Abstract
Buprenorphine HCl is a common analgesic for laboratory mice undergoing surgical procedures. The documented duration of action of buprenorphine HCl is as short as 3 to 5 h in mice, potentially necessitating readministration for continued analgesia. A long-acting buprenorphine formulation would reduce handling-associated stress and provide uninterrupted analgesia. This study used the hot-plate assay to assess the antinociceptive effects of a single injection of sustained-release buprenorphine (bup-SR), buprenorphine-HCl (bup-HCl), and saline over 72 h in young adult male BALB/cJ and SWR/J mice. SWR/J mice had shorter baseline latencies than did BALB/cJ mice, possibly reflecting greater sensitivity to thermal nociception. Relative increase from baseline latency (% maximal possible effect) was significant for buprenorphine-SR at 2, 6, and 12 h compared with saline. According to results from a hot-plate assay, the analgesic efficacy of buprenorphine-SR appears to last at least 12 h in male BALB/cJ and SWR/J mice.
Abbreviation: %MPE, % maximal possible effect; bup-HCl, buprenorphine hydrochloride; bup-SR, sustained-release buprenorphine
Analgesia of adequate efficacy and duration is necessary for laboratory animals undergoing painful procedures. In a survey of experiments reported in biomedical journals, buprenorphine was the analgesic used most often in rodents that underwent surgery.16 Buprenorphine is advantageous because it is relatively long-acting, has low potential for abuse, and has been studied extensively in mice and rats.9 Although longer-acting than other opioids, buprenorphine still can require repeated restraint and reinjection for full postsurgical analgesia, creating stress for animals and an inconvenience for personnel. A longer-acting buprenorphine formulation would overcome these drawbacks.
Buprenorphine HCl (bup-HCl) is an opioid and acts as a partial agonist at the μ-opioid receptor and as an antagonist at the κ-opioid receptor. Although its maximal analgesic effect is not as great as that of the complete μ-opioid agonist morphine,8 buprenorphine's slow dissociation from the μ-opioid receptor prolongs its activity.10 However, estimates of the buprenorphine's duration of action vary among studies, perhaps due to differences in dosage, nociceptive stimuli, and measured indicators of efficacy. One study concluded from tail-flick and hot-plate assays that buprenorphine's duration of action at a dosage of 2.0 mg/kg SC is 3 to 5 h in mice.8 Other investigators found that buprenorphine (3.0 mg/kg IP) provided marked antinociception in the tail-flick test for as long as 8 h in mice.11 Both studies prompt the concern that a single dose of buprenorphine at the end of the workday might not provide sufficient overnight analgesia for mice that have undergone major surgery.
A sustained-release formulation of buprenorphine would benefit both laboratory animals and researchers by providing consistent, long-lasting analgesia without the need for redosing. A veterinary compounding pharmacy has developed an injectable, patent-pending formulation of sustained-release buprenorphine (bup-SR). In laboratory rats, bup-SR appears to remain at therapeutic plasma levels for 72 h and to provide analgesia for the same duration in thermal nociception and surgical postoperative pain models.7 Similar duration and efficacy have been reported in a study of feline clinical patients.3 However, no published studies that compare bup-SR with bup-HCl in laboratory mice are available currently.
Opioids decrease thermal sensitivity in animals. Compare with untreated mice, mice treated with opioids have greater latency on thermal sensitivity tests, including the hot-plate and tail-flick tests, that is, opioid-treated mice are slower to perceive heat stimuli as unpleasant and to move away from them. As the opioid effect subsides, mice return to their normal preopioid thermal sensitivity. Although latency during the hot-plate assay may not translate directly into precise dosage recommendations for postsurgical analgesia in mice, these data are appropriate for preliminary comparisons of duration-of-action of bup-SR and conventional bup-HCl.
The current study examined thermal latencies in 2 inbred strains of mice, BALB/cJ and SWR/J, treated with 2 formulations of buprenorphine or with saline placebo. Two strains were used because mice exhibit interstrain differences in sensitivity to noxious stimuli and opioid analgesics.13 BALB/cJ mice are moderately responsive to opioids, whereas SWR/J mice are exceptionally sensitive.12 We hypothesized that bup-SR would have a longer duration of action than would bup-HCl or saline in both strains.
