Abstract
Purpose
Pulsatile movements of the dura mater have been interpreted as a sign that the cord is free within the subarachnoid space, with no extrinsic compression. However, the association between restoration of pulsation and adequate decompression of the spinal cord has not been established. The present study investigated the relationship between the extent of spinal cord decompression and spinal cord and dural pulsations based on quantitative analysis of intraoperative ultrasonography (US).
Methods
Eighty-five consecutive patients (55 males, 30 females; mean age, 64 ± 13 years) who underwent cervical double-door laminoplasty to relieve compressive myelopathy were enrolled. Spinal cord decompression status was classified as: Type 1 (non-contact), the subarachnoid space was retained on the ventral side of the cord, Type 2 (contact and apart), the cord showed both contact with and separation from the anterior element of the cervical spine, or Type 3 (contact), the cord showed continuous contact with the anterior element of the cervical spine. Spinal cord and dura mater dynamics were quantitatively analyzed using automatic video-tracking software. Furthermore, the intensity of spinal and dural pulsation was compared with the recovery of motor function at 1 year after surgery as measured by increase in the Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire (JOACMEQ).
Results
Spinal cord pulsation amplitude ranged from 0.01 to 0.84 mm (mean 0.30 ± 0.16 mm) and dural pulsation amplitude ranged from 0.01 to 0.38 mm (mean 0.14 ± 0.08 mm). Average spinal cord pulsation amplitude in Type 2 patients was significantly larger than that in the other groups, whereas, average dural pulsation amplitudes were similar for all three groups. There was a significant correlation between spinal cord and dural pulsation amplitudes in Type 1 patients, but not in Type 2 or Type 3 patients. Type 3 patients showed a particularly poor correlation between spinal cord and dural pulsations. Spinal cord pulsation amplitude was moderately correlated with the recovery of motor function evaluated by JOACMEQ.
Conclusion
The present results suggest that restoration of dural pulsation is not an adequate indicator of sufficient decompression of the spinal cord following a surgical procedure.
Keywords: Cervical spine, Myelopathy, Laminoplasty, Ultrasonography, Cerebrospinal fluid
Introduction
Periodic pulsations in the central nervous system are caused by pressure changes in the vascular system of the brain and spinal cord [9]. Pulsatile movements of the spinal cord, dura mater, and cerebrospinal fluid (CSF) have been widely recognized through intraoperative findings [5] and extensively evaluated by magnetic resonance imaging (MRI) [2, 9]. Surgeons have empirically interpreted the pulsatile movements of the dura mater as a sign that the cord is free within the subarachnoid space, with no extrinsic compression. However, it remains unclear whether restoration of dural pulsation is associated with adequate decompression of the spinal cord and whether dural and spinal cord motions correlate with each other.
The recent development of high-resolution intraoperative ultrasonography (US) allows real-time visualization of intraspinal abnormalities without dissection of the dura mater. Intraoperative US has been used to evaluate the decompression status of the spinal cord in patients with cervical compressive myelopathy [6, 10, 13, 15]. This real-time imaging technique, coupled with quantitative analysis software, permits objective measurements of the spinal cord and dural motion following decompression. In the present study, we investigated the relationships between the extent of spinal cord decompression and spinal cord and dural pulsations based on quantitative analysis of intraoperative US.
Methods
All patients who underwent cervical double-door laminoplasty for compressive myelopathy at our hospital between 2008 and 2010 were enrolled. The study group consisted of 85 consecutive patients (55 males, 30 females; mean age, 64 ± 13 years). The cause of cervical myelopathy was spondylosis in 56 patients and ossification of the longitudinal ligament in 29.
