and 2), mouse norovirus (MNV), pneumonia virus of mice, reovirus, mouse rotavirus, Sendai virus, and TMEV and were free of bacterial and parasitic infections at the time of shipment. In addition, for part 2, we obtained 2 to 4 mice from each of 6 local pet stores and tested them for ectromelia virus, lymphocytic choriomeningitis virus, MHV, MVM, MPV, MAV2, MNV, pneumonia virus of mice, reovirus, mouse rotavirus, Sendai virus, TMEV, Mycoplasma pulmonis, Clostridium piliforme, Helicobacter spp., pinworms and other intestinal parasites (based upon direct cecal–colonic examination and fecal flotation), fur mites (based on pelt examination), and bacterial cultures were performed on nasopharyngeal washes. We selected 2 of the target pet stores because their animals had greatest number of infectious agents of interest and obtained another 35 female mice in total from them. Mice were housed in individually ventilated cages (ACE, Allentown, NJ) in an animal room dedicated to working with murine pathogens, with a 12:12-h photoperiod and 10 to 15 air changes hourly. Cages contained corncob bedding (Harlan Teklad, Indianapolis, IN). All mice were provided ad libitum access to rodent chow (Global 2018S, Harlan Teklad) and hyperchlorinated water delivered by an automatic watering system. Mice were used in accordance with protocols and policies approved by the Yale University IACUC.
Necropsy.
All mice were euthanized by carbon dioxide overdose. Blood, feces, sections of the cecum and colon, a pelage swab, and the pelt were obtained from each mouse at the time of necropsy (except for mice that died prior to euthanasia). Sera, pelage swabs, and fecal pellets were stored at −70 °C until sample analysis was performed. Ceca, colons, and pelts were submitted for immediate analysis for intestinal parasites and fur mites.
Serology.
Sera from all mice in part 1 were tested for antibodies to MPV. Sera from all mice in part 2 were tested for antibodies to TMEV, MAV1, MAV2, MPV, MHV, ectromelia virus, rotavirus, lymphocytic choriomeningitis virus, MVM, pneumonia virus of mice, reovirus, Sendai virus, and Mycoplasma pulmonis by using immunofluorescent antibody assays as previously described.28
Parasitology.
Portions of the cecum and colon from each mouse were submitted for direct examination for intestinal parasites and for PCR assays specifically for pinworms. Immediately after collection of the cecum and colon, approximately 0.5 mL 0.9% saline was added to the sample in a culture dish, and the sample was mixed with a wooden applicator. A small amount of the sample was placed on a glass microscope slide, coverslipped, and examined under a microscope at 10× and 40× magnification for evidence of ova or adult endoparasites. Parasites were identified and speciated morphologically. The cecocolonic contents in the culture dish again were examined microscopically 10× magnification (24 h after the first examination) for evidence of adult endoparasites. Pelt examinations were performed after pelts were allowed to cool for detection of fur mites. Pelts were placed in a culture dish and examined microscopically under low power (magnification, 10×) for evidence of fur mites, which were identified and speciated morphologically. Pelage swabs were submitted for fur mite PCR.
PCR assays.
Fecal pellets were homogenized in PBS, and DNA and RNA were extracted by using DNAeasy or RNAeasy kits (Qiagen, Valencia, CA) according to the manufacturer's instructions. Ceca and colons were homogenized in minimal essential media, pelage swabs were digested for 14 to 18 h in ATL buffer, and DNA was extracted by using a DNAeasy kit (Qiagen) according to the manufacturer's instructions. DNA from MPV, MAV2, and Helicobacter spp. were detected with PCR by using the DyNAmo SYBR Green qPCR kit (MJ Research, Waltham, MA) and primers listed in Table 1. DNA from Clostridium piliforme, pinworms (Aspiculuris tetraptera, Syphacia obvelata), fur mites (Myobia musculi, Myocoptes musculinus, Radfordia affinis) and Rodentolepis nana were detected by using the Roche PCR core kit (Indianapolis, IN) and primers listed in Table 1. Amplification conditions for all PCR reactions, except for mite PCR assays, were: 2 min at 94 °C; 35 cycles of 30 s at 92 °C, 30 s at 50 °C, 60 s at 72 °C; and 5 min at 72 °C. Conditions for mite PCR assays were: 2 min at 94 °C; 35 cycles of 30 s at 92 °C, 30 s at 50 °C, 120 s at 72 °C; and 5 min at 72 °C. RNA from MHV, TMEV, MNV, reovirus, and murine rotavirus were detected via RT-PCR by using the Brilliant II SYBR Green qRT–PCR 1-step kit (Agilent, Santa Clara, CA). Reaction conditions were: 30 min at 50 °C, 2 min at 94 °C; 40 cycles of 30 s at 94 °C, 30 s at 50 °C, 90 s at 68 °C; and 10 min at 72 °C. Similar RT-PCR-based methods were used to amplify the VP0 and P2 regions of TMEV isolates from the ceca of 6 sentinels infected by contact with pet-store mice. Sequencing of PCR products was performed by the WM Keck Foundation Biotechnology Resource Laboratory at Yale University, and sequence homologies were determined by using BLAST (National Center for Biotechnology Information, Bethesda MD).
Table 1.
