Abstract
Purpose
New interspinous process decompression devices (IPDs) provide an alternative to conservative treatment and decompressive surgery for patients with neurogenic intermittent claudication (NIC) due to degenerative lumbar spinal stenosis (DLSS). APERIUS® is a minimally invasive IPD that can be implanted percutaneously. This multicentre prospective study was designed to make a preliminary evaluation of safety and effectiveness of this IPD up to 12 months post-implantation.
Methods
After percutaneous implantation in 156 patients with NIC due to DLSS, data on symptoms, quality of life, pain, and use of pain medication were obtained for up to 12 months.
Results
Early symptom and physical function improvements were maintained for up to 12 months, when 60 and 58 % of patients maintained an improvement higher than the Minimum Clinically Important Difference for Zurich Claudication Questionnaire (ZCQ) symptom severity and physical function, respectively. Leg, buttock/groin, and back pain were eased throughout, and the use and strength of related pain medication were reduced. Devices were removed from 9 % of patients due to complications or lack of effectiveness.
Conclusions
Overall, in a period of up to 12 months follow-up, the safety and effectiveness of the APERIUS® offered a minimally invasive option for the relief of NIC complaints in a high proportion of patients. Further studies are underway to provide insight on outcomes and effectiveness compared to other decompression methods, and to develop guidance on optimal patient selection.
Keywords: Lumbar stenosis, Neurogenic claudication, Interspinous device, Percutaneous
Introduction
Degenerative lumbar spinal stenosis (DLSS) is the most common type of spinal stenosis, with a reported incidence of 2–8 % in the general population [1]. Neurogenic intermittent claudication (NIC) is the cardinal symptom of DLSS, presenting as numbness, weakness and discomfort in the legs (with or without back pain) during walking or prolonged standing. One of the characteristic findings in NIC is the regression of symptoms when sitting or forward bending [2]. Lumbar extension decreases the cross-sectional area of the dural sac as well as the neural foramina, causing compression on neural and vascular structures, which triggers exacerbation of the symptoms [3–5].
Interspinous process decompression devices (IPDs) provide an alternative therapy to conservative treatment and are less invasive than open decompressive surgery for patients suffering from NIC. Biomechanical studies have shown that IPDs significantly reduce intradiscal pressure and facet load, and prevent narrowing of the spinal canal and neural foramen [6].
The APERIUS® device (Kyphon Sàrl, Neuchâtel, Switzerland) is an IPD that can be implanted percutaneously. It is indicated for the treatment of patients suffering from 1- or 2-level symptomatic DLSS (L1–L5) with NIC, exacerbated in the upright posture or by extension of the lumbar spine and relieved by flexion.
This prospective, single-arm, post-marketing study of consecutive eligible cases in 12 centers in Germany, Belgium, and the United Kingdom was designed to assess the safety and effectiveness of the IPD up to 12 months post-procedure.
Materials and methods
Surgical technique
After radiographic identification of the surgical level, a 1.5-cm incision is made at the affected level, 6–10 cm lateral to the midline. Under fluoroscopy, an 8-mm sharp trocar is introduced and advanced toward the interspinous space, followed by blunt trocars of increasing size. The percutaneous insertion of increasing size trocars allows a gradual distraction of the interspinous area to achieve optimal decompression and prevent over-distraction. An inserter is then used to achieve correct placement of the bullet-shaped implant. By turning the actuating handle of the inserter, a compressive force is created, retracting the outer shell and thereby deploying 2 × 3 “wings” (Fig. 1). The wings expand on each side of the spinous process, stabilizing the IPD on the midline and working with the intact supraspinous ligaments to keep the IPD in place.
Fig. 1.
