Abstract
Perioperative treatment of several rats in our facility with ketoprofen (5 mg/kg SC) resulted in blood loss, peritonitis, and death within a day to a little more than a week after surgery that was not related to the gastrointestinal tract. Published reports have established the 5-mg/kg dose as safe and effective for rats. Because ketoprofen is a nonselective nonsteroidal antiinflammatory drug that can damage the gastrointestinal tract, the putative diagnosis for these morbidities and mortalities was gastrointestinal toxicity caused by ketoprofen (5 mg/kg). We conducted a prospective study evaluating the effect of this therapeutic dose of ketoprofen on the rat gastrointestinal tract within 24 h. Ketoprofen (5 mg/kg SC) was administered to one group of rats that then received gas anesthesia for 30 min and to another group without subsequent anesthesia. A third group was injected with saline followed by 30 min of gas anesthesia. Our primary hypothesis was that noteworthy gastrointestinal bleeding and lesions would occur in both groups treated with ketoprofen but not in rats that received saline and anesthesia. Our results showed marked gastrointestinal bleeding, erosions, and small intestinal ulcers in the ketoprofen-treated rats and minimal damages in the saline-treated group. The combination of ketoprofen and anesthesia resulted in worse clinical signs than did ketoprofen alone. We conclude that a single 5-mg/kg dose of ketoprofen causes acute mucosal damage to the rat small intestine.
Abbreviation: NSAID, nonsteriodal antiinflammatory drug; FOBT, fecal occult blood test
This study was inspired by several clinical cases in our animal facility that involved perioperative treatment of rats with ketoprofen for postoperative pain. Several rats became ill with indications of blood loss, abdominal discomfort, and peritonitis within a day to a little more than 1 wk after surgery not related to the gastrointestinal tract. Gastrointestinal bleeding was diagnosed in some rats that had dark stools, pallor, and positive fecal occult blood tests (FOBT). Intestinal abnormalities (adhesions, fibrosis, ascites) were noted on necropsy of rats that died. The ketoprofen dose was 5 mg/kg SC administered either before or immediately after anesthesia with isoflurane gas. Most of these surgical cases involved removal of large muscle masses, but one involved a small dermal biopsy.
Surgical procedures and doses of all medications were checked for accuracy, and we determined that no related errors occurred. The putative diagnosis for these morbidities and mortalities was gastrointestinal toxicity caused by a therapeutic dose (5 mg/kg) of ketoprofen. We decided to conduct a prospective study to evaluate the effects of a 5-mg/kg SC dose of ketoprofen on the rat gastrointestinal tract within 24 h after administration.
Ketoprofen ([RS] 2-[3-benzoyl phenyl]-propionic acid) is a nonselective, nonsteroidal antiinflammatory drug (NSAID) that blocks both cyclooxygenase isoenzymes (COX1 and COX2) in the arachidonic acid cascade.6,11,19,32 Studies have shown that gastrointestinal damage occurs in some animals when both of these isoenzymes are blocked by nonselective NSAID.14,27,32 Blocking both COX1 and COX2 has been postulated to result in decreased synthesis of mucosal prostaglandins which protect the gastric mucosa.6,19,32 Other nonprostaglandin-related effects of nonselective NSAID include increased mucosal permeability in the intestines, elevation of lipooxygengase, elevation of bacterial numbers and their related toxic effects, infiltration of neutrophils into the mucosa, gastric hypermotility, uncoupling of mitochondrial oxidative phosphorylation, and calcium release into the cytosol of enterocytes.14,19,20,27,32
The 5-mg/kg dose we had used clinically and chosen for this experiment had been established as safe and effective for treatment of postoperative pain in rats.7,22 It currently is prescribed for rats in veterinary and laboratory animal formularies10,18 and used for the management of pain and inflammation in published reports.4,25 Here we administered ketoprofen (5 mg/kg SC) to a group of rats anesthetized for 30 min with isoflurane gas. A second experimental group received ketoprofen (5 mg/kg SC) but was not anesthetized. A control group received only saline (0.03 mL SC) prior to being anesthetized for 30 min. At the 24-h time point, animals and food were weighed, and fecal and blood samples taken for FOBT, PCV, and total protein concentration. Rats then were euthanized and tissues collected for microscopic evaluation.
Our primary hypothesis was that noteworthy gastrointestinal bleeding and lesions would occur in both groups of rats treated with ketoprofen but not in anesthetized rats that received saline. A related aim was to determine whether the stress of anesthesia combined with ketoprofen would be more deleterious to the rat gastrointestinal tract than ketoprofen was administered without subsequent anesthesia.
Materials and Methods
Animals and housing.
Groups of 14 or 7 female rats (Crl:CD[SD]; weight, 226 to 250 g; age, 71 to 84 d; Charles River Laboratories, Wilmington, MA) arrived at our facility, were singly housed and allowed to acclimate for 10 d before being handled for baseline evaluations. Rats were housed on 1/4-in. corncob bedding (Bed-o-Cobs, The Andersons, Maumee, OH) in static, polysulfone, microisolation cages (143 in.2; Allentown Caging, Allentown, PA). Standard irradiated rodent chow (Prolab Isopro RMH 3000 Diet 5P76, Lab Diets, Brentwood, MO) was provided ad libitum with bottled, ultrafiltered, and acidified water. Cages were cleaned and fresh food and water provided twice weekly. Daily enrichment consisted of nesting pads (Nestlets, Ancare, Bellmore, NY) and shelters (Rat Shacks, Shepherd Paper Products, Watertown, TN). The rats were kept in a conventional, AAALAC-accredited facility with a HEPA-filtered air supply, 12:12-h light:dark cycle, ambient room temperature of 68 to 79 °F (20.0 to 21.1 °C), and relative humidity of 30% to 70%. Sentinel rats from the related animal racks were tested every 10 wk and found to be negative for excluded pathogens: cilia-associated respiratory bacillus, Kilham rat virus, Mycoplasma pulmonis, pneumonia virus of mice, rat parvovirus, reovirus 3, sialodacryoadenitis virus (rat coronavirus), Sendai virus, Theiler murine encephalomyelitis virus (mouse poliovirus), fur mites, and pinworms. All procedures using animals were approved by the Animal Care and Use Committee of the University of Massachusetts Medical School.
Experimental design.
Twenty rats were assigned randomly to each treatment arm. Ketoprofen (Ketofen, Fort Dodge Animal Health, Fort Dodge, IA) was diluted 1:1 with sterile, preservative-free saline (0.9% Sodium Chloride for Injection, USP; Hospira, Lake Forest, IL) to a concentration of 50 mg/mL. Rats in group K+A were injected in the dorsal rump area with ketoprofen (5 mg/kg SC) 10 min prior to gas anesthesia with isoflurane for 30 min. Rats in group K were injected in the dorsal rump area with ketoprofen (5 mg/kg SC) without subsequent anesthesia. Rats in group S+A were injected SC in the dorsal rump area with 0.03 mL sterile saline 10 min prior to anesthesia with isoflurane gas for 30 min. We used 0.03 mL sterile saline because it approximated the volumes of the ketoprofen doses administered to the experimental groups. After anesthesia (groups K+A and S+A) or injection of ketoprofen (group K), rats were placed back into their cages. At 24 h after these experimental manipulations, rats were euthanized with CO2 gas (with bilateral pneumothorax as the second euthanasia method) for necropsy and tissue collection.