Primers used for PCR amplification
| Organism | Target | Left primer (5’ to 3’) | Right primer (5’ to 3’) | Amplicon (bp) |
| A. tetraptera + | 18S rRNA | SY1588 | SY2014 | 684/426 |
| S. obvelata | AGA TCG ATG AAG AAC GCA GT | CAG CGG GTA ATC ACG ACT GA | ||
| Clostridium | 16S RNA | CPIL2356 | CPIL3231 | 876 |
| piliforme | CGA GTT ACA TTT GCA AGC GA | TTC CTT TCC TCG GTT TTC CT | ||
| Helicobacter spp. | 16S RNA | HEP380 CGT GGA GGA TGA AGG TTT TAG | HEP1372 CCG ACT TAA GGC GAA TAC AAC | 992 |
| Rodentolepis nana | 18S rRNA | HNANAF GCG GAA GGA TCA TTA CAC GTT C | HNANAR GCT CGA CTC TTC ATC GAT CCA CG | 664 |
| MHV | Nucleocapsid | MHVN512 GTC ATG AGG CTA TTC CTA CTA | MHVN1027 ATA CAC ATC TTT GGT GGG | 533 |
| MPV/MVM | NS | MPV1059 CAC TGC GCA GGA AAC TAA G | MPV1812 CAA AGT CAC CAG GCA ATG TA | 773 |
| MAV2 | Hexon | K87H310 AAG CGC ACC TAC GAT TAC AT | K87H562 CTT CCT GAA AGC CCA CTC | 252 |
| MNV | Capsid | MNV5033 GGA ACG CTC AGC AGT CTT TG | MNV5542 CAA GAA GAG GGA GTT GAA TG | 509 |
| Murine rotavirus | VP7 | EDIM8 AAA GAG AGA ATT TCC GTT TG | EDIM939 GTA GAA CAC TTG CCA CCA TT | 930 |
| M.musculi + | 18S rRNA | MBO465 | MBO1429 | 964 |
| R. affinis | TAC CCA ATC CCG GCA CGG GG | CTG AAA CGC CGC CTG TCC CT | ||
| M. musculinus | 18S rRNA | COP328 TCG ATT CCG GAG AGG CAG CCT | COP629 ACC ACC GAC AAG ATG GAC CGC | 301 |
| Reovirus | S4 | REO26 TGT CGC AAT GGA GGT G | REO1101 CGG ATC GCC AAT CAT | 1090 |
| TMEV | VP4 | TMEV84 GGC AGA CGG AGA ATG GT | TMEV325 CCA CTG GCA GAC AAA TCA AT | 261 |
| VP0 | TMEV1200 CGA TGA CGT CTT CTG GCC TTC G | TMEV1794 ACC CCT CCG TCC TCG CCA G | 594 | |
| P2 | TMEV4215 CCA CAA GGT GCG GTG CTA AC | TMEV5243 TCC AGG TGA GCC ATA TTC GG | 1018 |
Statistical analysis.
Two-tailed Fisher exact probability tests were performed by using an online statistics application (vassarstats.net).
Part 1: Detection of MPV in experimentally infected mice.
Forty-eight unanesthetized 4-wk-old female Swiss Webster index mice were inoculated orally with 300 ID50 of MPV1d (20 µL of a 10% spleen stock) and were housed in pairs (24 cages). Fresh feces were obtained from each index mouse at 1 wk after infection. Feces were pooled by cage to confirm infection with and shedding of MPV via fecal PCR. Each pair of index mice then was placed in a clean cage with a single contact sentinel. Eight cages each contained a single 4-wk-old contact sentinel, 8 each contained a single 12-wk-old contact sentinel, and 8 each contained a single 24-wk-old contact sentinel. In addition, each of the 24 cages was assigned a counterpart soiled-bedding sentinel of the corresponding age. All 24 soiled-bedding sentinels were singly housed. An approximately 100-mL aliquot of bedding29 was transferred from the soiled index cages to each of the soiled-bedding sentinel cages, which already contained approximately 400 mL of clean bedding.
One week after introduction to contact sentinels (that is, 2 wk after infection), index mice were euthanized and blood collected for MPV serology as described earlier. Contact and soiled-bedding sentinels were transferred to clean cages and left for 2 wk to allow for seroconversion. All sentinels were euthanized at 3 wk after exposure, and blood and tissues were collected as described earlier.
Part 2: Detection of multiple murine pathogens in naturally infected mice.
We obtained 2 or 4 mice from each of 6 local pet stores and immediately submitted them for necropsy. By using the techniques previously described, we determined the infectious agents carried by each of these mice. The 2 pet stores that collectively represented the greatest variety of infectious agents (MAV2, MHV, MPV, MNV, TMEV, Helicobacter spp., pinworms, fur mites, and Rodentolepis nana) were selected for part 2.
We obtained a total of 35 additional female mice (age unknown) from the 2 selected pet stores and 33 female Swiss Webster mice from Taconic. Pet-store mice were allocated into 9 cages (4 mice per cage, 2 from each store, except for one cage that had only 3 mice). Contact sentinels were used to determine which agents could be transmitted. A single 4-wk-old contact sentinel was added to each of the 9 cages, and 2 wk later, all pet-store mice were submitted for necropsy. Contact sentinels were placed in a clean cage for an additional week prior to being submitted for necropsy. Mice for necropsy were euthanized, and blood and samples were collected as described earlier. Soiled bedding from the 9 pet store cages was pooled, and an approximately 100-mL aliquot of soiled bedding was transferred to each of 24 cages containing 400 mL of clean bedding. A single sentinel was placed in each of the 24 soiled cages for 2 wk (8 soiled-bedding sentinels per age group: 4-, 12-, and 44-wk old). All soiled-bedding sentinels were euthanized, and blood and samples were collected at 3 wk after exposure as described earlier.
Results
Detection of MPV in experimentally infected mice.
To assess age-associated susceptibility to MPV under controlled conditions, 4-, 12-, and 24-wk-old contact sentinels were exposed to index mice that had been experimentally inoculated with MPV, and 4-, 12-, and 24-wk-old soiled-bedding sentinels were exposed to soiled bedding from cages that had housed the experimentally infected index mice. All 48 index mice were positive for MPV by both fecal PCR at 1 wk after infection and serology at 2 wk after infection. Almost all (23 of 24) contact sentinels and all (24 of 24) soiled-bedding sentinels were positive for MPV by serology at 3 wk after exposure. Only one 4-wk-old contact sentinel was seronegative. Therefore, we detected no difference in MPV transmission to contact and solid-bedding transfer sentinels depending on their age.