The procedure for double-door laminoplasty has been described in detail elsewhere [4, 7, 8]. The patient was placed prone with the neck in a neutral position on a horseshoe. In most patients, the cervical laminae from C3 to C7 were exposed laterally to the medial aspect of the facet joints through a conventional midline approach. For a C2 split, the semispinalis cervicis muscles were transiently detached. Bilateral gutters were made using a high-speed burr at the transitional area between the facet joint and the laminae, and the spinous processes were then split sagittally with a high-speed burr. The spinal canal was enlarged by opening the split laminae bilaterally with a spreader. To maintain the expanded position, hydroxyapatite spacers (Bonecerum; Olympus Co., Ltd., Tokyo, Japan) were placed between the split laminae and fixed with nonabsorbable sutures. In the case of C2 laminoplasty, detached muscles were repaired with nonabsorbable sutures.
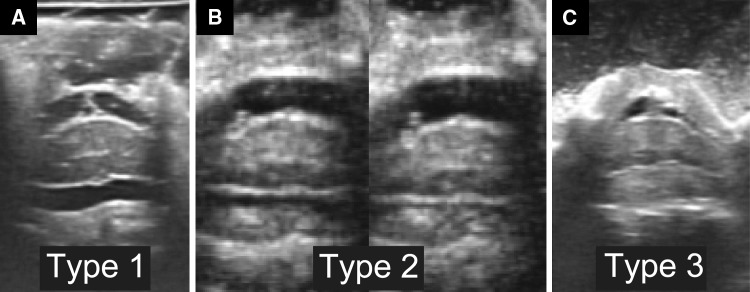
Immediately following expansion of the split laminae, intraoperative US was performed using a water-path imaging technique to investigate the dynamics of the spinal cord and dura mater using a digital echo camera (Prosound α10; Hitachi-Aloca Medical Co., Tokyo, Japan) and a 13 MHz linear array transducer. Pulsatile movements of the spinal cord and dura mater were videotaped in an axial view at the narrowest level of the cervical canal, which was identified on preoperative MRI. The ultrasound transducer was directed perpendicular to the spinal cord to obtain an accurate axial section and was stabilized for several seconds to prevent motion blur in the video. The decompression status of the spinal cord was classified into three types, as described previously [15]: Type 1 (non-contact), the subarachnoid space was retained on the ventral side of the spinal cord, Type 2 (contact and apart), the spinal cord showed both contact with and separation from the anterior element of the cervical spine in synchrony with cord pulsations, and Type 3 (contact), the cord showed continuous contact with the anterior element of the cervical spine (Fig. 1).
Fig. 1.
Decompression status of the spinal cord was classified into three types. In Type 1 (noncontact), the subarachnoid space was retained on the ventral side of the spinal cord (a). In Type 2 (contact and apart), the spinal cord showed both contact with and separation from the anterior element of the cervical spine in synchrony with cord pulsations (b). In Type 3 (contact), the cord showed continuous contact with the anterior element of the cervical spine (c)
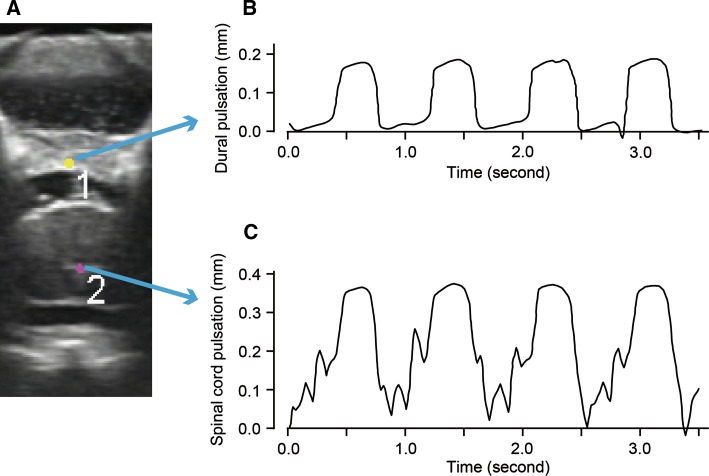
The dynamics of the spinal cord and the dura mater were quantitatively analyzed using automatic video-tracking software (VW-H1MA; Keyence, Osaka, Japan). Briefly, regions of interest (ROI) were set at the central echo of the spinal cord, a constant feature of the normal cord [14], and at the dorsal midpoint of the dura mater (Fig. 2a). Each ROI was automatically tracked on the US movie, and a time–motion chart was created based on the location of the ROI in the vertical axis (Fig. 2b, c). Pulsation amplitude was measured as the average of at least three consecutive pulsatile waves on the chart.
