Abstract
Tail biopsy in mice is a common procedure in genetically modified mouse colonies. We evaluated the anesthetic and analgesic effects of various agents commonly used to mitigate pain after tail biopsy. We used a hot-water immersion assay to evaluate the analgesic effects of isoflurane, ice-cold ethanol, ethyl chloride, buprenorphine, and 2-point local nerve blocks before studying their effects on mice receiving tail biopsies. Mice treated with ethyl chloride spray, isoflurane and buprenorphine, and 2-point local nerve blocks demonstrated increased tail-flick latency compared with that of untreated mice. When we evaluated the behavior of adult and preweanling mice after tail biopsy, untreated mice demonstrated behavioral changes immediately after tail biopsy that lasted 30 to 60 min before returning to normal. The use of isoflurane, isoflurane and buprenorphine, buprenorphine, 2-point nerve block, or ethyl chloride spray in adult mice did not significantly improve their behavioral response to tail biopsy. Similarly, the use of buprenorphine and ethyl chloride spray in preweanling mice did not improve their behavioral response to tail biopsy compared with that of the untreated group. However, immersion in bupivacaine for 30 s after tail biopsy decreased tail grooming behavior during the first 30 min after tail biopsy. The anesthetic and analgesic regimens tested provide little benefit in adult and preweanling mice. Given that tail biopsy results in pain that lasts 30 to 60 min, investigators should carefully consider the appropriate anesthetic or analgesic regimen to incorporate into tail-biopsy procedures for mice.
Abbreviation: HWI, Hot water immersion
Genetically modified mice continue to advance the study of human disease. To obtain DNA for verification of genotype, mice may undergo tissues sample collection from multiple sites, including blood, saliva, hair, and stool. However, tail biopsy of the distal 5 mm continues to be the preferred location of obtaining sufficient DNA for genotyping.14,19 Tail biopsy involves transecting multiple tissue types, including skin, nervous tissue, muscle, tendons, vasculature, cartilage, and bone,14 and this technique has the potential to cause short-term pain and distress. Several studies have demonstrated that tail biopsy without anesthesia causes short-term pain or distress that is greatest within the first 1 to 2 h but that may last as long as 5 h.1,4,20,26 Similar physiologic changes associated with pain and distress were apparent and prolonged in anesthetized control mice.1
Analgesic protocols at the time of biopsy vary among institutions. IACUC policies from several institutions3,10,18,28,29 recommend but do not require anesthesia or analgesia for tail biopsy of mice 10 to 21 d old but do require anesthesia or analgesia for older mice. The anesthetic regimens to mitigate the temporary pain associated with tail biopsy at these institutions include immersion in ice-cold ethanol, topical ethyl chloride spray, and isoflurane inhalant anesthesia. Because of their short duration of action, we hypothesized that these agents provide poor management of the postprocedural pain associated with tail biopsy, and we decided to explore other practical options. The use of traditional drugs such as lidocaine, bupivacaine, or buprenorphine may provide more appropriate analgesia for tail biopsy. Here we examined the anesthetic and analgesic effects on mouse behavior after tail biopsy in adult and preweanling outbred NSA mice that had been treated with various analgesics and anesthetics.
Materials and Methods
Mice.
Non-Swiss Albino (NSA) outbred mice from an inhouse breeding colony were used in this study. Mice were free of Sendai virus, mouse hepatitis virus, minute mouse virus, mouse parvovirus, mouse norovirus, Theiler murine encephalitis virus, rotavirus, Mycoplasma pulmonis, pinworms, and ectoparasites. Mice were housed 5 per group in individually ventilated caging (Thoren Caging System, Hazelton, PA), had unlimited access to a commercial rodent chow (Teklad Irradiated Diet 2918, Harlan Laboratories, Madison, WI) and filter-sterilized water, and were allowed to acclimate for 3 to 7 d before studies were initiated. All mice were maintained under a 12:12-h light:dark cycle at temperatures of 21 to 24 °C. All animal experiments were IACUC-approved.
Baseline hot-water immersion (HWI) assay of mice without tail biopsy.
To evaluate whether topical application of lidocaine or bupivacaine to a mouse's tail provides analgesia, 8 male NSA mice were randomized by body weight into 2 groups of 4. Analgesic effect was measured by using HWI test.25 Four baseline measurements of time (in seconds) until tail flick after immersion in a hot-water bath (51 °C) were obtained for each mouse; the first measurement was discarded, and the remaining 3 times averaged to obtain baseline responses. Treatments consisted of submerging each mouse's tail for 2 min in either 5 mM lidocaine (L7757, Sigma-Aldrich, St Louis, MO) or 15 mM bupivacaine base (Santa Cruz Biotechnology, Santa Cruz, CA) in DMSO. Measurements of tail-flick latency were taken at 2, 5, 10, 20, and 30 min after drug treatment. Measurements were normalized by dividing them by the average baseline response, and data were compared by using repeated-measures ANOVA (www.r-project.org). The effect of treatment in the repeated-measures ANOVA was assessed as the significance of the treatment × time interaction. When treatment × time differences were observed, Tukey HSD posthoc testing (R Project) was used to evaluate differences between groups. P values less than 0.05 were considered statistically significant.