Detection of multiple murine pathogens in naturally infected mice.
MPV.
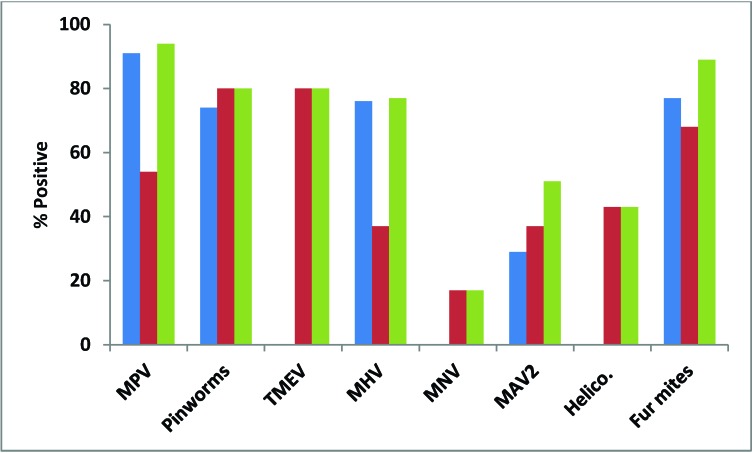
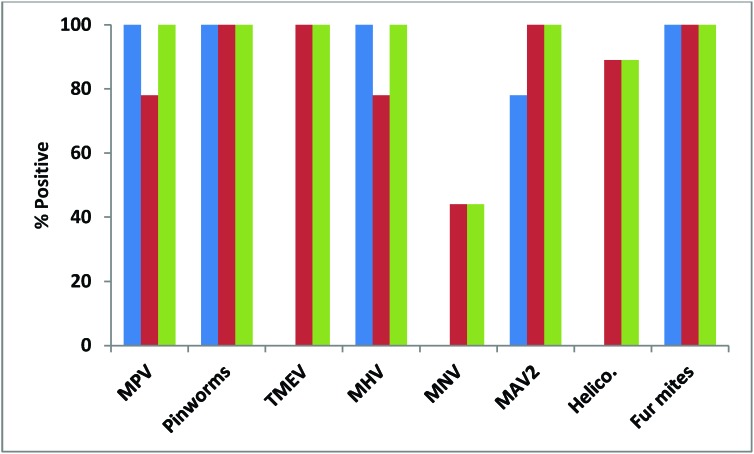
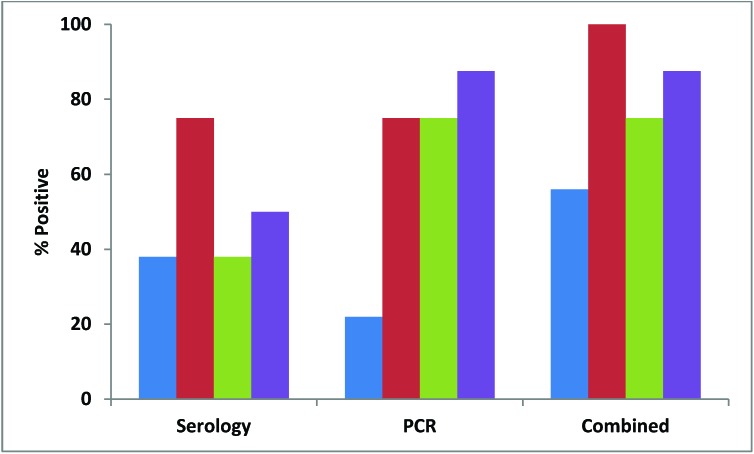
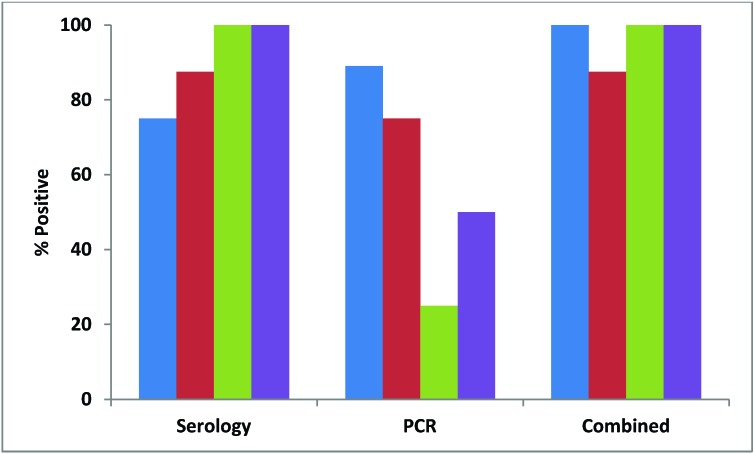
Almost all (91%) pet-store mice were positive for MPV by serology, but only about half (54%) were positive by fecal PCR (Figure 1). Although all of the pet-store cages contained mice that were seropositive for MPV, only 7 of the 9 (78%) cages had at least one mouse positive by PCR (Figure 2). MPV was detected in 38% of contact sentinels by serology and 22% by fecal PCR, with an overall detection rate of 56% (Figure 3). Detection in soiled-bedding sentinels was higher by both serology and PCR, ranging from 75% to 100% for both methods of detection overall (Figure 3). However, there was no significant difference in detection between age groups. Unlike for contact sentinels, PCR was equally or more effective than serology for detection of MPV in soiled-bedding sentinels (Figure 3).
Figure 1.
Percentages of pet-store mice testing positive for each of 8 infectious agents by serology or direct examination (blue), PCR (red), and both methods combined (serology–direct examination and PCR; green). Helico., Helicobacter spp.
Figure 2.