Positioning of the IPD between the spinous processes and showing deployment of wings Top pictures show positioning of the IPD in the interspinous space while the bottom pictures show the IPD with wings deployed (indicated by arrows)
Population
The IPD was implanted at symptomatic levels in 156 patients, 78 men and 78 women, aged 64.8 ± 11.9 years (range 19–84 years). At baseline, all patients had to have a history of DLSS (L1–L5), confirmed by MRI, with symptoms of NIC, including leg/buttock/groin pain, with or without back pain, relieved by flexion. If back pain was also present, it was to be partially relieved when flexed. Patients had to be able to sit for 50 min without pain and walk a distance of 20 m without pain.
Follow-up
The primary effectiveness endpoint was the change from baseline in Zurich Claudication Questionnaire (ZCQ) symptom severity at 6 weeks [7]. The mean change in ZCQ symptom severity and physical function [7] from baseline and mean improvements in EuroQoL 5 dimension (EQ5D) [8] questionnaire scores up to 12 months post-procedure were secondary effectiveness endpoints. Changes in physical examination data, visual analogue scale (VAS) pain assessments (for leg, buttock/groin, and back), walking condition and distance, use of pain medication, and adverse events (AEs) were also assessed. Immediate post-procedure radiographs were compared with follow-up radiographs by an independent radiologist to document maintenance of the position of the implant.
Statistical analysis
Results were expressed as means ± standard deviation (SD) for quantitative variables while counts and percentages were used for categorical findings. In figures, mean values were also reported with SD. Percent changes in pain VAS, ZCQ and EQ5D scores from baseline at subsequent time-points were associated with a two-sided 95 % confidence interval (95 % CI). Changes from baseline were also tested for significance by the classical Student’s t test and the Wilcoxon signed-rank test.
Results were considered to be statistically significant at the 5 % critical level (p < 0.05). All calculations were done with the SAS (Version 9.1) and S-PLUS (Version 6.2) statistical packages.
Clinical significance thresholds
Improvements in ZCQ, VAS and EQ5D scores were considered clinically significant when they reached a threshold defined in earlier publications [7, 9, 10], as reported in Table 1. This is described as the Minimum Clinically Important Difference (MCID).
Table 1.
MCID: cut-off points and clinically irrelevant and important changes 12 months post-procedure
| Outcome variable | Cut-off used | N | Clinically irrelevant (%) | Clinically important (%) |
|---|---|---|---|---|
| VAS back pain improvement | 1.2 | 153 | 70 (45.8) | 83 (54.2) |
| VAS leg pain improvement | 1.6 | 153 | 58 (37.9) | 95 (62.1) |
| ZCQ symptom severity | 0.48 | 155 | 62 (40.0) | 93 (60.0) |
| ZCQ physical function | 0.52 | 153 | 65 (42.5) | 88 (57.5) |
| EQ5D | 0.08 | 155 | 55 (35.5) | 100 (64.5) |
Results
All patients were treated in the prone position (lying flat, face downwards): 97 % under general anesthesia and 3 % receiving a local anesthetic. Seventy-two patients (46 %) were treated at 1 level and 74 patients (47 %) at 2 levels; contrary to the protocol and approved use, 10 additional patients (6 %) were treated at 3 levels. At entry to the study, 50 % of patients had two levels of lumbar spinal stenosis and 44 % had one level, most commonly at L4–L5 (94 %) and/or L3–L4 (59 %). One patient suffered a spinous process fracture during the distraction phase of surgery and did not have an IPD implanted. The maximum trocar size ranged from 8 mm (1 % of cases) to 14 mm (48 % of cases). The IPD size also ranged from 8 to 14 mm, the most common being 12 mm (39 %). In 62 % of cases, the IPD implanted was the same size as the maximum trocar size used. The mean duration of the surgical procedure was 15.5 min for 1 level, 24.6 min for 2 levels and 39.1 min for 3 levels. No particular problems were encountered at L4–L5 in patients with a prominent iliac crest, and an oblique entry was sufficient to ease implantation. Baseline patient data are summarized in Table 2.
Table 2.