Materials and Methods
Animal care and use program.
The protocol was approved by the IACUC at University of California San Francisco, as part of an AAALAC-accredited care and use program. Mice were housed singly in ventilated cage racks (Lab Products, Seaford, DE) with UV-sterilized, filtered, dechloraminated, purified water. They were fed irradiated commercial chow (PicoLab Mouse Diet 20 5058, PMI LabDiet, Brentwood, MO) and maintained on paperchip bedding (Sheppard Specialty Paper, Richland MI) with nesting pads (Nestlets, Ancare, Bellmore, NY) and toilet paper rolls for nesting material. Room conditions were 12:12-h light:dark cycle, ambient temperature of 68 to 70 °F (20.0 to 21.1 °C), and ambient humidity of 30% to 40%. Cage changes occurred every other week, 7 to 8 d before injections were given. Injection sites were monitored daily during data collection. Throughout the study, sentinels in a soiled-bedding sentinel program were negative for the following pathogens: epizootic diarrhea of infant mice, mouse hepatitis virus, mouse parvoviruses, Theiler murine encephalomyelitis virus, Mycoplasma pulmonis, pneumonia virus of mice, Sendai virus, fur mites, and pinworms.
Animals and acclimation.
Subjects were 8 male BALB/cJ and 7 male SWR/J mice (Jackson Laboratories, Bar Harbor, ME). The mice were 13 wk old at the start of the 5-wk data collection period. During the first of 3 wk of acclimation, each mouse was handled and restrained 3 times, for 1 to 3 min per session, and was placed on the nonheated testing apparatus for 1 min. During the remainder of the acclimation period, each mouse received a saline injection and was tested on the heated testing apparatus, with the schedule of baseline latencies and postinjection time points in accordance with the experimental protocol. The data collected during the acclimation period were not included in the analysis.
Treatment groups and pharmaceutical compounds.
For the experimental period, mice were stratified by strain and divided randomly into treatment groups (3 groups per strain) and then received each of the 3 treatments in a cross-over design. Total sample size was 7 or 8 mice per strain per treatment. Each mouse was assigned a distinct study identification number before each treatment.
Two buprenorphine formulations were evaluated. Bup-HCl was obtained in a 0.3-mg/mL formulation from a veterinary compounding pharmacy (Diamondback Drugs, Scottsdale, AZ). Bup-SR is a proprietary patent-pending formulation provided at a concentration of 1.0 mg/mL in a solvent containing N-methyl-2-pyrrolidone (ZooPharm, Fort Collins, CO). Treatment consisted of a subcutaneous injection (0.5-mL syringe, 28-gauge needle) of one of bup-HCl (0.1 mg/kg), bup-SR (1.0 mg/kg), or sterile saline, with a 2-wk washout between treatments. In this way, mice were treated and tested on the hot plate during weeks 1, 3, and 5 only. Treatments were administered in one of the following sequences: saline, bup-HCl, and bup-SR; bup-HCl, bup-SR, and saline; or bup-SR, saline, and bup-HCl.
The concentration of bup-SR was 1 mg/mL, and bup-HCl was diluted from a stock concentration of 0.3 mg/mL to a final concentration of 0.1 mg/mL, so that the volume injected was 1 mL/kg for all treatments. Injections were administered at approximately 0900 by a handler who was blinded to strain and who was not involved in data collection. The skin of the scruff was tented on a restrained, unanesthetized mouse, and the injection was administered. In 2 instances, the contents of the injection spilled onto the subject's fur, and the injection was successfully given on the second attempt.
Hot-plate apparatus.
The assay used to test the antinociceptive effect of treatment was a thermal test, the hot-plate assay (Ugo Basile, Comerio, Italy). The aluminum plate was heated to 55 °C. A clear acrylic glass open-top cylinder of the same diameter as the plate contained the mouse. A video camera was directed at the subject and hot plate, and a mirror was placed on the opposite side of the cylinder (Figure 1).
Figure 1.