Percentages of pet-store cages housing mice that tested positive for each of 8 infectious agents by serology or direct examination (blue), PCR (red), and both methods combined (serology–direct examination and PCR, green). Helico., Helicobacter spp.
Figure 3.
Percentages of contact (blue) and 4-wk-old (red), 12-wk-old (green), and 44-wk-old (purple) soiled-bedding sentinels testing positive for MPV by serology or PCR and both methods of detection combined.
Pinworms.
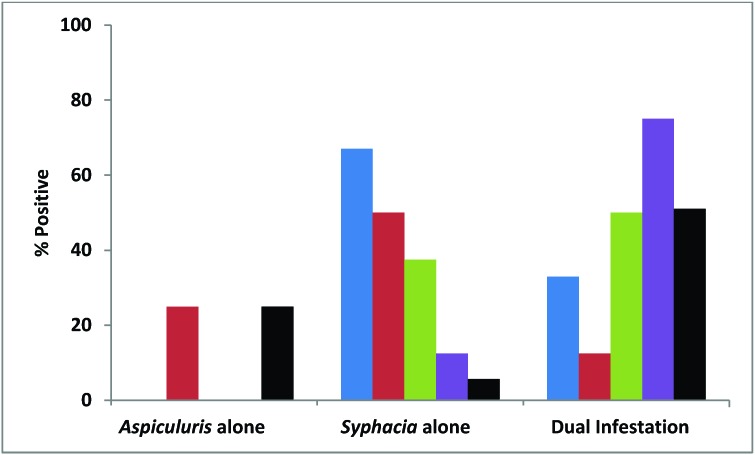
Pinworm detection and speciation was performed by both direct examination and PCR of the cecal and colonic contents. Results for direct exam and PCR agreed in terms of the presence or absence of infestation. However, PCR was superior for speciation of pinworms. The vast majority (29 of 35) of pet-store mice were infested with pinworms, representing all of the pet-store index cages. Of the 29 mice positive for pinworms, 9 were positive for A. tetraptera alone, 2 were positive for S. obvelata alone, and 18 were positive for both agents (Figure 4).
Figure 4.
Percentages of pet-store mice (black), contact sentinels (blue), and 4-wk-old (red), 12-wk-old (green), and 44-wk-old (purple) soiled-bedding sentinels positive for Aspiculuris tetraptera alone, Syphacia obvelata alone, and for both organisms concurrently (dual infestation).
Pinworms were transmitted to all contact sentinels. Despite dual infestation in 8 of the 9 cages of pet-store mice, only S. obvelata was detected in 5 of 8 contact sentinels. Transmission of pinworms did not differ significantly among the 3 age groups of soiled-bedding sentinels. Although all soiled-bedding sentinels were exposed to both S. obvelata and A. tetraptera, several of these mice were infested with only S. obvelata or A. tetraptera. Notably, detection of dual infestation increased with increasing age: 12.5% of 4-wk-old mice, 50% of 12-wk-old mice, and 75% of 44-wk-old mice had dual pinworm infestation (Figure 4); the incidence of occurrence of dual infestation differed significantly (P < 0.05) between 4- and 44-wk-old mice.
TMEV.
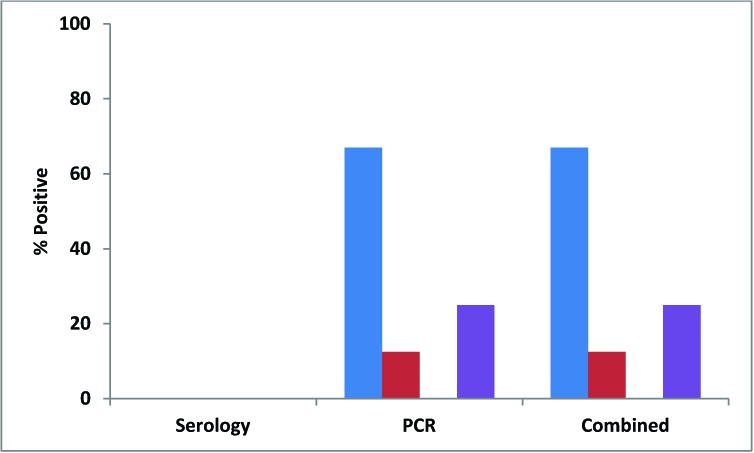
All of the pet-store mice were seronegative for TMEV, but the majority (80%) were positive by fecal RT-PCR, with 100% of cages containing TMEV-positive mice (Figures 1 and 2). Similarly, all contact and soiled-bedding sentinels were negative for TMEV by serology (Figure 5). Despite the fact that all pet-store cages contained at least one TMEV-positive mouse, only 67% of the contact sentinels were positive by fecal PCR (Figure 5). TMEV was transmitted to less than a quarter of the soiled-bedding sentinels, and detection by fecal RT-PCR showed no significant variation in transmission according to age (Figure 5).
Figure 5.
Percentages of contact (blue) and 4-wk-old (red), 12-wk-old (green), and 44-wk old (purple) soiled-bedding sentinels testing positive for TMEV by serology or PCR and both methods of detection combined.
RT-PCR amplification and sequencing of 2 regions from viral strains present in the ceca of 6 sentinels in contact with pet-store mice indicated 93% to 97% nucleotide identity in the VP0 region and 86% to 97% nucleotide identity in the P2 region among the 6 isolates. BLAST analysis showed that these isolates had slightly lower levels of nucleotide identity (89% to 94% nucleotide identity in the VP0 region and 86% to 89% nucleotide identity in the P2 region) with TMEV-BeAN, -DA, -GDVII, and -Yale.
MHV.