Baseline data of patients
| No. patients with IPD(s) | Age (years) | Male/female | Complaint duration (months) | Levelsa | No. levelsb | No. IPDs | Size of IPD(s) |
|---|---|---|---|---|---|---|---|
| 156 | 64.8 (range 19–84) | 78/78 (50.0 %/50.0 %) | 40.0 (range 0.8–477) | L1–L2: 0.6 % (1 pt) | 1 level: 46.1 % (72 pts) | 250 | 8 mm: 6.4 % (16 pts) |
| L2–L3: 9.6 % (15 pts) | 2 levels: 47.4 % (74 pts) | 10 mm: 24.8 % (62 pts) | |||||
| L3–L4: 58.6 % (92 pts) | 3 levels: 6.4 % (10 pts) | 12 mm: 39.2 % (98 pts) | |||||
| L4–L5: 93.6 % (147 pts) | 14 mm: 29.6 % (74 pts) |
aLevels of lumbar spinal stenosis at study entry
bNumber of levels at which patients had the IPD implanted
Of the 156 patients with the device implanted, 154 were in the study at 6 weeks, 144 at 6 months, and 128 (82 %) completed the study to 12 months.
Six weeks post-procedure, the mean percentage change in ZCQ symptom severity score (Table 3) indicated a statistically significant improvement from baseline (p < 0.001; 95 % CI 26.0–33.5 %), with 77 % of patients meeting the MCID threshold. The statistically significant improvement from baseline was maintained up to 12 months (p < 0.001; 95 % CI 22.6–30.8 %), with 60 % of patients meeting the MCID threshold.
Table 3.
Mean percentage change from baseline in ZCQ symptom severity and physical function
| ZCQ symptom severity | ZCQ physical function | |||
|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | |
| Baseline | 157 | 3.0 ± 0.61 | 155 | 2.5 ± 0.51 |
| 7 days post-procedure score | 156 | 2.1 ± 0.53 | 155 | 2.3 ± 0.67 |
| Percentage change from baseline | 156 | −27.4 % ± 21.7** | 153 | −5.5 % ± 33.7* |
| 6 weeks post-procedure score | 155 | 2.1 ± 0.62 | 152 | 1.8 ± 0.65 |
| Percentage change from baseline | 155 | −29.7 % ± 23.4** | 150 | −27.1 % ± 28.2** |
| 6 months post-procedure score | 151 | 2.2 ± 0.74 | 149 | 1.8 ± 0.67 |
| Percentage change from baseline | 151 | −26.8 % ± 24.3** | 147 | −26.6 % ± 29.5** |
| 12 months post-procedure score | 155 | 2.2 ± 0.85 | 155 | 1.9 ± 0.73 |
| Percentage change from baseline | 155 | −26.7 % ± 25.8** | 153 | −25.3 % ± 27.7** |
SD standard deviation, ZCQ Zurich Claudication Questionnaire
* Student’s t test p = 0.047; signed-rank test, p = 0.008
** Student’s t test, p < 0.001; signed-rank test, p < 0.001
For the ZCQ Physical Function scores (Table 3), a highly statistically significant improvement was observed from baseline from 6 weeks (p < 0.001; 95 % CI 22.6–31.7 %) up to 12 months post-procedure (p < 0.001; 95 % CI 20.8–29.7 %). At 12 months, 58 % of patients met the MCID threshold.
The evolution of ZCQ symptom severity and physical function scores up to 12 months post-procedure is presented in Figs. 2 and 3. The ZCQ symptom severity score decreased from 3.0 ± 0.6 at screening to 2.1 ± 0.5 at 7 days and to 2.2 ± 0.9 at 12 months, while the mean ZCQ Physical Function score decreased from 2.5 ± 0.5 at screening to 2.3 ± 0.7 at 7 days and to 1.9 ± 0.7 at 12 months.
Fig. 2.
Mean (±SD) evolution of the ZCQ symptom severity score after the procedure Student’s t test, p < 0.001; signed-rank test, p < 0.001 at all time-points for both the continuous change from baseline and the mean percentage change
Fig. 3.