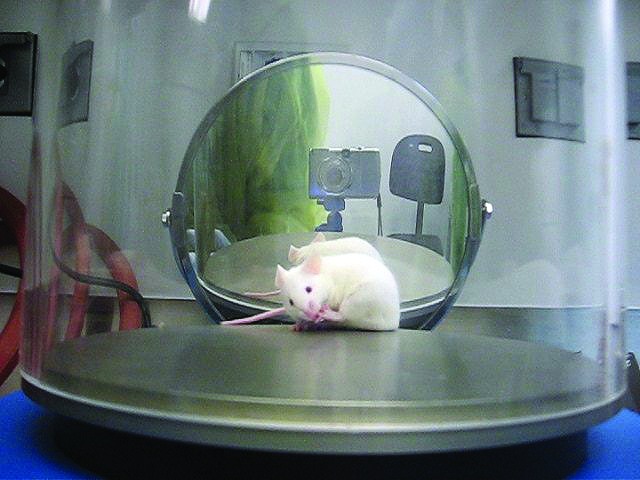
Hot-plate apparatus and endpoint behavior. Two observers were present during data collection, and a digital camera and mirror were set up to allow subsequent review of video recordings. This mouse is licking his hind paw, a nocifensive behavior.
Data collection.
Each mouse was placed on the plate and watched by 2 observers (who remained blind to strain and treatment) until the mouse displayed one of the following behaviors: licking a hindpaw (Figure 1), jumping, lifting a hindpaw (entire paw or toes only, without taking a step), or fluttering a hindpaw (shaking the paw while standing or stepping). The same observer controlled the timer pedal for all sessions. The mouse was removed from the plate upon showing any of these behaviors, or at the cut-off time of 30 s. Latency (time to endpoint behavior or time to cut-off) was recorded to the nearest 0.1 s. Videos of the testing sessions then were reviewed by one of the session observers to verify the observed latencies. If a nociceptive behavior missed by the observers was confirmed during viewing of the video, the recorded latency was adjusted accordingly.
The mice were tested 3 times before treatment (twice on the day before treatment, and once during the morning just before treatment) to establish the mean baseline latency for that week's data collection. In addition, mice were tested at 2, 6, 12, 24, 48, and 72 h after treatment. The observers did not participate in the injections and thus were blinded to the subjects’ strain and treatment. During the acclimation period, and again after all data collection was complete, the mice were placed on the unheated testing apparatus (21 °C) for 30 s, and their behaviors were observed.
Statistical analysis.
Baseline latencies for each strain were compared using an unpaired Student t test (normal data, equal variances). For this test, each mouse was represented by its mean baseline latency, determined by averaging the 9 baseline latencies measured over the course of the study.
For subsequent analysis, latencies were converted to percentages of maximal possible effect (%MPE), a way of expressing the absolute latencies relative to baseline (0% MPE) and to cut-off time (100% MPE), according to the formula
Postinjection latencies were analyzed separately at each time point by using the Friedman test. This test ranks nonnormal data and is the nonparametric alternative to repeated-measures ANOVA. %MPE data were analyzed for carryover effect by using the Kruskal–Wallis test (3 treatment sequences) and for strain effect by using the Wilcoxon ranked-sum test (2 strains). Data were grouped by treatment (n = 15, repeated measures) at each time point. The analysis was first performed independently for each strain and then repeated with pooled data from the 2 strains. A significant result after the Friedman test at any time point was followed by posthoc pairwise comparisons between treatment groups by using the Wilcoxon signed-ranks test with Bonferroni correction. Data were analyzed by using Stata 11.0 (StataCorp, College Station TX). An α level of P ≤ 0.05, or P < 0.017 for analyses in which Bonferroni correction was applied, was considered to indicate a statistically significant difference.
Results
A total of 9 baseline latencies were recorded for each mouse over the course of data collection. The baseline latency (mean ± 1 SD) of BALB/cJ mice (16.5 ± 3.8 s) was significantly (P < 0.002) greater than that of SWR/J mice (9.8 ± 2.1 s).
Neither treatment sequence nor strain had significant effect on %MPE. When the Friedman test was performed separately for each strain (SWR/J, n = 7; BALB/cJ, n = 8), the only significant result was for BALB/cJ at 2 h, when %MPE was higher (P = 0.014) for bup-SR than for saline.