HWI assay of mice without tail biopsy.
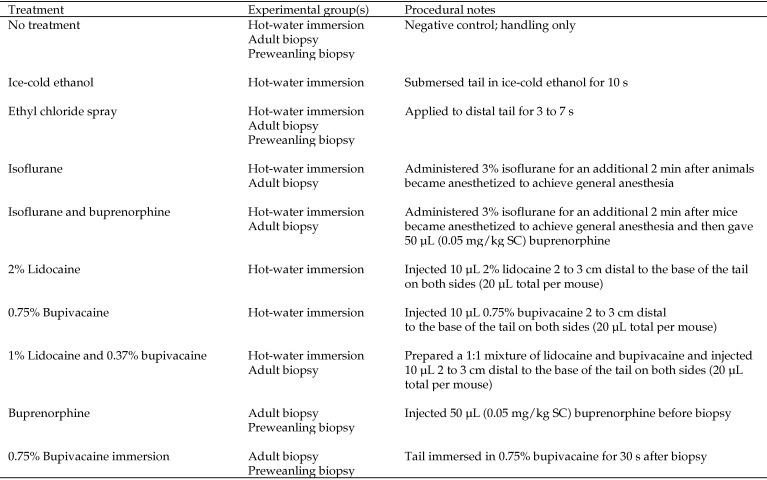
We randomized 35-day-old outbred NSA mice by body weight into 8 groups of 6 (3 male and 3 female mice each). Mice were treated with 1 of 8 anesthetics or analgesics as described in Figure 1. Topical anesthetics delivered via tail immersion was not assessed in this portion of the study due to their failure to provide appropriate analgesia in the preliminary HWI assay in unoperated, normal mice. Analgesic effect was measured by using the HWI test as described earlier. Treatment order was randomized. Measurements of tail-flick latency were taken at 0, 2, 5, 10, 20, 30, 45, 60, 90, 120, 150, 180, 210, and 240 min after treatment. Measurements for nerve-block groups concluded when analgesia dissipated. Response times greater than 15 s were considered maximal; tails then were withdrawn from the water to prevent tissue damage. Statistical analysis was performed as described for the preliminary HWI assay.
Figure 1.
Treatments and experimental groups.
Behavioral evaluation of adult mice after tail biopsy.
We randomized 45-day-old outbred NSA mice by body weight into 8 groups of 8 (4 male and 4 female mice per group). Treatments included 3% isoflurane delivered via vaporizer, 3% isoflurane and buprenorphine (0.05 mg/kg SC), buprenorphine (0.05 mg/kg SC), a 2-point nerve block of lidocaine and bupivacaine (MWI Veterinary Supply, Boise, ID), ethyl chloride spray (Med-Vet International, Mettawa, IL), a 30-s postbiopsy immersion in 0.75% bupivacaine, no treatment, and no biopsy (Figure 1). Ice-cold ethanol was not assessed during this portion of the study due to its failure to provide analgesia in the previous HWI assay. Mice were group-housed by sex in their home cages on the videotaping platform for at least 24 h before treatment. All treatments were conducted within the same 30 min of the light cycle. Mice were removed from their home cages once to receive both treatment and tail biopsy. Tail biopsies (5 mm) were taken, mice were returned to their home cage, and behaviors over time were videotaped from above the cage. Video footage was scored at 0, 0.5, 1, 2, 3, and 6 h after biopsy by an observer blinded to treatment. Each mouse was scored for 5 min during each observation period. Behaviors scored as indicators of pain or distress included tail grooming, twitching, writhing, circling, tail flicking, and staggering.21 Exploratory behaviors (normal gait, rearing, and climbing) and other behaviors (head and body grooming, eating, nesting or rooting in bedding, and social interactions such as aggression and examining cagemates) were also scored . The frequency of each behavior was tallied. The untreated control group was included to differentiate behavioral changes associated with the procedure compared with the potentially confounding behavioral effects of the analgesics and anesthetics and their effectiveness at mitigating pain after tail biopsy. Repeated-measures ANOVA was performed by using the statistical program R (www.r-project.org). When treatment × time differences were observed, Tukey HSD posthoc testing was used. P values less than 0.05 were considered statistically significant. Because preliminary analyses of anesthetic and analgesic found no differences between sexes, we combined these data for subsequent analysis.
Behavioral evaluation of preweanling mice after biopsy.
Four litters of 17-d-old outbred NSA mice were randomized into 5 groups of 8; each litter had 2 mice (one male, one female) assigned to each treatment. Treatments included buprenorphine (0.05 mg/kg SC), ethyl chloride spray, 30-s immersion in 0.75% bupivacaine after biopsy, no treatment, and no biopsy. Because one male pup died on arrival, the no-biopsy group had a group size of 7 mice. Mice were group-housed with their dam and littermates in their home cages, which were placed on the videotaping platform for at least 24 h prior to treatment. Handling, biopsy, videotaping, and scoring were performed as described previously for adult mice.
Results
Preliminary HWI assay with tail-submersion anesthesia in nonbiopsied mice.