Fig. 2.
Representative images of spinal cord and dural pulsations tracked on intraoperative US. Regions of interest were set at the central echo of the spinal cord, a constant feature of the normal cord, and on the dorsal midpoint of the dura mater (a). Pulsatile movements of the dura mater (b) and the spinal cord (c) were automatically traced on the intraoperative US. Each pulsation was synchronized with the heart beat
We investigated whether the pulsatile movement of the spinal cord and dura mater is associated with imaging parameters, including cervical alignment and the severity of spinal cord compression. Preoperative cervical alignment was measured as the Cobb angle between C2 and C7 (C2–7 angle) on a lateral radiograph in the neutral position. Intraoperative cervical alignment was also measured with the C2–7 angle on the operating table. Preoperative cervical range of motion was evaluated on functional radiographs. Preoperative severity of spinal cord compression was assessed by measuring the cross-sectional area of the cervical canal at each intervertebral level on magnetic resonance imaging (MRI).
Neurological outcome was evaluated preoperatively and at 1 year after surgery based on upper- and lower-extremity motor function as measured by JOACMEQ [3, 12]. JOACMEQ is a patient-oriented outcome measure for cervical myelopathy comprised 24 question items. The questions are categorized into five functional domains: cervical spine function, upper-extremity function, lower-extremity function, bladder function, and quality of life. JOACMEQ provides functional scores ranging from 0 to 100 for each of the five functional domains. We employed postoperative increases in JOACMEQ upper- and lower-extremity motor function scores as an indicator of neurological recovery. The increase in JOACMEQ was compared with various imaging parameters, including the C2–7 angle, narrowest canal area on MRI, and measurements in the intraoperative US.
SPSS version 17 software (SPSS, Chicago, IL, USA) was used for all statistical analyses. Differences in pulsation amplitudes among groups were compared using one-way ANOVA followed by the Bonferroni post hoc test. Correlations between the spinal cord and dura mater pulsations were evaluated using Pearson’s correlation coefficients.
Results
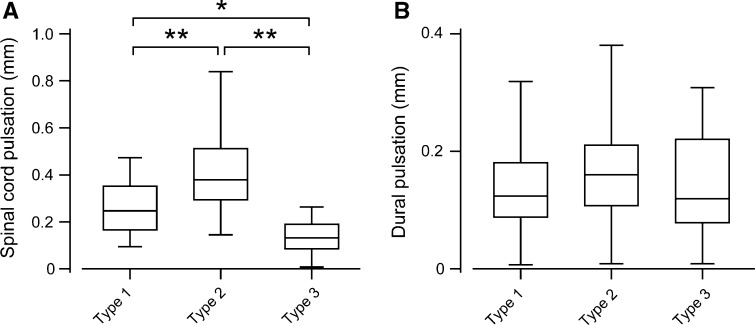
Intraoperative US demonstrated a decompression status of Type 1 in 44 patients (52 %), Type 2 in 26 (31 %), and Type 3 in 15 (18 %). Quantitative analysis of intraoperative US revealed that spinal cord pulsation amplitude ranged from 0.01 to 0.84 mm (mean 0.30 ± 0.16 mm) and dural pulsation amplitude ranged from 0.01 to 0.38 mm (mean 0.14 ± 0.08 mm). The average dural pulsation amplitude was approximately half of the average spinal cord pulsation amplitude. Spinal cord pulsation amplitude differed significantly depending on decompression status (Fig. 3a); the average amplitude in Type 3 patients was significantly smaller than that in the other groups, and the average amplitude in Type 2 patients was significantly larger than that in Type 1 patients. However, there were no significant differences in the average dural pulsation amplitudes among the three groups (Fig. 3b).
Fig. 3.