Mean (±SD) evolution of the ZCQ physical function score after the procedure Student’s t test, p < 0.001; signed-rank test, p < 0.001 at all time-points for both the continuous change from baseline and the mean percentage change except for the mean percentage change 7 days post-procedure (Student’s t test, p = 0.047; signed-rank test, p = 0.008)
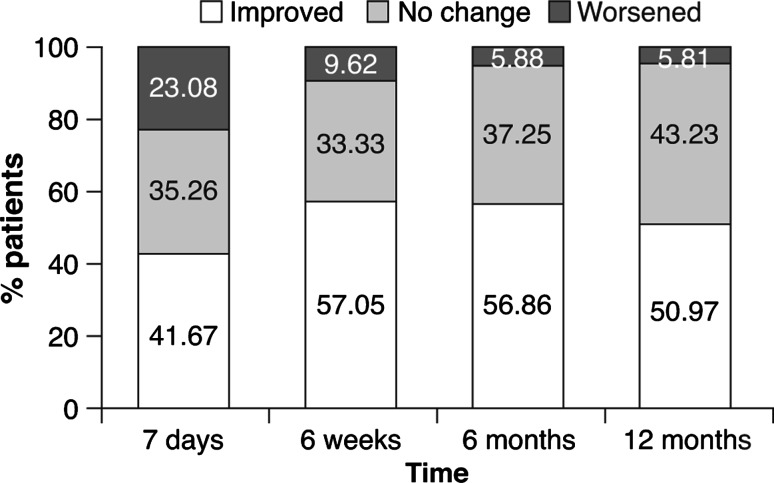
Twelve months post-procedure, 74 % of patients with IPDs implanted were judged by their physician to walk “fluently” versus 43 % of patients at baseline. An improvement in walking distance was also observed up to 12 months post-procedure (Fig. 4), when 57 % of patients were able to cover >1,000 m versus 22 % at baseline (Table 4).
Fig. 4.
Walking distance up to 12 months; categorized change
Table 4.
Walking components of physical function
| Parameter | Screening, n (%) | 7 days, n (%) | 6 weeks, n (%) | 6 months, n (%) | 12 months, n (%) |
|---|---|---|---|---|---|
| Walking condition fluent | 68 (43.3) | 108 (69.2) | 115 (73.7) | 114 (74.5) | 114 (74.0) |
| Walking distance 0–100 m | 36 (22.9) | 24 (15.4) | 15 (9.62) | 16 (10.5) | 19 (12.3) |
| 100–500 m | 58 (36.9) | 52 (33.3) | 26 (16.7) | 20 (13.1) | 23 (14.8) |
| 500–1,000 m | 28 (17.8) | 37 (23.7) | 26 (16.7) | 29 (19.0) | 24 (15.5) |
| >1,000 m | 35 (22.3) | 43 (27.6) | 89 (57.1) | 88 (57.5) | 89 (57.4) |
| No walking aids used | 127 (80.9) | 138 (87.9) | 139 (88.5) | 131 (83.4) | 133 (85.3) |
| Walking distance from baseline improved | – | 65 (41.7) | 89 (57.1) | 87 (56.9) | 79 (51.0) |
| No change in walking distance from baseline | – | 55 (35.3) | 52 (33.3) | 57 (37.3) | 67 (43.2) |
| Walking distance from baseline worsened | – | 36 (23.1) | 15 (9.62) | 9 (5.88) | 9 (5.81) |
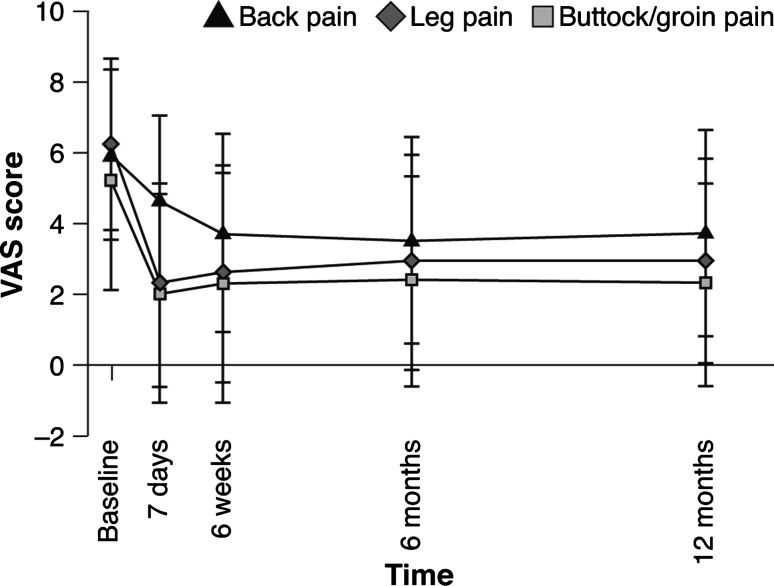
The VAS pain scores for leg, buttock/groin and back showed that pain decreased significantly (p < 0.001) from baseline at all time-points. Immediate improvements were seen in leg and buttock/groin pain post-procedure and were maintained up to 12 months (Fig. 5). The mean score for leg pain decreased from 6.2 ± 2.4 at screening to 2.3 ± 2.8 at 7 days, while the mean score for buttock/groin pain decreased