When data from the 2 strains and 3 treatment sequences were pooled, the Friedman test identified statistically significant differences among treatment groups 2 (P = 0.001), 6 (P = 0.031), 12 (P = 0.027), and 72 (P = 0.016) h after treatment (Figure 2). Posthoc pairwise comparisons showed that %MPE (relative thermal latency) for the bup-SR treatment group was significantly higher than those for the saline (P < 0.001) and bup-HCl (P = 0.002) groups at 2 h. %MPE for the bup-SR group was significantly higher than for the saline group at 6 (P = 0.007) and 12 h (P = 0.011). %MPE was significantly (P = 0.009) higher for the bup-SR treatment group than for the bup-HCl group at 72 h. No significant difference among treatment groups was found at 24 or 48 h after treatment.
Figure 2.
Percentage of maximal possible effect (%MPE; mean ± SEM, n = 15) for each treatment group at each time point. Significant differences were present at 2 (sustained-release buprenorphine [bup-SR] > buprenorphine-HCl [bup-HCl]; bup-SR > saline), 6 (bup-SR > saline), 12 (bup-SR > saline), and 72 (bup-SR > bup-HCl) h. *, Significant (P < 0.05) difference between bracketed values.
Of the 15 mice, 12 developed lesions at the site of injection within 1 to 7 d after receiving bup-SR (3 of 5 mice after the first round of injections, 3 of 4 after the second, and 6 of 6 after the third). Lesions appeared as scabs that were visible for as few as 3 d to as long as 20 d. One mouse developed a mild lesion after a bup-HCl injection, but no other mice had lesions after injections of bup-HCl or saline.
Discussion
The current study evaluated the duration of action of 2 formulations of buprenorphine in mice by using a thermal analgesiometric assay, the hot-plate assay. %MPE (relative thermal latency) was greater for bup-SR compared with saline at 2, 6, and 12 h and compared with bup-HCl at 2 and 72 h. These data indicate that bup-SR at a dose of 1.0 mg/kg has an analgesic effect for at least 12 h in mice. At no time point was the effect of bup-HCl significantly different from that of the control.
Direct extrapolation of doses and durations effective in hot-plate assays to clinical application requires caution. Higher doses of buprenorphine have been required in analgesiometric assays of phasic nociception than during controlled clinical trials.15 In another study, the ED50 of bup-HCl for mice on a 55 °C hot plate was 1.5 mg/kg.18 In the current study, we used the recommended clinical doses for bup-HCl (0.1 mg/kg) and bup-SR (1.0 mg/kg). The bup-SR dose approximated the cumulative dose of bup-HCl that a mouse would receive from 3 or 4 daily administrations over 72 h, so that we could compare the relative effects of the 2 formulations at equivalent doses. However, bup-HCl at the clinical dose does not appear to provide sufficient analgesia to mice during the hot-plate assay. Bup-SR did have a significant effect relative to saline at 2, 6, and 12 h after treatment, and these data may indicate that bup-SR at 1.0 mg/kg reaches higher plasma concentrations during the first 12 h than does bup-HCl at 0.1 mg/kg at any time.
We noted a significant difference at 72 h between the bup-HCl and bup-SR groups, but neither of these groups was significantly different from the control group. Without statistical significance, whether the negative %MPE at 72 h truly represents thermal hyperalgesia is unclear. Hyperalgesia is a known phenomenon of buprenorphine, although the pharmacodynamics are complex. One group reported apparent hyperalgesia in rats after single or repeated subanalgesic doses of buprenorphine, suggesting an even more complicated pharmacology than what had been described previously.19 The cited study did not find delayed hyperalgesia after single administration of an analgesic dose during the 72-h period investigated; rats returned to baseline and were not studied beyond this point.19 The possibility of rebound hyperalgesia in our mice at the dose used warrants further exploration.
SWR/J mice had significantly (P < 0.002) shorter baseline latencies than did BALB/cJ mice. This finding suggests that SWR/J mice are more sensitive to thermal stimuli or that they at least are quicker to show the endpoint behaviors. In addition, we noted differences in temperament between the strains, but the relationship between temperament and latency during the hot-plate assay is speculative. For example, we noted that SWR/J mice were more aggressive and more difficult to restrain manually for transport to the hot plate. Perhaps the latency recorded was affected by differences in the levels of stress experienced by the 2 strains at the start of each hot-plate assay. We did not analyze strain-associated differences in the analgesic efficacy of bup-SR in the current study, because the sample size was too low to provide adequate power.