Spinal cord and dural pulsation amplitudes for each type of decompression. Average spinal cord pulsation amplitudes were significantly larger in Type 2 patients than in patients with other types of decompression (a); (one-way ANOVA with Bonferroni’s post hoc test, *P < 0.05, **P < 0.001). However, there were no significant differences in dural pulsation amplitudes among the different decompression groups (b)
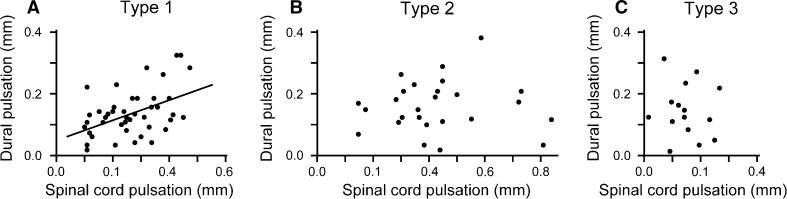
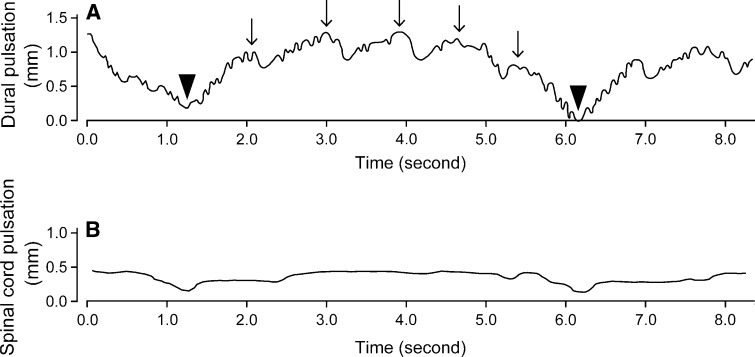
The relationship between the pulsation amplitudes of the spinal cord and dura mater was analyzed for each decompression type. Type 1 patients showed a significant correlation between the spinal cord and dural pulsation amplitudes (Pearson’s correlation coefficient = 0.487, P = 0.0012; Fig. 4a), whereas, no significant correlation was observed in Type 2 and 3 patients (Fig. 4b, c). The correlation between spinal cord and dural pulsations was particularly poor in Type 3 patients. The dural pulsation amplitude was larger than the spinal cord pulsation amplitude in 7 of 15 patients with Type 3 decompression, and some patients with Type 3 decompression demonstrated obvious dural pulsations despite having minimal spinal cord pulsations (Fig. 5). In addition to pulsations with a frequency corresponding to the heart rate, the patient in Fig. 5 also showed large slow waves with a frequency corresponding to the respiratory rate.
Fig. 4.
Relationship between spinal cord and dural pulsations. A significant correlation was observed between spinal cord and dural pulsation amplitudes in Type 1 patients (a); (correlation coefficient = 0.487, P = 0.001). However, there was no correlation in Type 2 (b) and Type 3 patients (c)
Fig. 5.
The dura mater can show obvious pulsations despite minimal spinal cord pulsations. This patient with Type 3 decompression showed dural pulsations that were synchronized with the heartbeat (arrows), even though the spinal cord demonstrated minimal pulsations. Note that the dural pulsations are combined with large slow waves with a frequency corresponding to the respiratory rate (arrowheads)
The average preoperative C2–7 angle was 12.4 ± 10.3°, 12.1 ± 7.7°, and 8.6 ± 10.3° in the Type 1, 2 and 3 groups, respectively. The preoperative C2–7 angle was the smallest in the Type 3 group, although there was no significant difference among the groups. The average intraoperative C2–7 angle was 3.7 ± 5.3°, 3.1 ± 5.2°, and 0.6 ± 4.1° in the Type 1, 2, and 3 groups, respectively. Similarly, there was no significant difference among the groups. The intraoperative C2–7 angle showed no significant correlation with spinal cord and dural pulsation amplitude. However, there was a weak correlation between preoperative C2–7 angle and spinal cord pulsation amplitude (correlation coefficient = 0.251, P = 0.009). The average preoperative spinal cord area was smallest in the Type 3 group (64.6 ± 13.8, 65.1 ± 16.1, and 54.4 ± 12.7 mm2 in the Type 1, 2 and 3 groups, respectively), however, there was no significant difference among the groups. The preoperative spinal cord area showed no significant correlation with spinal cord or dural pulsation amplitudes.