from 5.2 ± 3.1 at screening to 2.0 ± 2.8 at 7 days. A gradual improvement in back pain was observed (Fig. 5): the mean score decreased from 5.9 ± 2.4 at screening to 4.6 ± 2.4 at 7 days and 3.7 ± 2.8 at 6 weeks, maintained thereafter up to 12 months. At 12 months, 54 and 62 % of patients met the MCID threshold for back and leg pain, respectively.
Fig. 5.
Mean (±SD) evolution of the VAS leg, buttock/groin and back pain score after the procedure Student’s t test, p < 0.001; signed-rank test, p < 0.001 at all time-points for continuous change from baseline in leg, buttock/groin and back pain scores
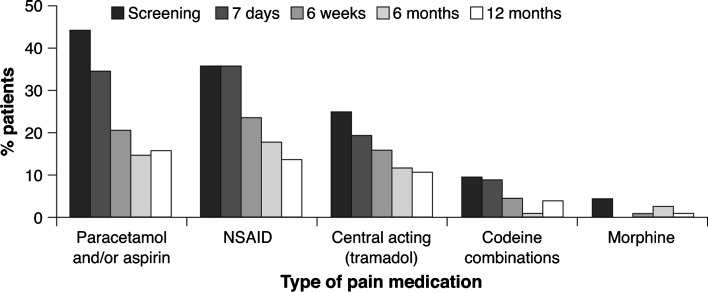
While pain scores decreased over time, the use of pain medication (including strong pain medication such as opioids) decreased. The number of patients requiring 3-times daily dosing rose 7 days post-procedure, but subsequently fell (Fig. 6). Twelve months post-procedure, the percentage of patients requiring stronger pain medication such as codeine combinations or morphine was reduced and a high proportion of patients did not require any medication at all (65 % of patients vs. 25 % at baseline).
Fig. 6.
Medication intake up to 12 months; stenosis-specific medication
The EQ5D data showed a statistically significant improvement from baseline up to 12 months post-procedure (p < 0.001; 95 % CI 0.6–0.7). The EQ5D score improved from 0.4 ± 0.3 at screening to 0.6 ± 0.2 at 7 days and 0.7 ± 0.3 at 12 months. At 12 months, 65 % of patients met the MCID threshold for EQ5D.
The number of levels treated had no significant impact on changes in pain or ZCQ scores. Quality-of-life mean scores (EQ5D) were lower at baseline in patients treated at 2 or 3 levels (0.35 ± 0.32) than at a single level (0.48 ± 0.28), but showed greater improvement, with mean scores similar in the two sub-groups after surgery.
The effect of surgery on lordosis was estimated by measuring the L1–S1 angle from lateral view X-rays taken before and after implantation. From a mean (95 % CI) of 54.1° (51.2–57.0) at screening, the mean angle decreased to 49.8° (46.7–52.9) at 6 weeks, 51.6° (48.8–54.4) at 6 months, and 52.4° (49.1–55.6) at 12 months (46 patients).