Anesthesia.
Rats were anesthetized with isoflurane gas by using a Lab Research Anesthesia System (SurgiVet, Waukesha, WI). Anesthesia was induced by using 4% to 5% isoflurane delivered in 100% oxygen (1 L/min) inside the attached chamber. Rats were removed from the induction chamber after loss of righting reflexes, and anesthesia was maintained by using isoflurane between 1.5% to 3% delivered in 100% oxygen (1 L/min) through a diaphragm-covered nose cone. Heart rate and hemoglobin oxygen saturation were monitored (PulseSense VET Portable Tabletop Pulse Oximeter, Nonin Medical, Plymouth, MN) with the probe placed on one of the rat's feet. Respiration rate, heart rate, hemoglobin oxygen saturation were recorded every 5 min. Rats were kept warm by using recirculating-water heating blanket (T Pump, Gaymar Industries, Orchard Park, NY) covered with a cloth diaper and placed directly beneath the animals. Rats recovered inside their cages while being placed half on, half off of the recirculating-water heating blanket.
Fecal collection and FOBT.
Ten days after the rats arrived, fresh fecal samples were collected while the rats were weighed for baseline evaluations. Rats voided voluntarily, or stools were gently massaged from their rectums. Baseline FOBT were conducted according to the manufacturer's instructions (Sure-Vue Fecal Occult Blood Test, Fisher HealthCare, Houston, TX). This qualitative test for the detection of fecal occult blood consists of 2 paper panels impregnated with guaiac resin contained within a cardboard casing. Briefly, thin smears taken from different parts of a fecal pellet were applied with a wooden applicator to the 2 test panels inside the casing. The windows were covered with the cardboard flap, and a separate flap on the opposite side of the casing was opened to reveal the back sides of the testing panels. Two drops of the developer, which consisted of 6% hydrogen peroxide and denatured alcohol, were applied to each panel. After 30 s, the panels were observed for the development of a blue color, which indicated a positive reaction. Positive and negative control monitors below the test panels were tested in a similar manner. Because we were not blinded to treatment group, only tests with an obvious blue color were recorded as positive; equivocal or trace reactions were deemed negative. FOBT on fresh fecal samples were performed again 5 d later, immediately prior to injection with ketoprofen or saline, and then again in 24 h, just before euthanasia.
Blood sampling and measurement of PCV and total protein concentration.
Preexperimental blood samples were drawn from the rat lateral tail veins 15 to 20 min prior to injection with ketoprofen or saline. A 24-h sample was drawn in the same manner, prior to euthanasia. Both samples were assayed immediately after being drawn. Tail veins were dilated by dipping the tails into warm tap water for about 1 min. Blood samples (50 to 100 µL) were drawn into heparinized, 0.3-mL U100 insulin syringes with 29-gauge, 0.5-in. needles (Terumo Medical Corp, Elkton, MD) and injected into heparinized, microhematocrit capillary tubes (Fisher Scientific, Pittsburg, PA). Samples were spun for 1 min in a Micro Hematocrit Centrifuge (United Products and Instruments, Dayton, NJ) and measured against a standardized chart to obtain readings (in percentages). The tubes then were broken, and the serum portion of the sample was applied to a refractometer (Schuco Clinical Refractometer, Tokyo, Japan) to obtain the total protein concentration (in g/dL).
Food intake and body weight.
Ten days after the rats arrived, during the same time period that the first baseline FOBT and body weights were obtained, rodent chow pellets were placed into to a stainless steel bowl and weighed (in grams) by using a digital baby scale (Tanita, Arlington Heights, IL) before providing them to rats. Five days later, before the preexperimental blood draws, the remaining food was weighed, and the individual mean daily intakes over 5 d were calculated. Twenty-four hours later, during postexperimental evaluations, the remaining pellets were weighed. Body weights were measured (in grams) by using the same baby scale at 10 d after arrival and then 5 d later, immediately before animals were injected with ketoprofen or saline. The 5-d individual mean body weight changes were calculated. Twenty-four hours after ketoprofen or saline injection, body weights were measured prior to euthanasia.
Necropsy and histology.
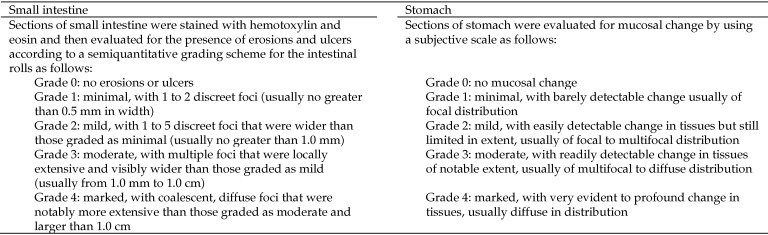
Rats were necropsied immediately after euthanasia. Organs and tissues were observed in situ, and relevant images were obtained (Optio E 50 Digital Camera, Pentax, Tokyo, Japan) by using the macro lens setting both with and without flash. The stomach and intestines subsequently were removed in toto and immediately flushed with 10% neutral buffered formalin. The stomach was removed from the intestines, and the small intestine was removed from the large intestine. Intestinal rolls of the small bowel were created according to a described technique17 with modifications. Briefly, intestines were cut into 12-cm sections, incised along the mesenteric border, and rolled onto a wooden dowel with the mucosa facing outward. Rolls were removed from the dowel and placed into individually numbered cassettes. There were 7 to 9 rolls per rat, with the final roll sometimes a few centimeters longer or shorter than the previous rolls. The cecum and colon were saved for future examination. The stomachs were cut in half after being opened along the greater curvature. Half of the stomach was placed into a cassette for microscopic evaluation, and the other half was saved in 10% formalin. Prepared tissues were fixed in 10% formalin for 7 to 10 d and then routinely processed for hematoxylin and eosin staining. Two 5-µm, serial sections were applied to a single glass slide per intestinal roll and stomach for each rat. The slides were examined in a blinded fashion by a board-certified veterinary pathologist using a microscope (BX40, Olympus, Tokyo, Japan). Images were captured (Qicam Fast 1394 Digital Camera with QCapture Pro Version 5.1.1.14 Imaging Software for Windows XP, QImaging, Surrey, British Columbia, Canada). The severity of mucosal erosions and ulcerations was graded according to 5-point scales (grade, 0 to 4; Figure 1).
Figure 1.
Grading system (scale, 0 to 4) used to categorize the severity of gastrointestinal histologic lesions (ulcers and erosions) in the small intestines and stomach.
Statistical analysis.
Results for continuous values (PCV, total protein concentration, food intake, and body weight) are presented as means with the bounds of their respective 95% confidence intervals. Binary data (FOBT) are summarized by using the proportion of positive results per group. The numbers of rats with ulcers or erosions are expressed as the proportions of rats per group.