Administration of bup-SR resulted in scabby lesions at the injection site in 12 of the 15 mice. Other colleagues have remarked that when bup-SR seeped out of the injection site onto the skin, it caused local erythema and scabbing; the investigators resolved this problem by slowly withdrawing the needle after injection and by pinching the injection site for 15 s.7 This refinement proved difficult to implement in unanesthetized mice. The lesions were first noted as early as the day of injection and as late as 7 d after injection and lasted from 3 to 21 d. One mouse developed an open wound (diameter, approximately 3 mm) on the day of the injection. None of the mice were observed to display behaviors such as scratching or licking that might indicate pain or pruritus at the injection site lesion.
Because of the current study's crossover design and multiple time points, each mouse underwent repeated trials on the hot plate and received 2 doses of buprenorphine. Other studies6,20 showed decreases in hot-plate latencies with repeated testing; hyperalgesia, learned response, and habituation (reduction of stress) were proposed as possible underlying mechanisms. In the current study, comparing the buprenorphine treatment groups with the saline group at each time point was meant to control for any effect of repeated testing. That the mice received 2 doses of buprenorphine raises the question of opioid tolerance. One group noted that twice-daily dosing of buprenorphine induced tolerance in mice but more slowly than did morphine.5 It does not appear from the cited study that 2 doses of buprenorphine were sufficient to change the ED50 in the mice. In addition, only half of the possible sequences of treatments were included in the study, leaving some potential for bias due to an order effect.5
Studies using hot-plate assays vary in the endpoint behaviors that are monitored. Licking of the hindpaw has the advantage of being unequivocal and is recommended by some authors as the sole endpoint.1 We attempted to use this criterion in the present study, but our pilot studies revealed that the BALB/cJ mice often remained on the hot plate for 30 s (the cut-off time) without showing this behavior. Because this outcome might mask any analgesic effect, we broadened our endpoint criteria to include hindpaw shaking, hindpaw lifting, and jumping as well as hind paw licking and applied these criteria to both strains.
Determining endpoint behaviors that were sensitive, specific, and unambiguous to the observers was a major challenge of this study. For the data collected for this report, the endpoint behaviors consisted of licking a hindpaw, lifting a hindpaw (lifting and holding or lifting independent of taking a step), fluttering a hindpaw (while standing or stepping), and jumping. These endpoints have been used in various combinations in other studies.6,8,14 In pilot work for the current study, licking of a hindpaw was the most consistent endpoint behavior among SWR/J mice but was rarely the first endpoint behavior shown by BALB/cJ mice. For data collection, the broader range of endpoint behaviors was used. None of these behaviors were displayed when we placed our mice on an unheated plate. To the observers, lifting and fluttering were sometimes obvious and sometimes subtle, requiring subjective assessment.
Behaviors displayed by mice on both heated and unheated plates were presumed to be exploratory or nonspecific and were not considered to be endpoints. These behaviors included walking, sniffing, urination or defecation, rearing, and lifting a front paw. Additional behaviors observed on the heated plate but not the unheated plate included running, freezing, circling backward, and grooming the front paws. In the current study, these additional behaviors may have been elicited by heat, but because they are not described as nocifensive behaviors in the literature, we did not include them as endpoints. For example, grooming the front paws is considered to be a grooming behavior that is not specific to nociception, although this behavior has been suggested to aid in heat dissipation,14,17 a reason that might explain why the mice displayed this behavior frequently during trials on the heated plate.
The SWR/J and BALB/cJ strains appeared to differ regarding the relative frequency with which they displayed various endpoint behaviors, as described previously.2 In the 150 hot-plate trials in the last week of data collection (10 trials for each of 15 mice, of which 7 were SWR/J and 8 were BALB/cJ), hindpaw licking was the endpoint in 15 trials of SWR/J mice but in only 3 trials of BALB/cJ mice. Two potentially nocifensive responses, backward circling seen mainly in SWR/J mice and running with frequent rearing seen mainly in BALB/cJ mice, were not used as endpoints. Choice of strain may explain why some studies can use hindpaw licking as the sole endpoint behavior.