The anesthetic effectiveness of submerging mouse tails in 5 mM lidocaine or 15 mM bupivacaine for 2 min was evaluated by using the HWI test. The average baseline responses for lidocaine and bupivacaine treatment were 3.8 and 4.6 s, respectively. The lidocaine-treated mice became hyperalgesic immediately after treatment, and their tail-flick latencies returned to baseline measurements by 5 min after treatment (P < 0.01). Neither of the topical treatments evaluated (5 mM lidocaine or 15 mM bupivacaine) prolonged the tail flick latency time. We therefore discarded this route of administration as a treatment in subsequent studies.
HWI assays after various anesthetics and analgesics in nonbiopsied mice.
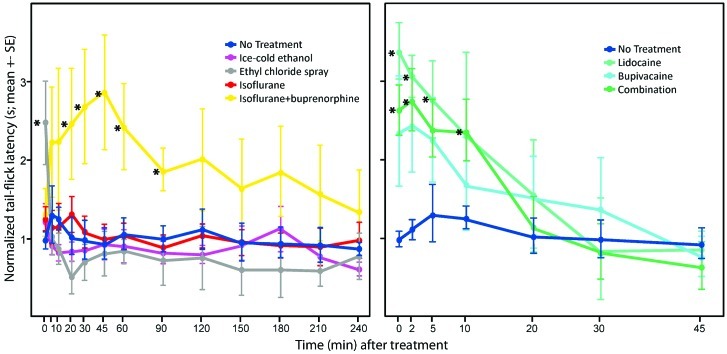
Only 2-point nerve block treatments and isoflurane and buprenorphine resulted in tail-flick latencies that were significantly (P < 0.05) higher than those found with no treatment (Figure 2). Isoflurane and buprenorphine had the longest duration of effect (3 to 4 h), and was significant for up to 90 min into the experiment (P < 0.05, posthoc test). The 2-point nerve blocks exhibited greater tail flick latency than the no treatment group from 0 to 10 min (posthoc tests P < 0.05), and did not differ from control mice beyond 10 min, and wore off after 10 to 20 min. There was an initial increased latency for ethyl-chloride spray treated mice, that dissipated within 5 min (P < 0.05, Figure 2), but did not differ from the no treatment control after 5 min. There was no significant difference between isoflurane and ice-cold ethanol compared with the no treatment control.
Figure 2.
Effect of treatment on hot-water–immersion tail-flick latencies (mean ± SE) of mice without tail biopsy. (A) Mice treated with isoflurane with and without buprenorphine, ice cold ethanol, and ethyl chloride spray. (B) Mice received a 2-point nerve block treatment at the base of the tail. There were significant (repeated-measures ANOVA, P < 0.05 for both panels) effects of treatments over time. Ethyl chloride spray resulted in an immediate increase in tail-flick latency, which was significant at the initial time point but did not differ from that for isoflurane, ice-cold ethanol, ethyl chloride spray, and no treatment thereafter. Isoflurane and buprenorphine increased tail-flick latency for 30 to 90 min post tail biopsy, and the 2-point nerve blocks resulted in increased tail flick latency for as long as 10 min after tail biopsy. *, Value significantly (P < 0.05, Tukey post hoc testing) different compared with that for the no-treatment groups.
Behavioral evaluation of mice after biopsy.
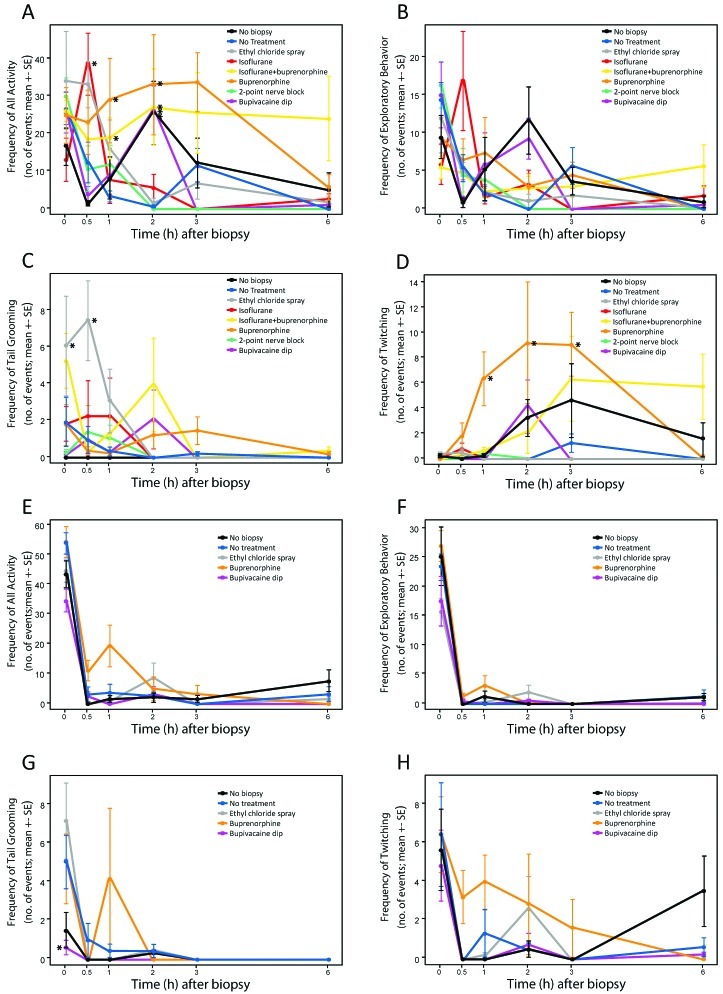
To determine the efficacy of anesthetics and analgesics after tail biopsy, groups of mice received 1 of 6 treatments as outlined in Figure 1. Two additional groups included no-biopsy and no-treatment mice to serve as controls. Adult mice that had undergone tail biopsy were most active during the first 2 h after handling or treatment and generally became less active over time (P < 0.001, Figure 3 A). Biopsied mice treated with isoflurane were more (P < 0.05, posthoc test) active at 30 min than was the no-treatment group, but these groups did not differ thereafter. Mice treated with buprenorphine, isoflurane and buprenorphine, or bupivacaine immersion after biopsy and those that received no biopsy were more (P < 0.05, posthoc tests) active at 2 h than was the no-treatment group, but all groups did not differ thereafter. The 2 groups that received buprenorphine treatment tended to exhibit greater (P < 0.001) activity on average than did the other treatment groups (Figure 3 A). Exploratory behaviors waned significantly (P < 0.001) over time, and there was no significant difference between groups (Figure 3 B). We considered that the primary behaviors indicative of potential pain or distress were tail grooming and twitching (Figure 3 C and D). Other behaviors associated with pain in rodents (writhing, circling, tail flicking, and staggering) were infrequent.