The majority of pet-store mice (76%), including mice from all cages, were positive for MHV by serology, but fewer (37%) were positive by fecal RT-PCR (Figures 1 and 2). Serology results for one pet-store mouse were inconclusive, and serology was not performed on another mouse due to its unexpected death. All of the contact sentinels and the majority of soiled-bedding sentinels were positive for MHV by serology, RT-PCR, or both (one 4-wk-old mouse was negative for MHV by both serology and RT-PCR; Figure 6). There was no significant difference in transmission of MHV among the 3 ages of soiled-bedding sentinels.
Figure 6.
Percentages of contact (blue) and 4-wk-old (red), 12-wkd-old (green), and 44-wk-old (purple) soiled-bedding sentinels testing positive for MHV by serology or PCR and both methods of detection combined.
MNV.
Only 17% of pet-store mice (44% of pet-store cages) were positive for MNV RNA (Figures 1 and 2). Transmission to contact or 4- and 12-wk-old soiled bedding sentinels was not detected, although MNV was transmitted to 25% of 44-wk-old soiled bedding sentinels. The difference in transmission between the 3 ages of soiled bedding sentinels was not significant.
MAV2.
According to results from both serology and fecal PCR, 51% of the pet-store mice (100% of cages) were positive for MAV2 (Figures 1 and 2). Only 56% of contact sentinels showed evidence of MAV2 infection, and all soiled-bedding sentinels were negative for MAV2 by both serology and fecal PCR, indicating a lack of transmission of MAV2 by soiled-bedding transfer.
Helicobacter spp.
Among the pet-store mice, 43% (89% of cages) were positive by PCR for a variety of Helicobacter species (H. bilis, H. typhlonius, H. muridarum, and H. rappini; Figures 1 and 2). Only 22% of contact sentinels and none of the soiled-bedding sentinels were positive for DNA from Helicobacter spp., indicating that a single soiled-bedding exposure was insufficient for transmission of Helicobacter spp.
Fur mites.
Almost all (94%) pet-store mice were positive for fur mites (100% of cages had mice with various combinations of Myobia musculi, Myocoptes musculinus, and Radfordia affinis) detected by direct pelt examination or PCR of pelage swabs or both methods (Figures 1 and 2). There was 100% transmission to contact sentinels, with equivalent transmission of M. musculi and M. musculinus. Transmission of fur mites to soiled-bedding sentinels did not occur.
Discussion
Our studies found no significant difference in transmission of MPV to outbred bedding sentinels ranging in age from 4 to 44 wk that were exposed to either index mice experimentally inoculated with MPV or to pet-store mice naturally infected with MPV. A previous study4 suggested that MPV infection may go undetected in rodent facilities when sentinels are 12 wk of age or older when they are exposed to MPV1e, but only when the rNS1 ELISA or the MVM immunofluroescent assays (which detect antibodies to parvoviral nonstructural antigens) are used. However, there was no indication that age of exposure of ICR mice to MPV was a critical factor when MPV immunofluroescent assays, MPV hemagglutination inhibition tests, or MPV PCR of intestinal or mesenteric lymph node DNA were used. Another study8 found that C57Bl/6Arc mice inoculated as adults (8 wk old) with MPV1f did not have detectable DNA in the feces or spleen, whereas those inoculated as juveniles (4 wk old) did. There was no age-related difference seen in Arc/Arc or Balb/cArc mice.
Our data agrees with those from an earlier study4 in that we saw no significant difference in MPV infection of 4- and 12-wk-old mice by using MPV immunofluorescent or fecal PCR assays and extends the age range over which susceptibility to MPV infection is essentially equivalent. Our studies differ from the aforementioned studies in that we used Swiss Webster mice, a strain frequently used for sentinels, and the ages we compared were considerably older than those used in previous studies. Although mice younger than 4 wk are more susceptible to persistent viral infection than older mice, it is impractical to submit sentinels at 4 wk of age. The purpose of our current study was to compare the susceptibility of sentinel mice submitted at approximately 3 and 6 mo of age to see whether earlier submission of sentinels improves the detection of MPV. Our study results indicate that it does not.
The difference observed between transmission of MPV from experimentally inoculated index mice and from naturally infected pet-store mice could be explained by the fact that all experimentally infected index mice were acutely infected when contacts were added and soiled bedding was collected, whereas many pet-store mice likely were chronically infected and may have been shedding lower levels of virus at the time of sentinel exposure. However, the soiled-bedding sentinels from part 2 of our studies also were exposed to feces from the acutely infected contact sentinels in addition to feces from chronically infected pet-store mice. Ideally, we would have liked to compare the rate of shedding between the 2 studies, but the fecal samples were taken at different times (that is, at the time of sentinel placement in part 1 but at the time of sentinel removal in part 2), so direct comparison was not possible.
For contact sentinels but not soiled-bedding sentinels in the pet-store mouse part of the study, PCR was equally or more effective than was serology for detection of MPV; this result may reflect when the mice became infected. Contact sentinels may have become infected more rapidly, and several of the mice may have stopped shedding by 3 wk. Conversely, the soiled-bedding sentinels may have taken longer to become infected and were still shedding (but had not yet seroconverted) after 3 wk.