Safety
Twelve serious adverse device effects (SADEs) were reported in 11 patients during the study.
One day after the procedure, 4 SADEs were reported: spinous process fracture, back pain, hematoma, and pain in extremity, each in 1 (0.6 %) patient. Between 2 and 7 days after the procedure, back pain was reported in 2 (1.3 %) patients. From 7 days post-procedure, spinous process fracture and back pain [each in 1 (0.6 %) patient], spinal claudication [3 (1.9 %) patients], and sciatica [1 (0.6 %) patient] were reported.
Overall, 58 serious AEs (excluding SADEs) were reported in 39 patients, most commonly arthralgia [6 (3.8 %) patients], lumbar spinal stenosis [3 (1.9 %) patients], spinal claudication [3 (1.9 %) patients] and social stay hospitalization [3 (1.9 %) patients]. No other serious AEs were reported in more than 2 (1.3 %) patients. One patient died from esophageal cancer (unrelated to the procedure or IPD).
Device position and removal
The final position of the IPD in the interspinous space was assessed by X-ray in the anterior–posterior and lateral views, which confirmed that the IPD position was correct and was maintained throughout the study, with no cases of implant dislocation observed.
Eleven patients (7 %) had a reported spinous process fracture, 3 noted by the surgeons and a further 8 only by the central reader at a later stage. In one of these patients a fracture occurred during surgery, and the implant was not inserted. The implant was not removed from patients whose fracture was detected by later X-ray and who were asymptomatic.
During the 12-month post-procedure period, 14 (9 %) patients had their IPD removed (2 after up to 3 months, 3 after 3–6 months, and 9 after 6–12 months) due to persistent or recurring symptoms (10 cases), spinous process fracture (3 cases), or malpositioning (1 case). Two of the 3 spinous process fractures had been identified by the surgeons and one only by the central reader. Ten of the 14 patients had implants at one level only. No baseline risk factors for removal were identified other than gender (11/14 were male).
Discussion
In this multicenter, prospective study, the safety and effectiveness of the IPD was evaluated for 12 months in DLSS patients with NIC. The IPD effectively and quickly alleviated the symptoms of spinal stenosis as demonstrated by changes in ZCQ symptom severity and physical function scores, VAS pain scores, the use of pain medication, and improved walking distance.
The patient’s outcome at 12 months post-procedure was based on the use of the MCID thresholds for score improvement [7, 9, 10], considered to be the most objective parameter in the assessment of clinical outcome, providing an immediate evaluation of the effectiveness of the treatment. The data show that the IPD can be beneficial for up to 12 months after implantation in a high proportion of patients, with the majority of patients showing clinically important improvements in ZCQ symptom severity and physical function scores, VAS scores for pain, and EQ5D scores at 12 months versus baseline.
Following surgery, an immediate improvement was observed in leg and buttock/groin pain and ZCQ Symptom Severity; however, back pain and ZCQ Physical Function showed a more gradual improvement, which may suggest that patients with classical claudication and leg symptoms as their predominant complaints are the optimal target population for this IPD.
We did not see a significant long-term effect on the degree of lordosis in the patients with data in this study.
The most commonly reported SADEs up to 12 months post-procedure were back pain, spinal claudication and spinous process fracture. Spinous process fracture, observed here and in other studies of IPDs, may be considered as potentially risk related specifically to interspinous process decompression, both in terms of the procedure and the device itself [11–14]. Fracture rates, and the means of detecting fractures, vary between studies, making direct comparisons difficult. The rate of 7 % overall (including 2 % reported by the surgeons) in this study was less than has been reported in a similar study of other IPDs (29 %) [15], but is higher than in other studies where no central analysis of X-rays was undertaken [15, 16].