The average upper- and lower-extremity motor function scores measured as JOACMEQ (JOACMEQ-U and JOACMEQ-L) were 69.9 ± 23.1 and 51.1 ± 28.4 before surgery, and these improved to 84.5 ± 18.3 and 68.0 ± 25.7 at 1 year after surgery, respectively. The postoperative increase in JOACMEQ-U was 16.2 ± 18.6, 16.3 ± 21.2, and 3.0 ± 17.1 and in JOACMEQ-L was 12.8 ± 23.6, 26.5 ± 25.9, and 10.6 ± 21.3 in Type 1, 2 and 3 patients, respectively. Patients with Type 3 decompression showed the smallest increase both in JOACMEQ-U and JOACMEQ-L, although there was no statistically significant difference among the groups. Preoperative and intraoperative imaging parameters were compared with the recovery of motor function measured as increase in JOACMEQ (Table 1). Increases in both JOACMEQ-U and JOACMEQ-L are moderately correlated with spinal cord pulsation amplitudes. The preoperative C2–7 angle was weakly correlated with increase in JOACMEQ-L.
Table 1.
Correlations between various imaging parameters and the recovery of motor function as measured by increases in JOACMEQ scores
| Increase in JOACMEQ-U | Increase in JOACMEQ-L | |||
|---|---|---|---|---|
| R (95 % CI) | P | R (95 % CI) | P | |
| Preoperative C2–7 angle | 0.211 (−0.06 to 0.46) | 0.132 | 0.291 (0.02 to 0.52) | 0.036* |
| Preoperative canal area | 0.123 (−0.15 to 0.38) | 0.382 | −0.008 (−0.28 to 0.26) | 0.958 |
| Spinal cord pulsation amplitude | 0.371 (0.11 to 0.58) | 0.006** | 0.406 (0.15 to 0.61) | 0.003** |
| Dural pulsation amplitude | 0.155 (−0.12 to 0.41) | 0.267 | −0.005 (−0.27 to 0.23) | 0.970 |
JOACMEQ the Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire, JOACMEQ-U upper-extremity motor function score of JOACMEQ, JOACMEQ-L lower-extremity motor function score of JOACMEQ, R Pearson’s correlation coefficient, CI confidence interval, C2–7 angle Cobb angle between C2 and C7
* P < 0.05, ** P < 0.01
Discussion
The present study demonstrated that spinal cord pulsation amplitude differs significantly depending on the decompression status of the spinal cord; the highest spinal cord pulsation amplitude was observed in patients with Type 2 decompression, in which the spinal cord showed periodic contact with the anterior element of the cervical spine. No significant differences were observed in dural pulsation amplitude among the different types of decompression. These results suggest that restoration of dural pulsation does not necessarily indicate sufficient decompression of the spinal cord following a surgical procedure.
Despite the fact that Type 1 decompression represents continuous separation of the spinal cord from the anterior element, indicating that the cord is completely free of extrinsic compression, the average spinal cord pulsation amplitude in Type 1 patients in the present study was significantly lower than that in Type 2 patients. This paradoxical finding could be explained by a posterior shift of the spinal cord away from the anterior element, leading to tethered nerve roots, consequently restricting pulsatile movement of the spinal cord. Tethering of the nerve root due to excessive posterior movement of the spinal cord is suggested to be the pathophysiologic mechanism for segmental motor paresis (C5 palsy) after laminoplasty [17].