Figure 3.
Behavioral evaluations (mean ± SE) of mice after tail biopsy. Overall activity includes exploratory behaviors, tail grooming, and twitching. Exploratory behaviors include normal gait, rearing, and climbing. Adult mice are shown in panels A through D; preweaning mice are shown in panels E through H. (A) Treatment × time effect shows that buprenorphine treatment, with or without isoflurane, increased overall activity over time (P < 0.001, repeated-measures ANOVA). Mice treated with buprenorphine had increased activity at 1 to 2 h after tail biopsy. Isoflurane resulted in increased activity at 30 min after tail biopsy, and the no-biopsy control mice had increased activity at 2 h after the procedure. (B) Regardless of treatment, exploratory behaviors declined over time, with no difference among treatment groups. (C) The effect of treatments on tail grooming differed over time (P < 0.05, repeated-measures ANOVA). Ethyl chloride spray increased initial tail grooming behavior significantly during the first 30 min after tail biopsy, whereas local 2-point nerve block and bupivacaine immersion initially tended to decrease tail grooming behavior. (D) Twitching behavior did not differ between treatments, except for buprenorphine, which caused an increase in twitching behavior (P < 0.001, repeated-measures ANOVA) at 1 to 3 h after tail biopsy. (E) Overall activity and (F) exploratory behaviors decreased over time in all preweanling treatment groups, but no difference between treatments was seen. (G) There were significant (P < 0.05, repeated-measures ANOVA) differences among treatments over time on tail grooming behavior in preweaning mice. Frequency of tail grooming initially was decreased for the bupivacaine immersion and no-biopsy groups. After the initial time point, treatments did not differ. (H) Frequency of twitching behavior decreased over time, and buprenorphine caused an increase in twitching behavior relative to other treatments (P < 0.05, repeated-measures ANOVA). *, Value significantly (P < 0.05, Tukey post hoc testing) compared with that of the no-treatment group.
Patterns of tail grooming and twitching behaviors differed from each other; therefore, these 2 behaviors were analyzed separately. There was a significant (P < 0.001) effect of treatment over time for tail grooming (Figure 3 C). Tail grooming in biopsied adult mice that received isoflurane, bupivacaine immersion, or the 2-point nerve block did not differ from that in the no-treatment group throughout the experiment. Mice treated with ethyl chloride spray groomed their tails more (posthoc tests P < 0.05) often during the first 30 min after treatment than did other treatment groups, and they did not differ from the no-treatment group thereafter. The frequency of tail grooming when mice were treated with buprenorphine or isoflurane and buprenorphine varied somewhat but did not differ significantly from that of the no-treatment group. Twitching behaviors were increased markedly (P < 0.001) in buprenorphine-treated mice compared with the other treatment groups (Figure 3 D). Mice treated with buprenorphine twitched more (P < 0.05, posthoc tests) between 1 and 3 h than did the no-treatment group. Differences among other groups were not significant over time.
Behavioral evaluation of preweanling mice after biopsy.