In addition to MPV, we investigated the transmissibility of 8 other infectious agents to sentinels via natural infection. Mice from pet-stores served as the source of infection for Swiss Webster contact and soiled-bedding sentinels, providing a realistic construct for assessing the transmission and subsequent detection of infectious agents within a murine research colony, because both acutely and chronically infected mice were represented. In addition, the use of pet-store mice allowed for the assessment of susceptibility to infectious agents for which experimental stocks are not readily available (for example, pinworms). We used 4-wk-old contact sentinels to confirm active shedding of infectious agents by pet-store mice. Under these relatively natural conditions, there was no significant difference in transmission via soiled bedding of MPV, pinworms, TMEV, MHV, and MNV to 4-, 12- and 44-wk-old mice. There is scant to no recent literature regarding age susceptibility to infection with these agents, and the studies that appear to be congruent with our current findings looked at much younger mice. A significant reduction in both the frequency of virus isolation and virus titers was reported in mice inoculated with TMEV after 3 wk of age.31 Literature relative to age susceptibility to MHV deals only with mice less than 6 wk of age, indicating that disease is more severe in neonates, despite equivalent levels of virus in the intestines.34
Although the different age groups showed no significant difference in the overall level of pinworm infestation, aged sentinels were significantly more susceptible to dual infestation than were 4-wk-old sentinels. There is a lack of recent publications regarding pinworms and age susceptibility, but data from older literature do not correspond to our findings. One review article indicated that S. obvelata infestation decreases with increasing age, whereas Aspiculuris spp. is uncommon in young mice but increases in incidence with age.35 This review further indicates that after 10 wk of age, equilibrium is reached, and no further variation in age susceptibility occurs. This conclusion is contrary to the findings of another study, in which the Aspiculuris spp. worm burden was equivalent among female mice infected at 4- to 24-wk of age with 500 eggs.22 According to the results of our current study, there is no significant difference in susceptibility between mice older than 4 wk of age. The differences seen in our data as compared with information presented in older literature could be explained by the presence or absence of underlying undetected infections, given that several agents currently detected in mouse colonies (MPV, MNV, Helicobacter spp.) had not yet been discovered. In addition, previous research may have been confounded by the presence of multiple infections, given that many of the agents that are excluded today were not excluded when the earlier studies were performed.
Pinworm infestations of laboratory rodents are generally nonpathogenic and are regarded as being asymptomatic, despite reports of subsequent rectal prolapse, intestinal impaction and intussusceptions, mucoid enteritis, and unthriftiness associated with heavy worm burdens.35 In the current study, one pet-store mouse and 2 sentinels became unexpectedly ill; of the 3, 2 were found dead, and one was euthanized due to poor condition. Necropsy of these 3 mice revealed heavy parasite burdens, including R. nana in the mouse that was found dead.
Heavy oxyurid infestation may explain the lack of tapeworm transmission in our current study. Despite the presence of R. nana in the ceca and colons of 20% of the pet-store mice, this tapeworm was not transmitted to any of the contact or soiled-bedding sentinels. This outcome may be due to the environmental instability of R.nana.2 Moreover an antagonism between different types of worms has been reported previously. For example, mice carrying natural infections of oxyurids exhibit a low susceptibility to infestation with Trichuris muris.15 Similarly, Trichinella spiralis and S. obvelata infestations both result in an increase in resistance of mice to Aspiculuris spp.30 In our study, although S. obvelata occurred more frequently as a single-worm infestation than did A. tetraptera, dual infestation was more common than infestation with either agent alone. Furthermore, our study demonstrated a significantly increased occurrence of dual infestation with increasing age of the mice. This finding was the only significant difference in terms of age-associated susceptibility among all of the agents that were investigated in this study.
The discrepancy between TMEV detection by serology compared with fecal RT-PCR was notable. Serologic methods currently offer the principal way to assess TMEV infection in mice but proved to be ineffective for detection in our study. All pet-store mice were seronegative for TMEV, but 80% were positive by fecal RT-PCR. The same was true of detection of TMEV in sentinels, in that 67% of contact sentinels and 12.5% of soiled bedding sentinels were positive by RT-PCR, but all were seronegative. To clarify these results, sera from 18 TMEV RT-PCR positive mice and 2 TMEV RT-PCR negative mice were submitted to Charles River Laboratories (Wilmington, MA) for serologic testing. The 2 RT-PCR negative mice were seronegative. None of the sera from the TMEV RT-PCR positive mice were seropositive by multiplex fluorometric immunoassay or immunofluorescent assay, although 13 of the 18 mice gave nonspecific reactions with the tissue control in multiplex fluorometric immunoassays, and 3 of 18 gave nonspecific reactions with the tissue control in the immunofluorescent assay. Some of these sera may have included antiTMEV antibodies, but the reaction of antibodies crossreactive with the tissue control antigens may have overwhelmed any TMEV-specific reaction. At least 4 viruses are members of the Theilovirus genus (TMEV, rat theilovirus, and the human viruses Saffold and Vilyuisk), and they may have distinct serotypes.5 RT-PCR amplification, sequencing, and multiple-sequence alignment of 2 regions from viral strains present in the ceca of 6 contact sentinels indicated that the TMEV strains detected were closely related to each other; were slightly less similar to TMEV-BeAN, -DA, -GDVII, and -Yale; and showed much lower levels of nucleotide identity with rat and human theiloviruses. Further investigation is necessary to determine the cause of this incongruity between results from serologic and molecular tests.
MNV was not transmitted to contact sentinels, but was transmitted to 8.3% of soiled-bedding sentinels. Although soiled-bedding transmission was not significantly different between age groups, 25% of 44-wk-old sentinels were positive for MNV and this was the only age group that detected infection. However, MNV may not have been transmitted effectively in our current study, as repeated exposure and longer seroconversion times may be necessary to effectively detect infection.21 One study found that 12-wk-old sentinels with 6 wk of exposure to MNV were more sensitive to MNV transmission than were 24-wk-old sentinels with 18 wk of exposure.9 Infectious titers of MNV in stool suspensions have been reported to drop by more than 100,000 within 24 h at 30 °C.7,16 In addition, the levels of MNV shed during the chronic phase of the infection are frequently below the level necessary to transmit infection.7,12 In a previous study, the minimal time necessary to detect seroconversion in soiled-bedding sentinels was 2 wk, although the level of detection was low (MNV was detected in only a single sentinel).21 By the end of 10 wk, 80% of soiled-bedding sentinels tested positive serologically for MNV.21
At least one mouse in all pet-store cages was positive for MAV2 by PCR, but only 56% of contact sentinels were positive. MAV2 was not transmitted to soiled-bedding sentinels, but they probably were exposed to a low viral dose, given that feces from only 37% of the pet store mice were MAV2 PCR positive. Natural infection with MAV2 has proven difficult to replicate, but successful experimental infection has been demonstrated. After oral inoculation, 4-wk-old DK1 mice reportedly shed MAV2 in the feces for 3 wk, and 7-wk-old mice shed for one less week.32 Outbred 4-wk-old Sencar mice, as compared with 2-d-old mice, have been shown to require more than 4000-fold higher dose orally of MAV2 to become infected, and weanling AKR/J, BALB/c, C57BL/6, and SJL/J could not be infected.27 We do not know the strain or background of the pet store mice or their exact age, though we would assume they are outbred and greater than 4-wk of age based on size.