Categorical data (FOBT; rats with gastrointestinal lesions) were compared by using a 2-tailed χ2 test (Fisher exact test for marginal totals less than 5). PCV percentages were arcsine-transformed to compare groups by using 2-tailed, repeated-measures ANOVA. The remaining continuous variables (total protein concentration, food intake, and body weight) were compared directly by using 2-tailed, repeated-measures ANOVA. Spearman nonparametric correlation (r) was calculated to detect a possible linear relationship between body weight at time 0 and PCV at the 24-h time point in the 3 study groups. The threshold for statistical significance for all analyses was set at a P of less than 0.05.
Where significance was achieved for continuous variables, post hoc analyses using Scheffé adjustment were carried out to detect differences between the subgroups. SPSS (version 19, IBM, New York, NY) software was used for statistical calculations and chart construction.
Results
FOBT and fecal color.
The two baseline screening tests for fecal occult blood were negative in all 3 study groups (data not shown). At 24 h, 100% of the rats in group K+A, 90% in group K, and 10% in group S+A tested positive for fecal occult blood, yielding a highly significant difference among the groups (χ2, 43.8; df, 2; P < 0.001). Post hoc comparisons revealed highly significant differences between the positive FOBT in groups K+A and S+A (P < 0.0001) and groups K and S+A (P < 0.0001). There was no significant difference between the positive FOBT in groups K+A and K (P = 0.487). Most stools that tested positive were dark brown; some were black (melena). Some stools that tested positive did not appear abnormally dark.
PCV and total protein concentration.
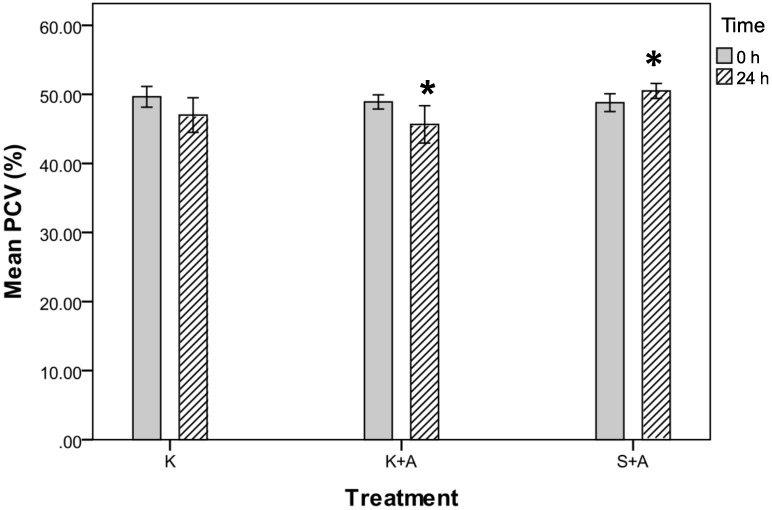
PCV showed a significant difference among the 3 groups at 24 h (P = 0.039, repeated-measures ANOVA; Figure 2). The mean PCV declined in groups K+A and K but increased in group S+A. Post hoc comparisons yielded a significant difference between the PCV in groups K+A and S+ A (P = 0.034) but not between groups K+A and K (P = 0.591) and S+A and K (P = 0.382; Figure 2).
Figure 2.
Packed cell volumes (%) for groups K, K+A, and S+A before (0 h) and after (24 h) injection of ketoprofen. *, Post hoc comparisons found a significant difference between group K +A and S+A (P = 0.034) but not between K+A and K (P = 0.591) or S+A and K (P = 0.382). Data are presented as mean values with the bounds of their respective 95% confidence intervals (n = 20 per group).
There were clinically relevant differences in gastrointestinal bleeding among the 3 groups at 24 h. In group K+A, the mean PCV decreased by 3.3% to 46% (95% confidence interval [CI], 43% to 49%). 60% of the PCV in this group fell by 1% to 20%, with 3 down by 11%, 15% and 20%. In group K, the mean PCV fell by 2.7% to 47% (95% CI, 44% to 50%); 60% of the PCV values in this group decreased by 1% to 22%, with 2 rats showing decreases of 10% and 22%. In group S+A, the mean PCV increased by 1.7% to 51% (95% CI, 49% to 52%); 75% of the PCV values in this group increased by 1% to 7%. Three rats in this group showed declines of 1% to 5% (Table 1).
Table 1.
Trends in PCV (%) and total protein concentration (g/dL;n = 20 per group) at 24 h
| Group K+A | Range of change | Group K | Range of change | Group S+A | Range of change | ||
| PCV | |||||||
| Decreased | 12 | 1 to 20 | 12 | 1 to 22 | 3 | 1 to 5 | |
| Increased | 6 | 1 to 4 | 5 | 1 to 8 | 15 | 1 to 7 | |
| Unchanged | 2 | 0 | 3 | 0 | 2 | 0 | |
| Total protein | |||||||
| Decreased | 14 | 0.2-3.7 | 17 | 0.2-4.6 | 7 | 0.1-0.6 | |
| Increased | 2 | 0.2-0.6 | 1 | 1.5 | 9 | 0.1-1.0 | |
| Unchanged | 4 | 0 | 2 | 0 | 4 | 0 |
Data shown are the numbers of rats in each group that showed each change in the indicated index.
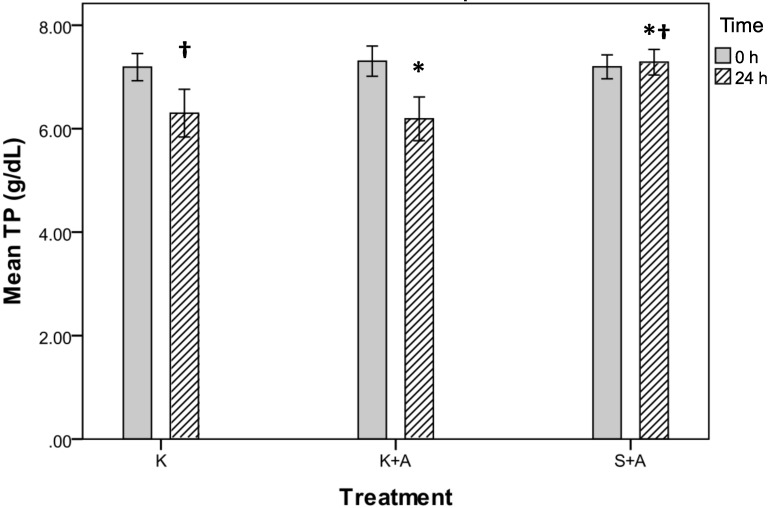
Total protein values at 24 h showed a significant difference among all 3 groups (P = 0.003, repeated-measures ANOVA; Figure 3). The mean total protein concentration declined in groups K+A and K but increased in group S+A. Post hoc comparisons found a significant difference between the total protein values in groups K+A and S+A (P = 0.013) and S+A and K (P = 0.012) but not between groups K+A and K (P = 1.00; Figure 3).
Figure 3.
Total protein (g/dL) for groups K, K+A, and S+A before (0 h) and after (24 h) injection of ketoprofen. Post hoc comparisons found a significant difference between groups K+A and S+A (*, P = 0.013) and K and S+A (†, P = 0.012) but not between K+A and K (P =; 1.00). Data are presented as mean values with the bounds of their respective 95% confidence intervals (n = 20 per group).