The effects of anesthetic and analgesic treatments after tail biopsy were evaluated in 17-d-old preweanling mice. In light of the results from adult mice, the isoflurane and isoflurane and buprenorphine groups were not assessed in preweanling mice. In addition, the 2-point nerve block was not assessed in preweanling mice because it did not show effectiveness in adult mice and because it is impractical in a research environment. Preweanling mice received 1 of 3 treatments: ethyl chloride spray, buprenorphine, and bupivacaine immersion. No-treatment and no-biopsy control groups were included. Except for the buprenorphine group, preweanling mice became mostly inactive within 30 min after handling and treatment (P < 0.001, Figure 3 E). Exploratory behaviors of preweanling mice decreased (P < 0.01) over time for all treatments, and there was no significant difference among the treatments over time (Figure 3 E and F); compared with all other groups, buprenorphine-treated preweanling mice showed a trend toward increased behaviors for all activities during the first hour (Figure 3 E). There was a significant effect of treatment over time on tail grooming (P < 0.05, Figure 3 G). Preweanling mice treated with ethyl chloride and buprenorphine groomed their tails as frequently as did mice that received no treatment. Preweanling mice treated with bupivacaine immersion after biopsy groomed their tail significantly (P < 0.05, posthoc test) less than did the no-treatment group but did not differ from the no-biopsy group. Twitching behaviors in preweanling mice were similar to those of adult mice, with buprenorphine treatment resulting in increased (P < 0.05) twitching over time compared with that of all other groups (Figure 3 H). Except for grooming or twitching in mice that underwent bupivacaine immersion, none of the treatment groups showed any significant, lasting effect on tail grooming or twitching.
Discussion
Although tail biopsy continues to be the preferred method for DNA analysis of genetically modified mice, the need and timing for anesthesia or analgesia to manage the potential pain and distress associated with the procedure remain uncertain. Most institutions require anesthesia for the procedure in mice older than weaning age but not typically prior to that age. Here we evaluated several anesthetics and analgesics to determine a rapid, practical, and effective pain management regimen for this common procedure.
The initial phase of this study determined whether simple submersion in local anesthetics would provide anesthesia for tail biopsies. Mice whose tails were pretreated topically by submersion in a solution of lidocaine, bupivacaine, or their combination did not seem to receive sufficient penetration into the tail tissues, and this technique provided insufficient analgesia in the HWI assay. This submersion technique might be enhanced by using iontophoresis to increase the penetration of local anesthetics to deeper tissues (maximum, 5 mm).7 This method uses a small electrical charge to actively transport drug across the skin; however, this procedure is impractical in a large production facility and was not pursued. Because the HWI assay was less invasive and less traumatic than was the actual tail biopsy, submersion in local anesthetics was not evaluated for efficacy after tail biopsy.
The effects of the common anesthetics and analgesics used for pain mitigation after tail biopsy were examined using the HWI assay. These common regimens include isoflurane, isoflurane and buprenorphine, ethyl chloride spray, and ice-cold ethanol. With the exception of buprenorphine as an adjunct to isoflurane anesthesia, these regimens did not control the potential pain after biopsy, because they rapidly lost anesthetic or analgesic effectiveness. Isoflurane administered for 2 min had no effect on tail-flick latency after recovery from anesthesia. This outcome is likely the result of the rapid elimination of isoflurane. When buprenorphine was administered in combination with 2 min of isoflurane, tail-flick latency was prolonged and lasted 3 to 5 h. This finding is consistent with other algesiometric studies that have found an effective duration of buprenorphine that lasted 3 to 5 h;11 we therefore further evaluated buprenorphine in biopsied mice. The onset of action of buprenorphine was not immediate, and if given simultaneously with a volatile anesthetic, might result in recovery from anesthesia prior to the onset of action of buprenorphine (Figure 2). Therefore, we recommend that buprenorphine is given 20 to 30 min prior to tail biopsy. The HWI assay demonstrated an initial latency when ethyl chloride spray was used, and this effect dissipated within 5 min (data not shown). Warming of the tail likely resulted in dissipation of the cryoanalgesic effects. Similar to isoflurane, ice-cold ethanol treatment failed to increase tail-flick latency. This finding may have been the result of warming of the tail or of the inadequacy of ice-cold ethanol as a cryoanalgesic. Other than IACUC policies that use ice-cold ethanol immersion for tail biopsy anesthesia, an Internet search yielded no references to its anesthetic properties, nor were there any references to the anesthetic properties of ice-cold ethanol after a PubMed search. A single reference suggests the use of ice-cold ethanol to test nociception during the evaluation of the actions of opioid analgesics.30 In addition, ice-cold ethanol was ineffective in the HWI assay, and we evaluated it no further.
We also evaluated local nerve blocks with lidocaine, bupivacaine, and their combination. Administering local anesthesia via a 2-point nerve block reliably provided measurable anesthesia in the HWI assay for 10 to 20 min, regardless of whether the nerve block was performed using lidocaine, bupivacaine, or their combination.
We evaluated several treatments to assess their effectiveness after tail biopsy in adult mice. Although isoflurane and ethyl chloride showed minimal effects in the HWI assay in unbiopsied mice, we evaluated these agents further because isoflurane is commonly available and used for mouse anesthesia and because the minimal effect of ethyl chloride, a cryoanalgesic, may have been due to warming from the water in the HWI. We then evaluated the effectiveness of the 2-point nerve block by using a combination of lidocaine and bupivacaine, with lidocaine providing rapid relief and bupivacaine prolonging the duration of action.