During the 12 months post-procedure, 14/156 (9 %) patients had the IPD removed. The incidence of device removal during this time was higher than in some other studies [14, 15] but lower than in others [17, 18]. In a recently published systematic review of the effectiveness of interspinous implant surgery in a total of 563 patients with NIC, 6 % of devices were found to have required replacement or reoperation [19]. Although it cannot be performed percutaneously, removal of the IPD is straightforward with minimal risk, and leaves other treatment options open (including open decompression surgery, with or without fusion), as fibrotic scar tissue in the spinal canal is not formed following the percutaneous approach. Reoperation does not always require removal of the IPD.
Detailed analyses on potential predictors for a successful outcome at 12 months in the present study did not reveal many new insights to aid patient selection in future, possibly because the number of patients not benefiting from the procedure was relatively low, and the range of baseline characteristics often wide. More research is needed to define the optimal patient population for this type of procedure. However, the minimally invasive IPD is a viable alternative for selected patients, is quick and simple to implant, and leaves options open for further treatments in future.
Conclusions
Overall, these data indicate that in a period of up to 12 months follow-up, the safety and effectiveness of the Aperius® procedure and device offered a minimally invasive option for the relief of NIC complaints in patients with symptomatic DLSS with NIC. Further studies are underway to provide insight on outcomes and effectiveness compared to other decompression methods, and to develop guidance on optimal patient selection.
Acknowledgments
The INCA study investigators and their staff for data collection: (1) Belgium: Dr Fransen and Dr Collignon, Brussels (35 patients); Dr Jodaitis and Dr Morelli, La Louviere (28 patients); Dr Van Meirhaeghe, Brugge (25 patients); Dr Scordidis, Montignies sur Sambre (10 patients); Dr Lambert, Mons (2 patients); Dr Hes, Antwerpen (1 patient). (2) Germany: Dr Stavros and Dr Richter, Schwandorf (19 patients); Dr Bachus and Dr Godde, Düsseldorf (13 patients); Dr Weisskopf, Polonius, and Dr Wierscher, Schwarzach (4 patients); Dr Kolvenbach, Erlangen (2 patients); Dr Sobottke and Dr Kaulhausen, Köln (1 patient). (3) United Kingdom: Mr Craig, Aberdeen (17 patients).
Prof Albert and Anne-Françoise Donneau (statisticians), Dr Brat (central reader), and Quintiles (writing assistance), all funded by Medtronic.
Conflict of interest
J.V.M. is a consultant with Medtronic, Synthes; P.F. is a consultant with Medtronic, SpineArt, Covidien; D.M. is a consultant with Medtronic, Stryker; N.C. is a consultant with Medtronic; A.M. is a former employee of Medtronic; G.G. is a consultant with Medtronic; and F.C. is a consultant with Medtronic, Alphatec. This study was sponsored by Medtronic Spinal and Biologics Europe BVBA (now Medtronic Bakken Research Center BV, Endepolsdomein 5, 6229 GW Maastricht, The Netherlands).