In group K+A, total protein dropped by 1.1 to 6.2 g/dL (95% CI, 5.7 to 6.7 g/dL) over the 24-h experimental period; 70% of the values fell by 0.2 to 3.7 g/dL, with 8 rats showing decreases of more than 1 g/dL. In group K, total protein fell by 0.9 to 6.3 g/dL (95% CI, 5.8 to 6.8 g/dL); 85% of the values fell by 0.2 to 4.6 g/dL, with 10 rats showing decreases of more than 1 g/dL. In group S+A, total protein concentration increased by 0.1 to 7.3 g/dL (95% CI, 7.0 to 7.6 g/dL); 45% of the values increased by 0.1 to 1.0 g/dL whereas 35% fell by 0.1 to 0.6 g/dL (Table 1).
Macroscopic pathology.
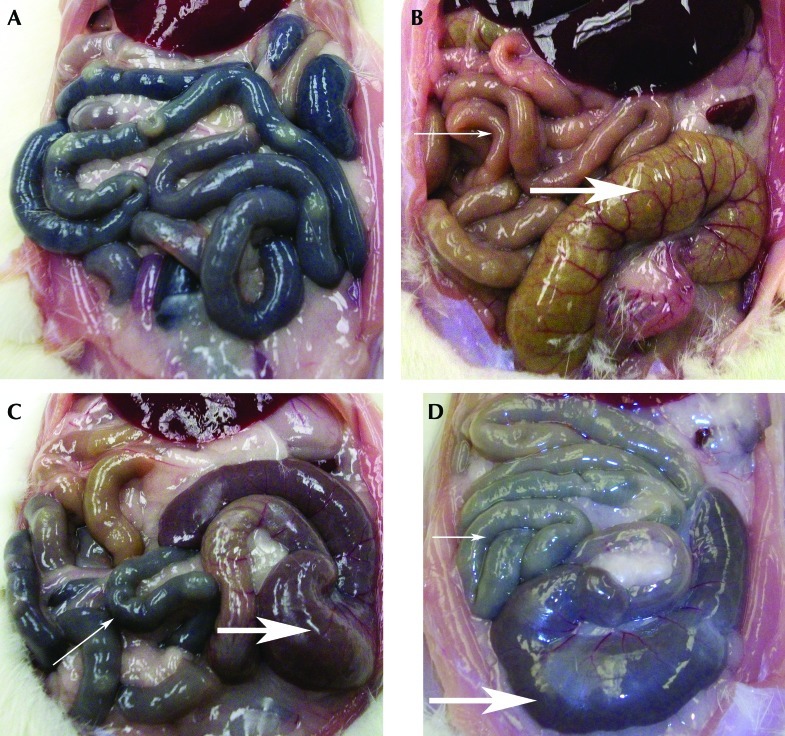
At necropsy, the small intestines of rats with the largest declines in PCV appeared dark when viewed from the serosal surface; this darkness was segmental to diffuse in distribution (Figure 4 A, C, and D). This observation is indicative of pronounced bleeding into the intestinal lumen. The small intestines of rats in groups K+A and K that showed modest declines in PCV and total protein concentration appeared light gray to tan, consistent with less bleeding into the small intestinal lumen. The serosal surface of the cecum was dark brown or dark red in rats with strongly positive FOBT, and frank blood was sometimes visible in the cecum of some of the most severely affected rats (Figure 4 B, C, and D). Necropsy of the stomach and small intestines revealed no obvious mucosal alterations in rats from all 3 groups.
Figure 4.
Photographs of intestines and selected hematologic indices. (A) Intestines from rat 49, group K, 24 h after ketoprofen injection. Darkened serosal surfaces indicative of bleeding into the intestines. PCV, 22% below baseline; total protein, 4.6 g/dL below baseline. (B) Normal intestines from rat 18, group S+A, 24 h after saline injection and anesthesia. The thin arrow points to the normal color of intestinal loops; the thick arrow indicates the normal color of the cecum. PCV, 2% below baseline; total protein, 0.1 g/dL below baseline. (C) Intestines from rat 21, group K+A, 24 h after injection of ketoprofen and anesthesia. The thin arrow points to darkened intestinal loops, indicative of blood in the intestines. The thick arrow points to the dark-red cecum, indicative of blood in the lower intestines. PCV, 15% below baseline; total protein, 3.7 g/dL below baseline. (D) Intestines from rat 15, group K+A, 24 h after injection of ketoprofen and anesthesia. The thin arrow points to darkened intestinal loops, indicative of blood in the intestines. The thick arrow points to the dark-red cecum, indicative of blood in the lower intestines. PCV, 20% below baseline; total protein, 2.6 g/dL below baseline.
Histopathology.
Microscopic evaluation of the small intestine showed significant (P < 0.05) differences in the numbers of rats with ulcers of varying grades (minimal to moderate) in groups K+A and K compared with group S+A . In group K+A, 9 of the 20 rats had ulcers, compared with 1 of the 20 rats in group S+A (χ2, 8.53; df, 1; P = 0.0035). In group K, 10 of the 20 rats had ulcers, compared with the 1 of 20 rats in group S+A (χ2, 10.16; df, 1; P = 0.0014). There was no statistical difference between the numbers of rats with ulcers in group K+A compared with group K (χ2, 0.1; df, 1; P = 0.7518).
Ulcers of grades 1 through 3 were observed throughout the small intestines of groups K+A and K, with most ulcers located in the jejunum (Table 2). No regional bias regarding ulceration was apparent along the length of the jejunum. The only lesion found in group S+A was a minimal ulcer located in the duodenum of a single rat. No gastric ulcers were found in any of the groups. Of the 11 ulcers found in rats in group K+A, 73% (8) were located in the jejunum and 27% (3) were in the ileum; 55% (6) of the total ulcers were grade 1, 18% (2) were grade 2, and 27% (3) were grade 3. Of the 11 ulcers found in rats in group K, 91% (10) were located in the jejunum, with 9% (1) in the ileum; 27% (3) of the total ulcers were grade 1, 55% (6) were grade 2, and 18% (2) were grade 3 (Table 2 and Figure 1).
Table 2.
Incidence of lesions per group (n= 20 each)
| Ulcers |
Erosions |
|||||||||||||
| Grade |
Grade |
|||||||||||||
| Group | 1 | 2 | 3 | 4 | Median | Total no. of ulcers | 1 | 2 | 3 | 4 | Median | Total no. of erosions | ||
| K+A | ||||||||||||||
| stomach | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 3 | |||
| duodenum | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 1.6 | 3 | |||
| jejunum | 3 | 2 | 3 | 0 | 2 | 8 | 2 | 4 | 3 | 0 | 2 | 9 | ||
| ileum | 3 | 0 | 0 | 0 | 1 | 3 | 3 | 0 | 0 | 0 | 1 | 3 | ||
| Total no. of lesions | 6 | 2 | 3 | 0 | 1 | 11 | 8 | 7 | 3 | 0 | 2 | 18 | ||
| per grade | ||||||||||||||
| K | ||||||||||||||
| stomach | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 3 | |||
| duodenum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| jejunum | 3 | 5 | 2 | 0 | 2 | 10 | 2 | 3 | 2 | 1 | 2 | 8 | ||
| ileum | 0 | 1 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | ||
| Total no. of lesions | 3 | 6 | 2 | 0 | 2 | 11 | 6 | 3 | 2 | 1 | 1.5 | 12 | ||
| per grade | ||||||||||||||
| S+A | ||||||||||||||
| stomach | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| duodenum | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |||
| jejunum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| ileum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Total no. of lesions | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |||
| per grade | ||||||||||||||
Some rats had multiple lesions of the same type or both ulcers and erosions.