Few studies have investigated the relationship between dural and spinal cord pulsations after decompressive procedures for cervical myelopathy [5, 6]. Kawakami et al. [6] classified the intensity of dural and spinal cord pulsations into four grades (absent, weak, fair, and strong) using intraoperative US during cervical laminoplasty, and concluded that dural pulsations do not always imply the absence of spinal cord compression. Jokich et al. [5] analyzed intraoperative US in patients with various pathological conditions and concluded that the dura and the spinal cord may move completely independently of each other. Although these results were obtained from a subjective and qualitative analysis of low-resolution US, they are consistent with our findings indicating a poor correlation between dural and spinal cord pulsation amplitudes in Type 2 and 3 patients. In addition, our quantitative study demonstrated that this relationship differs according to the decompression status of the spinal cord; dural and spinal cord pulsation amplitudes correlate significantly only in patients with Type 1 decompression.
It has been suggested that intraoperative US has value for predicting the neurological outcome of posterior decompression surgery in patients with cervical myelopathy [10, 13]. Mihara et al. [10] showed that restoration of the anterior subarachnoid space is a significant factor in neurologic improvement following posterior cervical decompression surgery. Consistent with their results, functional recovery in patients with Type 3 decompression was smallest among the groups although there was no statistical significance among them. We further investigated the relationship between quantitative spinal pulsation measures and functional recovery assessed with a patient-oriented measurement. The moderate but significant correlation between spinal cord pulsation amplitude and the functional recovery suggests that there is clinical significance for spinal cord dynamics evaluated by intraoperative US.
Recently, an increasing amount of emphasis has been placed on minimally invasive spine surgery (MISS), including the use of micro-endoscope, minimal incision, and muscle preservation techniques [1, 11, 16]. Because it is difficult to apply intraoperative US to confirm the decompression of the spinal cord during MISS, the only clue to predict the decompression status would be the surface appearance of the dura mater. Indeed, visualization of the posterior protrusion and dural pulsation is usually considered to indicate the completion of micro-endoscopic decompression for cervical myelopathy [11]. Considering the current results, however, caution should be exercised when interpreting dural pulsation as a sign of sufficient decompression.
In conclusion, the present results suggest that restoration of dural pulsation is not an adequate indicator of sufficient decompression of the spinal cord. The dura mater may show obvious pulsations independent of spinal cord pulsations, especially when the spinal cord shows continuous contact with the anterior element. These results highlight the importance of intraoperative US in confirming the decompression status of the spinal cord.
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Boehm H, Greiner-Perth R, El-Saghir H, Allam Y. A new minimally invasive posterior approach for the treatment of cervical radiculopathy and myelopathy: surgical technique and preliminary results. Eur Spine J. 2003;12:268–273. doi: 10.1007/s00586-002-0522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enzmann DR, Pelc NJ. Normal flow patterns of intracranial and spinal cerebrospinal fluid defined with phase-contrast cine MR imaging. Radiology. 1991;178:467–474. doi: 10.1148/radiology.178.2.1987610. [DOI] [PubMed] [Google Scholar]
- 3.Fukui M, Chiba K, Kawakami M, Kikuchi S, Konno S, Miyamoto M, Seichi A, Shimamura T, Shirado O, Taguchi T, Takahashi K, Takeshita K, Tani T, Toyama Y, Yonenobu K, Wada E, Tanaka T, Hirota Y. JOA back pain evaluation questionnaire (JOABPEQ)/JOA cervical myelopathy evaluation questionnaire (JOACMEQ). The report on the development of revised versions. April 16, 2007. The subcommittee of the clinical outcome committee of the japanese orthopaedic association on low back pain and cervical myelopathy evaluation. J Orthop Sci. 2009;14:348–365. doi: 10.1007/s00776-009-1337-8. [DOI] [PubMed] [Google Scholar]