References
- 1.Hilibrand AS, Rand N. Degenerative lumbar stenosis: diagnosis and management. J Am Acad Orthop Surg. 1999;7(4):239–249. doi: 10.5435/00124635-199907000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Boos N, Aebi M (eds) (2008) Spinal disorders: fundamentals of diagnosis and treatment. Springer
- 3.Katz JN, Dalgas M, Stucki G, et al. Degenerative lumbar spinal stenosis. Diagnostic value of the history and physical examination. Arthritis Rheum. 1995;38(9):1236–1241. doi: 10.1002/art.1780380910. [DOI] [PubMed] [Google Scholar]
- 4.Magnaes B. Clinical recording of pressure on the spinal cord and cauda equina. Part 1: the spinal block infusion test: method and clinical studies. J Neurosurg. 1982;57(1):48–56. doi: 10.3171/jns.1982.57.1.0048. [DOI] [PubMed] [Google Scholar]
- 5.Magnaes B (1982) Clinical recording of pressure on the spinal cord and cauda equina. Part 2: position changes in pressure on the cauda equina in central lumbar spinal stenosis. J Neurosurg 57(1):57–63 [DOI] [PubMed]
- 6.Zucherman JF, Hsu KY, Hartjen CA, et al. A multicenter, prospective, randomized trial evaluating the X STOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: two-year follow-up results. Spine. 2005;30(12):1351–1358. doi: 10.1097/01.brs.0000166618.42749.d1. [DOI] [PubMed] [Google Scholar]
- 7.Stucki G. Measurement properties of a self-administered outcome measure in lumbar spinal stenosis. Spine. 1996;21(7):796–803. doi: 10.1097/00007632-199604010-00004. [DOI] [PubMed] [Google Scholar]
- 8.Solberg TK, Olsen JA, Ingebrigtsen T, et al. Health-related quality of life assessments by the EuroQol-5D can provide cost-utility data in the field of low-back surgery. Eur Spine J. 2005;14(10):1000–1007. doi: 10.1007/s00586-005-0898-2. [DOI] [PubMed] [Google Scholar]
- 9.Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and Pain Scales. Spine J. 2008;8:968–974. doi: 10.1016/j.spinee.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14(6):1523–1532. doi: 10.1007/s11136-004-7713-0. [DOI] [PubMed] [Google Scholar]
- 11.Bowers C, Amini A, Dailey AT, et al. Dynamic interspinous process stabilization: review of complications associated with the X-stop device. Neurosurg Focus. 2010;28(6):E8. doi: 10.3171/2010.3.FOCUS1047. [DOI] [PubMed] [Google Scholar]
- 12.Chung KJ, Hwang YS, Koh SH. Stress fracture of bilateral posterior facet after insertion of interspinous implant. Spine. 2009;34(10):E380–E383. doi: 10.1097/BRS.0b013e31819fd3a0. [DOI] [PubMed] [Google Scholar]
- 13.Kim KA, McDonald M, Pik JH, et al. Dynamic intraspinous spacer technology for posterior stabilization: case control study on the safety, sagittal angulation, and pain outcome at 1-year follow-up evaluation. Neurosurg Focus. 2007;22(1):E7. [PubMed] [Google Scholar]
- 14.Kim DH, Tantorski M, Shaw J, et al. Occult spinous process fractures associated with interspinous process spacers. Spine. 2011;36(16):E1080–E1085. doi: 10.1097/BRS.0b013e318204066a. [DOI] [PubMed] [Google Scholar]
- 15.Nardi P, Cabezas D, Rea G, et al. Aperius PercLID stand alone interspinous system for the treatment of degenerative lumbar stenosis: experience on 152 cases. J Spinal Disord Tech. 2010;23(3):203–207. doi: 10.1097/BSD.0b013e31819b08da. [DOI] [PubMed] [Google Scholar]
- 16.Kuchta J, Sobottke R, Eyesel P, Simons P. Two-year results of interspinous spacer (X-Stop) implantation in 175 patients with neurologic intermittent claudication due to lumbar spinal stenosis. Eur Spine J. 2009;18:823–829. doi: 10.1007/s00586-009-0967-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kantelhardt SR, Török E, Gempt J, et al. Safety and efficacy of a new percutaneously implantable interspinous process device. Acta Neurochir. 2010;152:1961–1967. doi: 10.1007/s00701-010-0740-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson PA, Tribus CB, Kitchel SH. Treatment of neurogenic claudication by interspinous decompression: application of the X STOP device in patients with lumbar degenerative spondylolisthesis. J Neurosurg Spine. 2006;4:463–471. doi: 10.3171/spi.2006.4.6.463. [DOI] [PubMed] [Google Scholar]
- 19.Moojen WA, Arts MP, Bartels RHMA, Jacobs WCH, Peul WC. Effectiveness of interspinous implant surgery in patients with intermittent neurogenic claudication: a systematic review and meta-analysis. Eur Spine J. 2011;20:1596–1606. doi: 10.1007/s00586-011-1873-8. [DOI] [PMC free article] [PubMed] [Google Scholar]