The numbers of rats with erosions of varying grades (minimal to marked) involving the glandular stomach and small intestine were highly significantly different in groups K+A and K compared with group S+A, in which no erosions (0 of 20 rats) were found. In group K+A, 11 of the 20 rats had erosions (minimal to moderate) compared with none in group S+A (χ2, 15.17; df, 1; P < 0.001). In group K, 8 of the 20 rats had erosions (minimal to marked) compared with none in group S+A (P = 0.003). There was no statistical difference between the numbers of rats with erosions in group K+A compared with group K (χ2, 0.9; df, 1; P = 0.3428).
Most of the erosions in groups K+A and K were located in the jejunum. There was no consistency in the regional occurrence of erosions along the length of the jejunum. Only minimal erosions were observed in the stomachs in groups K+A and K. Of the 18 erosions found in group K+A, 9 (50%) were located in the jejunum and 3 (17%) were in each of the ileum, duodenum, and stomach; 44% (8) of the total erosions were grade 1, 39% (7) were grade 2, and 17% (3) were grade 3. Of the 12 erosions observed in group K, 67% (8) were in the jejunum, 8% (1) were in the ileum, and 25% (3) were in the stomach; 50% (6) of the total erosions were grade 1, 25% (3) were grade 2, 17% (2) were grade 3, and 8% (1) were grade 4 (Table 2 and Figure 1).
Food intake.
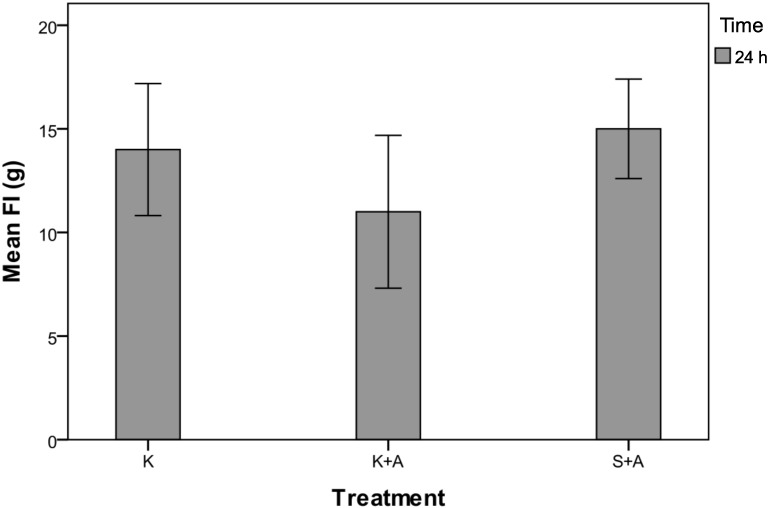
Mean food intake was significantly different among the rats in all 3 groups (P = 0.049, repeated-measures ANOVA; Figure 5), but post hoc comparisons could not detect differences between the subgroups. In the 24-h period after anesthesia and ketoprofen administration, rats in group K+A consumed 11 g (95% CI, 7 to 15 g), compared with 15 g (95% CI, 12 to 18 g) for group S+A and 14 g (95% CI, 10 to 18 g) for group K. Consumption in all 3 groups was down from the daily values of 19 g (95% CI, 17 to 20 g), 19 g (95% CI, 17 to 20 g), and 20 g (95% CI, 18 to 21 g) for groups K+A, S+A and K, respectively, that were calculated over a 5-d period before the experiment began. Five rats in group K+A and 2 rats in group K failed to consume measureable amounts of food, whereas all 20 rats in group S+A consumed measureable amounts of food.
Figure 5.
Food intake (g) for groups K, K+A and S+A at 24 h after injection of ketoprofen. There was a significant (P = 0.049) difference in mean food intake among the groups, but post hoc comparisons did not detect differences between subgroups. Data are expressed as mean values with the bounds of their respective 95% confidence intervals (n = 20 per group).
Body weights.
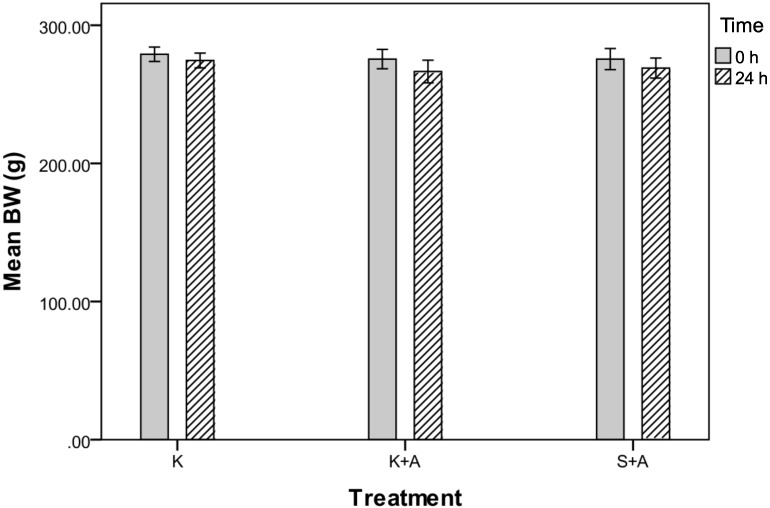
Mean body weight changes were not significantly different among the 3 groups (P = 0.414, repeated-measures ANOVA; Figure 6). Body weights fell by an average of 9 g (95% CI, −13 to −5 g) for group K+A, compared with declines of 6.5 g (95% CI, −10 to −3 g) and 4.5 g (95% CI, −7 to −2 g) in groups S+A and K, respectively. Body weights in all 3 groups had been increasing by a daily average of 3.3 g (95% CI, 2.5 to 4 g), 3.1 g (95% CI, 2 to 4 g), and 3.7 g (95% CI, 3 to 4.4 g) in groups K+A, S+ A, and K, respectively, during the 5-d preexperimental period. There was no linear relationship between body weight at time 0 and the 24-h measurement for PCV for all 3 groups (Spearman r, 0.0016; P = 0.749).
Figure 6.
Body weights (g) of groups K, K+A and S+A before (0 h) and after (24 h) injection of ketoprofen. Mean body weight did not differ (P = 0.414) among the 3 groups. Data are expressed as mean values with bounds of their their respective 95% confidence intervals (n = 20 per group).
Discussion
The main objective of this study was to determine whether a single, therapeutic dose of ketoprofen caused damage to the gastrointestinal mucosa in rats within 24 h after administration. A related aim was to determine whether anesthesia combined with ketoprofen in rats was more deleterious to the gastrointestinal tract than was ketoprofen administered without subsequent anesthesia. The results confirmed our primary hypothesis and suggested that the combination of anesthesia and ketoprofen in rats may be more toxic to the gastrointestinal tract than is ketoprofen treatment alone.