The effects of different anesthesia and analgesic regimens were assessed in 42-d-old mice. We used mature mice because ossification of the tail bones and maturation of sensory nerve fibers have been completed.14 In addition to the treatments mentioned earlier, the analgesic effects of 30-s immersion in bupivacaine and buprenorphine administered as a sole agent were evaluated. These regimens were added because the effects of isoflurane seemed to be negligible in the HWI assay.
Isoflurane administered without buprenorphine to mice at the time of tail biopsy increased the frequencies of tail grooming and other behaviors for at least 60 min after biopsy. These results suggest that although isoflurane may have resulted in sufficient anesthesia at the time of biopsy, it had little lasting effect and may have led to postanesthesia distress or anxiety. This result is similar to other studies that have found minimal benefit to the use of isoflurane or other inhalant anesthetics for tail biopsy. Evaluation of the effects of tail biopsy with and without methoxyflurane anesthesia in 12-wk-old mice demonstrated that tail biopsy with anesthesia induced a prolonged increase in heart rate, body temperature, and activity that returned to baseline 1 to 2 h after tail biopsy compared with tail biopsy without anesthesia.1 A more recent evaluation of the effects of isoflurane on mice that received tail biopsy also demonstrated minimal benefit to using the anesthetic.13 In these cited studies, BALB/c and C57BL/6 mice of various ages (21 to 45 d) were anesthetized prior to tail biopsy, and then their behavior was assessed through acute observations, an elevated plus-maze test of anxiety-like behavior, and locomotor activity as a measure of physical health and coordination. Their results demonstrated that biopsy with isoflurane anesthesia increased some behaviors including tail grooming and exploration in 42- to 45-d-old C57BL/6 mice at 10 min after tail biopsy, which was a common trend among all ages. The exception was 42- to 45-d-old BALB/c mice, in which anesthesia reduced the frequency of these behaviors. In the elevated plus-maze test, both strains of mice exhibited anxiety-like behavior that was enhanced with anesthesia, and both strains, regardless of age, exhibited decreased activity when given anesthesia. Anesthesia with isoflurane likely would be sufficient for the biopsy procedure itself, but because it is eliminated rapidly via respiration, it would not provide any analgesia during the recovery phase.
When buprenorphine was administered at the time of tail biopsy (with or without isoflurane), it failed to reduce tail grooming and increased other activities. Buprenorphine has been shown to cause increased activity in pain-free rats6,9,17,21 and mice,6,15,16 and we similarly found increased activity in buprenorphine-treated mice that lasted at least 6 h. Unlike buprenorphine in rats,22 buprenorphine increased twitching in mice. In addition, twitching may be normal in mice, given that the no-biopsy group showed increased twitching over time. This behavior therefore may be an inappropriate indicator of pain or distress in mice when buprenorphine has been administered. These behavioral changes may have been dose-related and not necessarily an adverse finding for the tail-biopsy procedure.
Local anesthetics were ineffective in alleviating pain associated with tail biopsy. Topical ethyl chloride spray provided temporary topical anesthesia that dissipated within a few minutes and resulted in an increase in tail grooming behavior for as long as 60 min when compared with that of other groups. This outcome may have been the result of inadequate anesthesia or due to some other sensory perception that the mice explored, including potential pain. The nociceptive properties of ethyl chloride are used to determine the efficacy and distribution of peripheral nerve blocks.24 Other risks associated with the use of ethyl chloride include frostbite when applied incorrectly and potentially painful thawing, and the compound is flammable and carcinogenic.23 Furthermore, ethyl chloride reportedly is used to provide cryoanalgesia in pediatric patients for venipuncture; however, the efficacy of this practice is controversial.5,27 These properties and the results of the current study suggest that ethyl chloride spray, as recommended by many institutions, provides inadequate anesthesia and may have some very serious drawbacks to its routine use.
The other local anesthetics we assessed were a 2-point nerve block of lidocaine and bupivacaine before tail biopsy and immersion in bupivacaine after biopsy. Neither treatment was effective after tail biopsy in adult mice. Both treatments increased tail grooming at 30 min in adult mice compared with nontreated controls, suggesting that the treatments were either ineffective or resulted in some other sensory perception. The 2-point nerve block may be impractical in a large production facility, because this procedure requires considerable mouse handling and restraint. Previous studies have demonstrated that handling of mice is a significant stressor that alters heart rate, body temperature, and motor activity as measured by telemetry and that has similar effects on these parameters as does tail biopsy, which resulted in physiologic changes that lasted only 1 h.1 The use of other topical treatments including bupivacaine and ethyl chloride spray has been demonstrated to be ineffective to manage pain after tail biopsy in preweanling mice.2 We similarly found no appreciable effect of local anesthetics on ameliorating biopsy pain in adult mice.