Similarly, Helicobacter spp. were not transmitted to the soiled-bedding sentinels, and transmission to only 22% of contact sentinels. Previous studies have indicated successful transmission of H. hepaticus by soiled bedding, although transmission rates were low.20,37 A potential limitation in our study may have been the short duration of exposure (2 wk) to feces carrying Helicobacter spp. Sentinels that are exposed to multiple aliquots of soiled bedding over several weeks or months may be more likely to acquire infection.26 Even for agents that are transmitted in soiled bedding, such as MPV, MHV, and MNV, the quantity of an infectious agent in bedding pooled from many cages can easily be diluted below a dose infective to sentinels. Alternatively, 3 wk after exposure may have been insufficient for Helicobacter spp. infection to become established at a level sufficient for detection, especially given that 2 of the Helicobacter species detected in the index mice were H. bilis and H. muridarum. Unlike most intestinal viruses in which shedding can be detected within days of infection, shedding of Helicobacter spp. usually cannot be detected for weeks. After experimental inoculation with H. bilis, Helicobacter spp. DNA was not detected in C57BL/6 mice until 3 wk after inoculation and in C3H mice until 4 wk after inoculation,13 and it took 6 mo for all mice exposed to soiled bedding from mice infected with H. bilis and H. muridarum to begin shedding detectable levels of Helicobacter spp.37
Fur mites were transmitted to all contact sentinels but were not transmitted to soiled-bedding sentinels. This result is not unexpected, given that fur mites are the only agent studied that are not transmitted via the fecal–oral route and are known to be transmitted almost exclusively by contact. Our findings are consistent with previous research, and the inefficiency of soiled-bedding sentinels to detect fur mite infestation within a colony is well-documented.17,18,25,36
In summary, we found no significant difference in detection of MPV, TMEV, MHV, MNV, and pinworms between 4-, 12-, and aged (24- or 44-wk-old) Swiss Webster sentinels exposed to a substantial dose of these infectious agents. Further experiments will be necessary to determine whether differences in detection occur when infectious agent levels are lower. The current data show no apparent benefit to submitting sentinels every 3 mo (that is, approximately 16 to 20 wk of age) rather than every 6 mo, yet the results do appear to support the judicious use of both serology and PCR on sentinels at the time of testing. PCR detects active infection with shedding and potential for transmission, whereas serology detects past infection, requiring at least 7 d for seroconversion. Performing these diagnostic tests simultaneously might improve the probability of detection.
Acknowledgments
We thank Gordon Terwilliger and Fu-chen Yang for assistance with necropsy, sample collection, and microbiology and Frank Paturzo for assistance with serology.
References
- 1.Baker DG. 1998. Natural pathogens of laboratory mice, rats, and rabbits and their effects on research. Clin Microbiol Rev 11:231–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker DG. 2007. Flynn's parasites of laboratory animals. Oxford (UK): Blackwell Publishing [Google Scholar]
- 3.Barthold SW, Beck DS, Smith AL. 1993. Enterotropic coronavirus (mouse hepatitis virus) in mice: influence of host age and strain on infection and disease. Lab Anim Sci 43:276–284 [PubMed] [Google Scholar]
- 4.Besselsen DG, Wagner AM, Loganbill JK. 2000. Effect of mouse strain and age on detection of mouse parvovirus 1 by use of serologic testing and polymerase chain reaction analysis. Comp Med 50:498–502 [PubMed] [Google Scholar]
- 5.Clifford CB, Watson J. 2008. Old enemies, still with us after all these years. ILAR J 49:291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Compton SR, Homberger FR, Paturzo FX, Clark JM. 2004. Efficacy of 3 microbiological monitoring methods in a ventilated cage rack. Comp Med 54:382–392 [PubMed] [Google Scholar]
- 7.Compton SR, Paturzo FX, Macy JD. 2010. Effect of murine norovirus infection on mouse parvovirus infection. J Am Assoc Lab Anim Sci 49:11–21 [PMC free article] [PubMed] [Google Scholar]
- 8.Filipovska-Naumovska E, Thompson MJ, Hopwood D, Pass DA, Wilcox GE. 2010. Strain- and age-associated variation in viral persistence and antibody response to mouse parvovirus 1 in experimentally infected mice. J Am Assoc Lab Anim Sci 49:443–447 [PMC free article] [PubMed] [Google Scholar]
- 9.Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL.2007. The mouse in biomedical research, 2nd ed. Boston (MA): Academic Press.
- 10.Fox JG, Ge Z, Whary MT, Erdman SE, Horwitz BH. 2011. Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal Immunol 4:22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen AX. 1996. Improvement of health monitoring and the microbiological quality of laboratory rats. Scand J Lab Anim Sci 23:1–70 [Google Scholar]
- 12.Henderson KS. 2008. Murine norovirus, a recently discovered and highly prevalent viral agent of mice. Lab Anim (NY) 37:314–320 [DOI] [PubMed] [Google Scholar]
- 13.Hodzic E, McKisic M, Feng S, Barthold SW. 2001. Evaluation of diagnostic methods for Helicobacter bilis infection in laboratory mice. Comp Med 51:406–412 [PubMed] [Google Scholar]
- 14.Institute for laboratory Animals Science. 1991. Infectious diseases of mice and rats. Washington (DC): National Academies Press.