Both of the experimental groups (K+A and K) showed appreciatively more intestinal lesions (ulcers and erosions) than did the control group (Table 2 and Figure 1), and there were highly significant differences between the numbers of rats with lesions in the experimental groups compared with the control group. In addition, the experimental groups showed gastrointestinal blood loss, exhibited by the predominantly positive FOBT, gross appearance of the intestines, and mean declines in PCV and total protein concentration, compared with the mostly negative FOBT and mean increases in PCV and total protein in the control group (Table 1 and Figures 2, 3, and 4).
The fact that the experimental groups did not differ significantly from each other in the measurements of bleeding and numbers of animals with gastrointestinal lesions suggests that the combination of isoflurane anesthesia and ketoprofen compared with ketoprofen alone is not the determining factor for gastrointestinal damage in rats. However, the rats in group K+A appeared clinically worse than did those in group K, evidenced by group K+A's comparatively larger mean drops in PCV and total protein, positive FOBT in all rats, and larger declines in mean body weight and food intake (Table 1 and Figures 2, 3, 5, and 6). In addition, 3 rats in group K+A showed very large drops in PCV and total protein compared with 2 rats in group K.
Anesthesia alone without surgery or postoperative pain has been reported to cause a stress reaction involving activation of the hypothalamic–pituitary axis and corticosterone release.12,31 Whether higher corticosterone during 30 min of anesthesia could lead to acute gastrointestinal ulcerations has not been shown but if present might suggest effects on coping and healing mechanisms. Another possible explanation is that anesthetic-related hypotension8,31 contributed to ketoprofen-induced gastrointestinal toxicity due to reduced perfusion of the gastrointestinal tract and kidneys. This effect could have led to higher blood levels of the drug and diminished washout from the gastrointestinal mucosa.
Anesthesia might be an additive component for the development of gastrointestinal toxicity after therapeutic ketoprofen administration in rats. Although the numbers of rats with intestinal ulcers in the K+A and K groups were nearly equal (9 rats in K+A and 10 rats in K) and the incidence of ulcers was equivalent, rats in group K+A had 33% more erosions in the stomach and intestines than did those in group K (Table 2).
The highly significant difference in positive findings of outcomes for fecal occult blood among the 3 groups was supported by differences in the other measures of gastrointestinal bleeding (Table 1 and Figures 2 and 3). The guaiac-based FOBT is used as a qualitative screening test in human medicine for gastrointestinal bleeding that might indicate gastrointestinal disease.2,21 This method detects hemoglobin or heme released after hemolysis of RBC in the gastrointestinal tract.2 Because heme is metabolized by intestinal bacteria as it passes through the gastrointestinal tract, the guaiac test is thought to be more sensitive for detecting lesions in the distal compared with the upper gastrointestinal tract.2,21 This assumption supports our data that show the majority of lesions throughout the jejunum (Table 2). This distribution is further supported by studies showing that oral or subcutaneous administration of various nonselective NSAID cause intestinal lesions in rats.1,6,19,23,27 In this regard, the lower gastrointestinal tract of rats is more sensitive to nonselective NSAID toxicity than are those of dogs, monkeys, and humans, whereas the reverse is true for the upper gastrointestinal tract.6,19 Our data show few and minimal lesions in the stomach and duodenum in both experimental groups. The melena noted in some of the stools from rats in groups K+A and K suggests some upper gastrointestinal bleeding, but the majority of the stools for the experimental groups were dark, reddish brown, indicative of bleeding in the lower gastrointestinal tract.
The 2 positive FOBT observed in group S+A may reflect effects of anesthesic stress, even though these rats lacked gastrointestinal lesions. Normal fecal occult blood loss in humans varies from 0.5 to 1.5 mL/d,21 but how this value translates to normal rats is unknown. The 2 rats in group K with negative FOBT also lacked gastrointestinal lesions. FOBT were positive in all the rats with lesions in groups K+A and K. The slight increases in mean PCV and total protein in group S+A might be explained by postanesthesia fluid shifts after isoflurane-induced hypotension. A similar effect may also have occurred in group K+A, partially masking the extent of intestinal bleeding in that group.
Some of the rats in the experimental groups showed marked individual drops in PCV and total protein concentrations. In group K+A, the PCV and total protein for 3 rats (2 of which are represented in Figure 4 C and D) fell by 11%, 15%, and 20% and 2.2, 3.7, and 2.6 g/dL, respectively. In group K, the PCV and total protein concentrations for 2 rats (1 of which is represented in Figures 4 A and 7 B, C and D) fell by 10% and 22% and 1.5 and 4.6 g/dL, respectively. Interestingly, 2 rats in group K + A (represented in Figure 4 C and D) had no histologic lesions, but their intestines showed darkened areas, similar to those in rats with histologic changes in their intestines. These darkened areas indicate bleeding into the intestines.
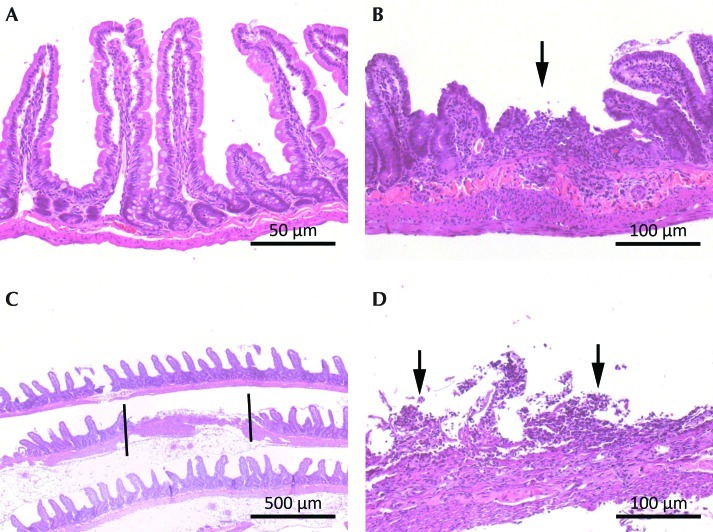
Figure 7.
Photomicrographs of intestines. Hematoxylin and eosin stain. (A) Normal jejunum, rat 18, group S+A, 24 h after ketoprofen injection. Grade 0; magnification, 40×. (B) Focal erosion, rat 49, group K, 24 h after ketoprofen injection. Grade 2; magnification. 20×. (C) Locally extensive mucosal ulcer of jejunum between bars, rat 49, group K, 24 h after ketoprofen injection. Grade 3; magnification, 4×. (D) Close-up of grade 3 ulcer shown in panel C. The arrows indicate denuded mucosal surface. Magnification, 40×.
The ceca from 2 representative rats (Figure 4 C and D) and from many others in groups K+A and K were dark red, and dark red-brown stool was observed in the ceca of these rats. We do not know whether this result was indicative of local lesions or was due to blood that traveled from more proximal areas of the gastrointestinal tract. Cecal and colonic lesions have developed in rats after the administration of nonselective NSAID.5,19,32
The anticoagulant effects of ketoprofen might have intensified gastrointestinal bleeding in some rats. Ketoprofen use in humans has caused significant decreases in platelet aggregation26,30 in ex vivo assays, but one study in ovariohysterectomized dogs showed that decreases in platelet aggregation were not accompanied by significant differences in the red blood indices or by evidence of prolonged bleeding between the experimental and control groups during or after surgery.15 Nevertheless, anticoagulant effects of ketoprofen cannot be ruled out in the current study.