Administering bup-SR to mice presented practical challenges. The drug is quite viscous and difficult to draw into a small-gauge needle on a small syringe. This formulation cannot be diluted, resulting in small injection volumes (0.03 mL for a 30-g mouse) that are difficult to administer accurately, especially in unanesthetized mice. Further dilution with the proprietary vehicle might increase dosing accuracy but would increase the volume of vehicle injected, the presumed cause of the skin lesions.
Further research is needed to continue characterizing the efficacy of bup-SR in mice. The current study had a small sample size and pronounced variability in latencies. Strategies for reducing variability include stricter standardization of handling, a longer acclimation period to reduce stress during trials, and refinement of endpoint criteria through pilot work. Selecting a strain that consistently shows an unambiguous endpoint behavior, such as licking a hindpaw, would simplify data collection but would lower applicability to strains with different opioid sensitivities. Reducing the number of hot plate trials undergone by each mouse may eliminate any confounding effect of repeated trials but would greatly increase the number of subjects necessary. If a cross-over design is used, treatment sequences should be balanced to minimize the potential for bias due to an order effect.
To derive clinical recommendations, bup-SR should be studied in pharmacokinetic studies and controlled clinical trials. Clinical trials use physiologic and behavioral parameters to assess analgesia, and doing so is necessary to determine the appropriate dosage of bup-SR for postsurgical analgesia. In our study, we used a clinical dose rather than a higher, thermal antinociceptive dose, so clinical trials may show that bup-SR has a longer duration of action than was indicated here by analgesiometry. In addition, the dose–response curve for buprenorphine is an inverted U-shape in some, but not all, models of pain.4,5 Therefore, the optimal dose of bup-HCl or bup-SR varies with the nature and severity of the pain model.
When multiple strains are compared, care should be taken to ensure that trials observers remain blinded to strain identity of subjects. In the current study, differences between SWR/J and BALB/cJ mice in size, temperament, nesting behavior, and hot-plate behavior compromised the observers’ blindedness.
Adding a treatment group that received only the vehicle of the bup-SR formulation would control for any possible effect of the vehicle itself. Although we saw evidence of potential hyperalgesia only after bup-HCl, it remains possible that the skin lesions, which developed over several days, made the mice slightly hyperalgesic and that stopping assays at 72 h before a 10-d intertrial period missed this effect. Administering the treatments to anesthetized mice may reduce the trauma of injection and potentially the incidence of injection site lesions after bup-SR. Because buprenorphine often is administered to mice during surgery, this modification is reasonable for clinically oriented studies. Research is needed to determine the incidence of lesions in mice that receive other formulations.
In conclusion, the sustained-release buprenorphine formulation that we tested has an apparent duration of action of at least 12 h in mice, according to the hot-plate assay. Clinically significant analgesia may last longer. Evidence of hyperalgesia at 72 h after administration of buprenorphine-HCl warrants further exploration. SWR/J mice had significantly shorter baseline thermal latencies than did BALB/cJ mice, providing additional evidence that strains vary in their responses to nociceptive stimuli. Different strains also may demonstrate nociception through different set of behaviors. The sustained-release buprenorphine formulation, which included N-methyl-2-pyrrolidone in the solvent, resulted in scabby injection sites lesions in most mice. Controlled clinical trials are needed to determine the appropriate dosage of this buprenorphine formulation for postoperative mice.
Acknowledgments
This research was supported, in part, by a NIH Short-Term Training Grant (RR029724) and the Neurobehavioral Core for Rehabilitation Research, Department of Physical Therapy and Rehabilitation Science. We thank Alicia Karas, DACVA, and Lara Helwig, DACLAM, for their assistance with the manuscript, and Sandra Canchola for her assistance in the Core.