The current studies were performed on mice with mature vertebrae. A recent study has shown that between 17 to 31 d of age, the vertebrae of the mouse tail undergo maturation and ossification,14 which findings are consistent with previous data demonstrating ossification before weaning.8,12 Because isoflurane, isoflurane and buprenorphine, and 2-point nerve block did not appear to have an effect in adult mice, we did not evaluate them in preweanling mice, because ossification and sensory nerve development would be more advanced in mature adults compared with preweanling mice. Tail biopsy was performed in 17-d-old preweanling mice treated with ethyl chloride spray, buprenorphine, or bupivacaine immersion, and behavior after tail biopsy was evaluated. The ethyl chloride spray and buprenorphine treatment groups had an increase in tail grooming behavior compared with that of no-biopsy controls. Immersion in bupivacaine after tail biopsy resulted in reduced tail grooming that lasted for 30 min. This result in preweanling mice is likely due to enhanced absorption of anesthetic through the tissues compared with that in adult mice. Nonetheless, after 30 min, there did not appear to be anesthetic or analgesic effects on behavior, suggesting that these regimens did not provide any sustained benefit to mice undergoing tail biopsy.
Given the onset of ossification prior to weaning and the presence of sensory nerve fibers, the potential for tail biopsy to be a painful procedure seems obvious. A few other studies have evaluated the behavioral response of mice after tail biopsy. A pilot study to assess mouse behavior after tail biopsy in 6-wk-old C57BL/6 mice found reduced eating, drinking and activity for as long as 5 h after tail biopsy.20 Similarly, 20-d-old C57BL/6 mice were observed to have decreased climbing activity at 30 min after tail amputation compared with that of mice of the same age that had not undergone tail amputation.26 These studies, as well as some of the others cited earlier, suggest that tail biopsy alters the physiologic and behavioral responses of mice.
The current study found that behavioral changes indicative of pain or distress, specifically tail grooming, subsided within 30 min and returned to normal within 60 min in both adult and 17-d-old mice for all treatment groups, including the untreated group. The exception was adult mice treated with ethyl chloride spray; these mice had an unexpected increased frequency of tail grooming for the first 60 min. In light of these observations, it appears that the pain associated with tail biopsy lasts 30 to 60 min. The anesthetic and analgesic regimens tested apparently provided little benefit in adult or preweanling mice that had undergone tail biopsy. However, the pain associated with the biopsy itself, the effects of recovery from anesthesia, and the potential long-term changes should be considered and the best management approach overall determined. Our results suggest that the most effective and practical treatment to manage pain in mice after tail biopsy was buprenorphine or immersion in bupivacaine. Although the effects lasted less than 30 min, a 30-s bupivacaine immersion after tail biopsy appeared to relieve the pain associated with the procedure in preweanling mice. The optimal concentration, duration, and composition of topically applied bupivacaine after biopsy require further evaluation to decrease the exposure time. In addition, buprenorphine given prior to tail biopsy may provide relief, owing to its known analgesic properties.
Acknowledgments
We are grateful to Dr Susan VandeWoude, Dr Winona Burgess, and Elisa French for their assistance. This work was supported by the Association for the Assessment and Accreditation of Laboratory Animal Care International Veterinary Student Summer Internship Program.
References
- 1.Arras M, Rettich A, Seifer B, Kasermann HP, Rulicke T. 2007. Should laboratory mice be anaesthetized for tail biopsy? Lab Anim 41:30–45 [DOI] [PubMed] [Google Scholar]
- 2.Braden-Weiss GC, Brice AK, Hankenson FC. 2011. Minimizing the impact of tail biopsy in preweanling laboratory mice: inhaled isoflurane compared with topical anesthetics. J Am Assoc Lab Anim Sci 50:736–737 [Google Scholar]
- 3.Case Western Reserve University School of Medicine Institutional Animal Care and Use Committee. [Internet]. 2011. CASE IACUC policies. [Cited 05 December 2011]. Available at: http://casemed.case.edu/ora/iacuc/policies/tail_biopsy.cfm.
- 4.Cinelli P, Rettich A, Seifert B, Bürki K, Arras M. 2007. Comparative analysis and physiological impact of different tissue biopsy methodologies used for the genotyping of laboratory mice. Lab Anim 41:174–184 [DOI] [PubMed] [Google Scholar]
- 5.Costello M, Ramundo M, Christopher NC, Powell KR. 2006. Ethyl vinyl chloride vapocoolant spray fails to decrease pain associated with intravenous cannulation in children. Clin Pediatr (Phila) 45:628–632 [DOI] [PubMed] [Google Scholar]
- 6.Cowan A, Doxey JC, Harry EJ. 1977. The animal pharmacology of buprenorphien, an oripavine analgesic agent. Br J Pharmacol 60:547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Draper DO, Coglianese M, Castel C. 2011. Absorption of iontophoresis-driven 2% lidocaine with epinephrine in the tissues at 5 mm below the surface of the skin. J Athl Train 46:277–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feik SA, Storey E. 1983. Remodelling of bone and bones: growth of normal and transplanted caudal vertebrae. J Anat 136:1–14 [PMC free article] [PubMed] [Google Scholar]
- 9.Flecknell PA, Liles JH. 1992. Evaluation of locomotor activity and food and water consumption as a method of assessing postoperative pain in rodents, p 482–488 In: Short CE, Van Poznak A. Animal pain. New York (NY): Churchill Livingstone [Google Scholar]
- 10.Florida State University Animal Care and Use Committee. [Internet]. 2011. FSU ACUC guidelines and policies. [Cited 05 December 2011]. Available at: http://www.research.fsu.edu/acuc/Policies_Guidelines.html.