The large drops in total protein concentration in the rats highlighted in Figure 4 and the general downtrend in total protein in both groups K+A and K provide more evidence for small intestinal pathologic changes, given that protein absorption occurs in the small intestine. Bleeding ulcers and erosions can cause vascular leakage of serum proteins into the intestinal lumen, in addition to potential acute protein malabsorption, as has been reported to occur in humans with gastrointestinal lesions due to chronic use of nonselective NSAID.3,14 Ketoprofen, like other NSAID, is highly protein-bound in the plasma.24 A large drop in serum proteins increases the levels of unbound drug. A positive feedback loop of intestinal mucosal damage, loss of serum proteins, and ensuing higher drug availability may have occurred in the most severe cases in groups K+A and K.
The majority of the small intestinal ulcers in groups K+A and K were located in the jejunum, with a few lesions in the ileum (Table 1). Ulcer severity ranged from minimal to moderate, with the moderate lesions encompassing 1 mm to 1 cm of the intestinal roll (Figures 1 and 7). No ulcers were found in the stomach or duodenum of the 2 experimental groups. Only one minimal ulcer, located in the duodenum, was observed in group S+A (Table 2). We do not know whether this ulcer was acute or chronic, but we saw no evidence of gastrointestinal bleeding in this rat, which had a negative FOBT, 2% increase in PCV at 24 h, and no change in total protein concentration.
Like the ulcers in our rats, most of the erosions in groups K+A and K were located in the jejunum, with a few in the stomach, duodenum, and ileum (Table 2). Severity ranged from minimal to marked (Figures 1 and 7). Few and minimal erosions were found in the stomach. The rats in this study were not fasted and had ad libitum access to rodent chow. A contributing factor for the scarcity of lesions in the stomach and duodenum might have been that the ingested chow acted as a buffer. This protective effect was demonstrated in one study that found fewer gastrointestinal tract lesions in fed rats treated with a nonselective NSAID compared with the number of gastric lesions in fasted rats that underwent the same treatment.23
In all 3 groups, body weight fell compared with the mean daily increases shown during the 5-d preexperimental period (Figure 6). Group K+A showed the largest decline, in line with their other generally worse clinical signs (including lower food consumption, Figure 5) compared with those of group K. The larger mean body weight drop in group S +A compared with group K may be an effect of anesthesia.
Although several studies have evaluated a single therapeutic dose or range of therapeutic doses of ketoprofen for pain management or potential toxicity in rats, they did not identify the apparent ulcerogenic effects of the 5-mg/kg dose.5,7,11,13,22,24,28 This outcome might be explained by small group sizes, uninformative time points, and different diagnostics used or questions asked.7,22 One report noted deleterious effects of ketoprofen (10 mg/kg SQ) on ovariectomized rats, finding ulcers, morbidity, and mortality for as long as 7 to 10 d after injection.13 The toxicity was attributed to the combination of ketoprofen and barrier housing for some of the rats. The mechanism for this action was not elucidated, but possibilities included the effects of microbial variability among the animals.13
Some reports have found a connection between the intestinal toxicity of nonselective NSAID and increased microbial numbers in the rat gastrointestinal tract.19,27,32 One study using germfree rats and indomethacin found that both germfree rats and their conventional counterparts developed intestinal ulcers, but ulcers were more severe in the conventional rats.16 Other studies contend that an important mechanism of nonselective NSAID-induced damage to the intestinal tract involves increased intestinal mucosal permeability that leads to increased bacterial numbers (which cause more mucosal damage), particularly with nonselective NSAID that undergo enterohepatic recirulation.20,32 Ketoprofen is excreted into the urine and bile in rats, and an enterohepatic cycle has been demonstrated in this species.9,11 Recirculation of the drug through the bile in the intestinal tract would prolong mucosal exposure to the toxic effects of the drug.
One potential limitation of the current study is the lack of a saline-only control group. Although handling and blood sampling might have been ulcerogenic in some rats, studies show that corticosterone levels do not increase from baseline in rats handled for less than 3 min.29 Our rats were not handled for blood sampling and other procedures for longer than 3 min. If we had observed more evidence of gastrointestinal bleeding in the S+A group, we might have added a saline-only control group. However other studies using oral or subcutaneous saline controls on operated or nonoperated rats did not find gastrointestinal lesions in these animals.5,7,13 From scientific and animal welfare standpoints, we could not justify including a saline-only group.
Another limitation may have been the single time point (24 h). A previous pilot study and our clinical experience with rats treated with 1 or 2 doses of ketoprofen (5 mg/kg SC) indicated that most rats survive the acute gastrointestinal insults but that mortality in some rats occurs within 1 to 2 wk. Some of the most profoundly affected rats in the current study might not have recovered from this single dose. In addition, the long-term consequences (possible fibrosis, adhesions, and strictures) of the acute gastrointestinal effects are unknown and should be investigated.
In conclusion, our data indicate that a single, therapeutic dose of ketoprofen (5 mg/kg SC) causes significant gastrointestinal bleeding, erosions, and ulcers of the small intestines of rats within 24 h after administration. The toxic effects of ketoprofen were evident in anesthetized and unanesthetized rats, although the anesthetized rats had worse clinical signs (larger drops in PCV, total protein, food intake, and body weight and a larger number of positive FOBT). The large drops in PCV and total protein concentration in some rats in both experimental groups suggested severe gastrointestinal mucosal damage. The guaiac-based FOBT was a useful screening tool for gastrointestinal bleeding and was positive in all rats in groups K+A and K that had gastrointestinal lesions. Given that ketoprofen is widely recommended, available, and used for pain management in rodents, these data have implications for both research and animal welfare. Directions for future investigation include additional long-term time points; using different rat stocks, strains, and sexes; and assessing the effects of therapeutic doses of other NSAID on the intestine of rats and perhaps mice.
Acknowledgments
We thank the Department of Animal Medicine (University of Massachusetts Medical School) for funding and supporting this research. Thanks to Dr Jerry Silverman for reviewing this manuscript.