References
- 1.Bannon AW, Malmberg AB.2007. Models of nociception: hot-plate, tail-flick, and formalin tests in rodents. Curr Protoc Neurosci Chapter 8:Unit 8.9. [DOI] [PubMed]
- 2.Belknap JK, Lame M, Danielson PW. 1990. Inbred strain differences in morphine-induced analgesia with the hot-plate assay: a reassessment. Behav Genet 20:333–338 [DOI] [PubMed] [Google Scholar]
- 3.Catbagan DL, Quimby JM, Mama KR, Rychel JK, Mich PM. 2011. Comparison of the efficacy and adverse effects of sustained-release buprenorphine hydrochloride following subcutaneous administration and buprenorphine hydrochloride following oral transmucosal administration in cats undergoing ovariohysterectomy. Am J Vet Res 72:461–466 [DOI] [PubMed] [Google Scholar]
- 4.Christoph T, Kogel B, Schiene K, Meen M, De Vry J, Friderichs E. 2005. Broad analgesic profile of buprenorphine in rodent models of acute and chronic pain. Eur J Pharmacol 507:87–98 [DOI] [PubMed] [Google Scholar]
- 5.Cowan A, Lewis JW, Macfarlane IR. 1977. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol 60:537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crockett RS, Bornschein RL, Smith RP. 1977. Diurnal variation in response to thermal stimulation: mouse hot-plate test. Physiol Behav 18:193–196 [DOI] [PubMed] [Google Scholar]
- 7.Foley PL, Liang H, Crichlow AR. 2011. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci 50:198–204 [PMC free article] [PubMed] [Google Scholar]
- 8.Gades NM, Danneman PJ, Wixson SK, Tolley EA. 2000. The magnitude and duration of the analgesic effect of morphine, butorphanol, and buprenorphine in rats and mice. Contemp Top Lab Anim Sci 39:8–13 [PubMed] [Google Scholar]
- 9.Heavner JE, Cooper DM.2008. Pharmacology of analgesics, p 97–124. In: Fish RE, Brown MJ, Danneman PJ, Karas AZ, editors. Anesthesia and analgesia in laboratory animals, 2nd ed. London (UK): Academic Press.
- 10.Johnson RE, Fudala PJ, Payne R. 2005. Buprenorphine: considerations for pain management. J Pain Symptom Manage 29:297–326 [DOI] [PubMed] [Google Scholar]
- 11.Kamei J, Saitoh A, Suzuki T, Misawa M, Nagase H, Kasuya Y. 1995. Buprenorphine exerts its antinociceptive activity via µ1 opioid receptors. Life Sci 56:PL285–PL290 [DOI] [PubMed] [Google Scholar]
- 12.Kest B, Palmese CA, Hopkins E, Adler M, Juni A, Mogil JS. 2002. Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: evidence for common genetic mechanisms in acute and chronic morphine physical dependence. Neuroscience 115:463–469 [DOI] [PubMed] [Google Scholar]
- 13.Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. 1999. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain 80:67–82 [DOI] [PubMed] [Google Scholar]
- 14.Mogil JS, Wilson SG, Wan Y.2001. Assessing nociception in murine subjects, p 11. In: Kruger L, editor. Methods in pain research. Boca Raton (FL): CRC Press.
- 15.Roughan JV, Flecknell PA. 2002. Buprenorphine: a reappraisal of its antinociceptive effects and therapeutic use in alleviating postoperative pain in animals. Lab Anim 36:322–343 [DOI] [PubMed] [Google Scholar]
- 16.Stokes EL, Flecknell PA, Richardson CA. 2009. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab Anim 43:149–154 [DOI] [PubMed] [Google Scholar]
- 17.Tjolsen A, Rosland JH, Berge OG, Hole K. 1991. The increasing-temperature hot-plate test: an improved test of nociception in mice and rats. J Pharmacol Methods 25:241–250 [DOI] [PubMed] [Google Scholar]
- 18.Tyers MB. 1980. A classification of opiate receptors that mediate antinociception in animals. Br J Pharmacol 69:503–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wala EP, Holtman JR., Jr 2011. Buprenorphine-induced hyperalgesia in the rat. Eur J Pharmacol 651:89–95 [DOI] [PubMed] [Google Scholar]
- 20.Wilson SG, Mogil JS. 2001. Measuring pain in the (knockout) mouse: big challenges in a small mammal. Behav Brain Res 125:65–73 [DOI] [PubMed] [Google Scholar]