- 15.Keeling JE. 1961. Experimental trichuriasis. I. Antagonism between Trichuris muris and Aspiculuris tetraptera in the albino mouse. J Parasitol 47:641–646 [PubMed] [Google Scholar]
- 16.Lee J, Zoh K, Ko G. 2008. Inactivation and UV disinfection of murine norovirus with TiO2 under various environmental conditions. Appl Environ Microbiol 74:2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindstrom KE, Carbone LG, Kellar DE, Mayorga MS, Wilkerson JD. 2011. Soiled-bedding sentinels for the detection of fur mites in mice. J Am Assoc Lab Anim Sci 50:54–60 [PMC free article] [PubMed] [Google Scholar]
- 18.Lipman NS, Homberger FR. 2003. Rodent quality assurance testing: use of sentinel animal systems. Lab Anim (NY) 32:36–43 [DOI] [PubMed] [Google Scholar]
- 19.Lipman NS, Perkins SE.2002. Microbiological quality control for laboratory rodents and lagomorphs. In: Fox JG, Loew FM, Quimby FW, editors. Laboratory animal medicine, 2nd ed. San Diego (CA): Academic Press.
- 20.Livingston RS, Riley LK. 2003. Diagnostic testing of mouse and rat colonies for infectious agents. Lab Anim (NY) 32:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manuel CA, Hsu CC, Riley LK, Livingston RS. 2008. Soiled-bedding sentinel detection of murine norovirus 4. J Am Assoc Lab Anim Sci 47:31–36 [PMC free article] [PubMed] [Google Scholar]
- 22.Mathies AW. 1959. Certain aspects of the host–parasite relationship of Aspiculuris tetraptera, a mouse pinworm. I. Host specificity and age resistance. Exp Parasitol 8:31–38 [DOI] [PubMed] [Google Scholar]
- 23.Nicklas W, Homberger F, Illgen-Wilcke B, Jacobi K, Kraft V, Kunstyr I, Mahler M, Meyer H, Pohlmeyer-Esch G, Hyg GWG. 1999. Implications of infectious agents on results of animal experiments. Report of the Working Group on Hygiene of the Gesellschaft fur Versuchstierkunde–Society for Laboratory Animal Science (GV–SOLAS). Lab Anim 33 Suppl 1:S39–S87 [PubMed] [Google Scholar]
- 24.Pritchett-Corning KR, Cosentino J, Clifford CB. 2009. Contemporary prevalence of infectious agents in laboratory mice and rats. Lab Anim 43:165–173 [DOI] [PubMed] [Google Scholar]
- 25.Ricart Arbona RJ, Lipman NS, Wolf FR. 2010. Treatment and eradication of murine fur mites: II. Diagnostic considerations. J Am Assoc Lab Anim Sci 49:583–587 [PMC free article] [PubMed] [Google Scholar]
- 26.Shek WR. 2008. Role of housing modalities on management and surveillance strategies for adventitious agents of rodents. ILAR J 49:316–325 [DOI] [PubMed] [Google Scholar]
- 27.Smith AL, Barthold SW. 1987. Factors influencing susceptibility of laboratory rodents to infection with mouse adenovirus strains K 87 and FL. Brief report. Arch Virol 95:143–148 [DOI] [PubMed] [Google Scholar]
- 28.Smith AL, Singleton GR, Hansen GM, Shellam G. 1993. A serologic survey for viruses and Mycoplasma pulmonis among wild house mice (Mus domesticus) in southeastern Australia. J Wildl Dis 29:219–229 [DOI] [PubMed] [Google Scholar]
- 29.Smith PC, Nucifora M, Reuter JD, Compton SR. 2007. Reliability of soiled bedding transfer for detection of mouse parvovirus and mouse hepatitis virus. Comp Med 57:90–96 [PubMed] [Google Scholar]
- 30.Stahl W. 1966. Experimental aspiculuriasis. II. Effects of concurrent helminth infection. Exp Parasitol 18:116–123 [DOI] [PubMed] [Google Scholar]
- 31.Steiner CM, Rozhon EJ, Lipton HL. 1984. Relationship between host age and persistence of Theiler's virus in the central nervous system of mice. Infect Immun 43:432–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugiyama T, Hashimoto K, Sasaki S. 1967. An adenovirus isolated from the feces of mice. II. Experimental infection. Jpn J Microbiol 11:33–42 [DOI] [PubMed] [Google Scholar]
- 33.Suto H, Zhang M, Berg DE. 2005. Age-dependent changes in susceptibility of suckling mice to individual strains of Helicobacter pylori. Infect Immun 73:1232–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki H, Kiatipattanasakul W, Kajikawa S, Tsutsui S, Nakayama H, Goto N, Doi K. 1997. Age-related changes in susceptibility of mice to low-virulent mouse hepatitis virus (MHV2-CC) infection. Exp Anim 46:211–218 [DOI] [PubMed] [Google Scholar]
- 35.Taffs LF. 1976. Pinworm infections in laboratory rodents: a review. Lab Anim 10:1–13 [DOI] [PubMed] [Google Scholar]
- 36.Thigpen JE, Lebetkin EH, Dawes ML, Amyx HL, Caviness GF, Sawyer BA, Blackmore DE. 1989. The use of dirty bedding for detection of murine pathogens in sentinel mice. Lab Anim Sci 39:324–327 [PubMed] [Google Scholar]
- 37.Whary MT, Cline JH, King AE, Hewes KM, Chojnacky D, Salvarrey A, Fox JG. 2000. Monitoring sentinel mice for Helicobacter hepaticus, H. rodentium, and H. bilis infection by use of polymerase chain reaction analysis and serologic testing. Comp Med 50:436–443 [PubMed] [Google Scholar]