References
- 1.Adams SS, Bough RG, Cliffe EE, Lessel B, Mills RF. 1969. Absorption, distribution, and toxicity of ibuprofen. Toxicol Appl Pharmacol 15:310–330 [DOI] [PubMed] [Google Scholar]
- 2.Beg M, Singh M, Saraswat MK, Rewari B. 2002. Occult gastrointestinal bleeding: detection, interpretation, and evaluation. J Indian Acad Clin Med 3:153–158 [Google Scholar]
- 3.Bjarnason I, Zanelli G, Prouse P, Smethurst P, Smith T, Levi S, Gumpel MJ, Levi AJ. 1987. Blood and protein loss via small-intestinal inflammation induced by nonsteroidal antiinflammatory drugs. Lancet 2:711–714 [DOI] [PubMed] [Google Scholar]
- 4.Burkholder TH, Niel L, Weed JL, Brinster LR, Bacher JD, Foltz CJ. 2010. Comparison of carbon dioxide and argon euthanasia: effects on behavior, heart rate, and respiratory lesions in rats. J Am Assoc Lab Anim Sci 49:448–453 [PMC free article] [PubMed] [Google Scholar]
- 5.Cabre F, Fernandez F, Zapatero MI, Arano A, Garcia ML, Mauleon D. 1998. Intestinal ulcerogenic effect of S(+)-ketoprofen in the rat. J Clin Pharmacol 38:27S–32S [PubMed] [Google Scholar]
- 6.Elliott GA, Purmalis A, VanderMeer DA, Denlinger RH. 1988. The propionic acids. Gastrointestinal toxicity in various species. Toxicol Pathol 16:245–250 [DOI] [PubMed] [Google Scholar]
- 7.Flecknell PA, Orr HE, Roughan JV, Stewart R. 1999. Comparison of the effects of oral or subcutaneous carprofen or ketoprofen in rats undergoing laparotomy. Vet Rec 144:65–67 [DOI] [PubMed] [Google Scholar]
- 8.Gaertner DJ, Hallman TM, Hankerson FC, Batchelder MA.2008. Anesthesia and analgesia for laboratory rodents, p 239. In: Fish RE, Brown MJ, Danneman PJ, Karas AZ, editors. Anesthesia and analgesia in laboratory animals. New York (NY): Elsevier.
- 9.Granero GE, Amidon GL. 2008. Possibility of enterohepatic recycling of ketoprofen in dogs. Int J Pharm 349:166–171 [DOI] [PubMed] [Google Scholar]
- 10.Hawk CT, Leary SL, Morris TH.2005. Ketoprofen, p 30. In: Formulary for laboratory animals. Ames (IA): Blackwell publishing.
- 11.Julou L, Guyonnet JC, Ducrot R, Fournel J, Pasquet J. 1976. Ketoprofen (19.583 R.P.) (2-(3-benzoylphenyl)-propionic acid). Main pharmacological properties—outline of toxicological and pharmacokinetic data. Scand J Rheumatol Suppl 1976:33–44 [PubMed] [Google Scholar]
- 12.Kurosawa S, Kato M. 2008. Anesthetics, immune cells, and immune responses. J Anesth 22:263–277 [DOI] [PubMed] [Google Scholar]
- 13.Lamon TK, Browder EJ, Sohrabji F, Ihrig M. 2008. Adverse effects of incorporating ketoprofen into established rodent studies. J Am Assoc Lab Anim Sci 47:20–24 [PMC free article] [PubMed] [Google Scholar]
- 14.Lanas A, Panes J, Pique JM. 2003. Clinical implications of COX1 and/or COX2 inhibition for the distal gastrointestinal tract. Curr Pharm Des 9:2253–2266 [DOI] [PubMed] [Google Scholar]
- 15.Lemke KA, Runyon CL, Horney BS. 2002. Effects of preoperative administration of ketoprofen on whole-blood platelet aggregation, buccal mucosal bleeding time, and hematologic indices in dogs undergoing elective ovariohysterectomy. J Am Vet Med Assoc 220:1818–1822 [DOI] [PubMed] [Google Scholar]
- 16.Melarange R, Moore G, Blower PR, Coates ME, Ward FW, Ronaasen V. 1992. A comparison of indomethacin with ibuprofen on gastrointestinal mucosal integrity in conventional and germ-free rats. Aliment Pharmacol Ther 6:67–77 [DOI] [PubMed] [Google Scholar]
- 17.Moolenbeek C, Ruitenberg EJ. 1981. The “Swiss roll”: a simple technique for histological studies of the rodent intestine. Lab Anim 15:57–59 [DOI] [PubMed] [Google Scholar]
- 18.Plumb DC.2011. Ketoprofen, p 579. In: Plumb's veterinary drug handbook. Ames (IA): Wiley-Blackwell.
- 19.Radi ZA, Khan NK. 2006. Effects of cyclooxygenase inhibition on the gastrointestinal tract. Exp Toxicol Pathol 58:163–173 [DOI] [PubMed] [Google Scholar]
- 20.Reuter BK, Davies NM, Wallace JL. 1997. Nonsteroidal anti-inflammatory drug enteropathy in rats: role of permeability, bacteria, and enterohepatic circulation. Gastroenterology 112:109–117 [DOI] [PubMed] [Google Scholar]
- 21.Rockey DC. 2005. Occult gastrointestinal bleeding. Gastroenterol Clin North Am 34:699–718 [DOI] [PubMed] [Google Scholar]
- 22.Roughan JV, Flecknell PA. 2001. Behavioural effects of laparotomy and analgesic effects of ketoprofen and carprofen in rats. Pain 90:65–74 [DOI] [PubMed] [Google Scholar]
- 23.Satoh H, Guth PH, Grossman MI. 1982. Role of food in gastrointestinal ulceration produced by indomethacin in the rat. Gastroenterology 83:210–215 [PubMed] [Google Scholar]
- 24.Satterwhite JH, Boudinot FD. 1992. Pharmacokinetics of ketoprofen in rats: effect of age and dose. Biopharm Drug Dispos 13:197–212 [DOI] [PubMed] [Google Scholar]
- 25.Shientag LJ, Rosenthal KL, Chandler HK, Wheeler SM. 2011. Bilateral traumatic temporomandibular joint luxation in a rat. Comp Med 61:510–513 [PMC free article] [PubMed] [Google Scholar]
- 26.Stichtenoth DO, Tsikas D, Gutzki FM, Frolich JC. 1996. Effects of ketoprofen and ibuprofen on platelet aggregation and prostanoid formation in man. Eur J Clin Pharmacol 51:231–234 [DOI] [PubMed] [Google Scholar]
- 27.Tanaka A, Hase S, Miyazawa T, Ohno R, Takeuchi K. 2002. Role of cyclooxygenase (COX) 1 and COX2 inhibition in nonsteroidal antiinflammatory drug-induced intestinal damage in rats: relation to various pathogenic events. J Pharmacol Exp Ther 303:1248–1254 [DOI] [PubMed] [Google Scholar]
- 28.Tsurumi K, Nozaki M, Nakano K, Go K, Fujimura H. 1977. Pharmacological studies of ketoprofen (19583RP) III. Antiinflammatory, analgesic, and antipyretic actions in subcutaneous administration (author's transl). Nippon Yakurigaku Zasshi 73:633–650 [DOI] [PubMed] [Google Scholar]
- 29.Vahl total protein, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D'Alessio DA, Herman JP. 2005. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab 289:E823–E828 [DOI] [PubMed] [Google Scholar]
- 30.Van Solingen RM, Rosenstein ED, Mihailescu G, Drejka ML, Kalia A, Cohen AJ, Kramer N. 2001. Comparison of the effects of ketoprofen on platelet function in the presence and absence of aspirin. Am J Med 111:285–289 [DOI] [PubMed] [Google Scholar]
- 31.Wade JG, Stevens WC. 1981. Isoflurane: an anesthetic for the 80s? Anesth Analg 60:666–682 [PubMed] [Google Scholar]
- 32.Wallace JL. 1997. Nonsteroidal antiinflammatory drugs and gastroenteropathy: the second 100 years. Gastroenterology 112:1000–1016 [DOI] [PubMed] [Google Scholar]