- 11.Gades NM, Danneman PJ, Wixson SK, Tolley EA. 2000. The magnitude and duration of the analgesic effect of morphine, butorphanol, and buprenorphine in rats and mice. Contemp Top Lab Anim Sci 39:8–13 [PubMed] [Google Scholar]
- 12.Garrard M, Harrison GA, Weiner JS. 1974. Genotypic differences in the ossification of 12-day-old mice at 23 °C and 32 °C. J Anat 117: 531–539 [PMC free article] [PubMed] [Google Scholar]
- 13.Hankenson FC, Braden Weiss GC, Blendy JA. 2011. Behavioral and activity assessment of laboratory mice (Mus musculus) after tail biopsy under isoflurane anesthesia. J Am Assoc Lab Anim Sci 50:686–694 [PMC free article] [PubMed] [Google Scholar]
- 14.Hankenson FC, Garzel LM, Fischer DD, Nolan B, Hankenson KD. 2008. Evaluation of tail biopsy collection in laboratory mice (Mus musculus): vertebral ossification, DNA quantity, and acute behavioral responses. J Am Assoc Lab Anim Sci 47:10–18 [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes KE, Raucci JA, Jr, Gades NM, Toth LA. 2000. An evaluation of analgesic regimens for abdominal surgery in mice. Contemp Top Lab Anim Sci 39:18–23 [PubMed] [Google Scholar]
- 16.Kuribara H, Uchihashi Y. 1994. Interactions of opioids with caffeine: evaluation by ambulatory activity in mice. J Pharm Pharmacol 46:141–144 [DOI] [PubMed] [Google Scholar]
- 17.Liles JH, Flecknell PA. 1992. The effects of buprenorphine, nalbuphine and butorphanol alone or following halothane anaesthesia on food and water consumption and locomotor movement in rats. Lab Anim 26:180–189 [DOI] [PubMed] [Google Scholar]
- 18.National Institutes of Health Office of Animal Care and Use. [Internet]. 2010. ARAC guidelines. [Cited 05 December 2011]. Available at: http://oacu.od.nih.gov/ARAC/index.htm.
- 19.Pinkert CA. 2003. Transgenic animal technology: alternatives in genotyping and phenotyping. Comp Med 53:126–139 [PubMed] [Google Scholar]
- 20.Puustinen K. 2004. Evaluation of the tail biopsy procedure on the behaviour and wellbeing of mice—a pilot study. [Dissertation]. Uppsala (Sweden): Uppsala University [Google Scholar]
- 21.Roughan JV, Flecknell PA. 2000. Effects of surgery and analgesic administration on spontaneous behaviour in singly housed rats. Res Vet Sci 69:283–288 [DOI] [PubMed] [Google Scholar]
- 22.Roughan JV, Flecknell PA. 2002. Buprenorphine: a reappraisal of its antinociceptive effects and the therapeutic use in alleviating postoperative pain in animals. Lab Anim 36:322–343 [DOI] [PubMed] [Google Scholar]
- 23.RxList The Internet Drug Index. [Internet] 2011. Ethyl chloride. [Cited 25 November 2011]. Available at: http://www.rxlist.com.
- 24.Sapir DA. 1995. Cutaneous application of ethyl chloride spray. Reg Anesth 20:260. [PubMed] [Google Scholar]
- 25.Sewell RD, Spencer PS. 1976. Antinociceptive activity of narcotic agonist and partial agonist analgesics and other agents in the tail immersion test in mice and rats. Neuropharmacology 15:683–688 [DOI] [PubMed] [Google Scholar]
- 26.Sørensen DB, Stub C, Jensen HE, Ritskes-Hoitinga M, Hjorth P, Ottesen JL, Hansen AK. 2007. The impact of tail-tip amputation and ink tattoo on C57BL/6JBomTac mice. Lab Anim 41:19–29 [DOI] [PubMed] [Google Scholar]
- 27.Soueid A, Richard B. 2007. Ethyl chloride as a cryoanalgesic in pediatrics for venipuncture. Pediatr Emerg Care 23:380–383 [DOI] [PubMed] [Google Scholar]
- 28.University of Kentucky ORI-IACUC. [Internet]. 2011. IACUC policies, procedures, and guidelines. [Cited 05 December 2011]. Available at: http://www.research.uky.edu/dlar/TissueCollectionForGeneticIdentificationofRodents.mht.
- 29.UT Health Science Center San Antonio. [Internet]. 2011. IACUC policies—identification and genotyping. [Cited 05 December 2011]. Available at: http://research.uthscsa.edu/iacuc/identification.shtml.
- 30.Wang JJ, Ho ST, Hu OY, Chu KM. 1995. An innovative cold tail-flick test: the cold-ethanol tail-flick test. Anesth Analg 80:102–107 [DOI] [PubMed] [Google Scholar]