- 4.Hirabayashi S, Yamada H, Motosuneya T, Watanabe Y, Miura M, Sakai H, Matsushita T. Comparison of enlargement of the spinal canal after cervical laminoplasty: open-door type and double-door type. Eur Spine J. 2010;19:1690–1694. doi: 10.1007/s00586-010-1369-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jokich PM, Rubin JM, Dohrmann GJ. Intraoperative ultrasonic evaluation of spinal cord motion. J Neurosurg. 1984;60:707–711. doi: 10.3171/jns.1984.60.4.0707. [DOI] [PubMed] [Google Scholar]
- 6.Kawakami N, Mimatsu K, Kato F, Sato K, Matsuyama Y. Intraoperative ultrasonographic evaluation of the spinal cord in cervical myelopathy. Spine (Phila Pa 1976) 1994;19:34–41. doi: 10.1097/00007632-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Kimura A, Seichi A, Inoue H, Hoshino Y. Long-term results of double-door laminoplasty using hydroxyapatite spacers in patients with compressive cervical myelopathy. Eur Spine J. 2011;20:1560–1566. doi: 10.1007/s00586-011-1724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luk KD, Kamath V, Avadhani A, Rajasekaran S. Cervical laminoplasty. Eur Spine J. 2010;19:347–348. doi: 10.1007/s00586-010-1314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maier SE, Hardy CJ, Jolesz FA. Brain and cerebrospinal fluid motion: real-time quantification with M-mode MR imaging. Radiology. 1994;193:477–483. doi: 10.1148/radiology.193.2.7972766. [DOI] [PubMed] [Google Scholar]
- 10.Mihara H, Kondo S, Takeguchi H, Kohno M, Hachiya M. Spinal cord morphology and dynamics during cervical laminoplasty: evaluation with intraoperative sonography. Spine (Phila Pa 1976) 2007;32:2306–2309. doi: 10.1097/BRS.0b013e318155784d. [DOI] [PubMed] [Google Scholar]
- 11.Minamide A, Yoshida M, Yamada H, Nakagawa Y, Maio K, Kawai M, Iwasaki H. Clinical outcomes of microendoscopic decompression surgery for cervical myelopathy. Eur Spine J. 2010;19:487–493. doi: 10.1007/s00586-009-1233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakashima H, Yukawa Y, Ito K, Machino M, Kanbara S, Morita D, Takahashi H, Imagama S, Ito Z, Ishiguro N, Kato F. Prediction of lower limb functional recovery after laminoplasty for cervical myelopathy: focusing on the 10 s step test. Eur Spine J. 2012;21(7):1389–1395. doi: 10.1007/s00586-012-2241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naruse T, Yanase M, Takahashi H, Horie Y, Ito M, Imaizumi T, Oguri K, Matsuyama Y. Prediction of clinical results of laminoplasty for cervical myelopathy focusing on spinal cord motion in intraoperative ultrasonography and postoperative magnetic resonance imaging. Spine (Phila Pa 1976) 2009;34:2634–2641. doi: 10.1097/BRS.0b013e3181b46c00. [DOI] [PubMed] [Google Scholar]
- 14.Quencer RM, Montalvo BM. Normal intraoperative spinal sonography. AJR Am J Roentgenol. 1984;143:1301–1305. doi: 10.2214/ajr.143.6.1301. [DOI] [PubMed] [Google Scholar]
- 15.Seichi A, Chikuda H, Kimura A, Takeshita K, Sugita S, Hoshino Y, Nakamura K. Intraoperative ultrasonographic evaluation of posterior decompression via laminoplasty in patients with cervical ossification of the posterior longitudinal ligament: correlation with 2 year follow-up results. J Neurosurg Spine. 2010;13:47–51. doi: 10.3171/2010.3.SPINE09680. [DOI] [PubMed] [Google Scholar]
- 16.Shiraishi T, Fukuda K, Yato Y, Nakamura M, Ikegami T. Results of skip laminectomy-minimum 2 year follow-up study compared with open-door laminoplasty. Spine (Phila Pa 1976) 2003;28:2667–2672. doi: 10.1097/01.BRS.0000103340.78418.B2. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji T, Asazuma T, Masuoka K, Yasuoka H, Motosuneya T, Sakai T, Nemoto K. Retrospective cohort study between selective and standard C3–7 laminoplasty. Minimum 2-year follow-up study. Eur Spine J. 2007;16:2072–2077. doi: 10.1007/s00586-007-0428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]