Abstract
Purpose
The objective of this study was to investigate thoracic myelopathy caused by ossification of the yellow ligament (OYL) in patients with posterior instrumented lumbar fusion.
Methods
Seven patients, who had undergone posterior instrumented lumbar fusion, presented with thoracic myelopathy caused by OYL. No patient had a history of thoracic myelopathy at previous surgery. Instrumented fusions were performed from L1–5 in two patients, L2–5 in three patients and L1–S1 and L2–S1 in one patient each, respectively. MRI and CT scans were performed to confirm cord compression by OYL. Of the seven patients, six patients underwent decompressive laminectomy and OYL removal while one was treated conservatively.
Results
The average time to presentation after first surgery was 63.4 months. OYL was located at T9–10 in two patients, T11–12 in three patients, and T10–11 and T9–11 in one patient each, respectively. All patients had a myelopathic gait and the average Japanese Orthopaedic Association (JOA) score was 3.9, preoperatively. The average JOA score improved from 3.7 to 8 and the average recovery rate was 58.9 % in the six patients who underwent surgical intervention. However, the JOA score fell from 5 to 4 in the one patient who was treated conservatively.
Conclusions
We report seven patients who suffered from thoracic myelopathy after instrumented lumbar fusion. Surgeons must be aware of the possibility of thoracic myelopathy caused by OYL at the thoracolumbar junction, especially in patients with a complaint of gait disturbance after long instrumented lumbar fusion.
Keywords: OYL, Adjacent segment disease, Lumbar fusion
Introduction
Convincing biomechanical and clinical data indicates that posterior instrumented spinal fusion creates a significant compensatory increase in motion at adjacent mobile segments by increasing stiffness at the fused segment [1–5]. Furthermore, these adjacent levels are thought to be subjected to higher loads during normal activities, because motions normally distributed over many levels must occur at fewer levels [1, 6]. The development of adjacent segment disease (ASD) after posterior instrumented lumbar fusion is a controversial issue. Some investigators have argued that radiographic changes ascribed to ASD are nothing more than normal age related changes [7, 8]. However, the incidence of ASD has been reported to range from 5.2 to 49 % [1].
To the author’s knowledge, no reports have been issued regarding ossification of the yellow ligament (OYL) development at adjacent segments after long instrumented lumbar fusion. Even though the pathogenesis of OYL is unclear, it would appear that mechanical stress is a main contributor. OYL might develop or further aggravate existing OYL as a result of the effect of localized mechanical stress on the yellow ligament [9]. However, no report has addressed thoracic myelopathy caused by OYL development at proximal segments after posterior instrumented lumbar fusion. Here we describe seven patients who experienced severe thoracic myelopathy associated with OYL after long posterior instrumented lumbar fusion.
Materials and methods
The study protocol was reviewed and approved by the Institutional Review Board of our facility. The study inclusion criteria were (1) thoracic myelopathy caused by OYL, (2) instrumented lumbar fusion, and (3) no history of thoracic myelopathy before instrumented lumbar fusion. Seven patients, three males and four females were enrolled in this study. Six of the seven patients were initially treated at our institution and one was referred post initial surgery. The mean patient age at the time of presentation with complaints of thoracic myelopathy and gait disturbance was 67.1 years (range 54–84). Previously treated levels and the average time laps between this presentation and the instrumented fusion for all seven patients was noted. Radiologic examinations included plain radiography, CT, and MRI in all patients prior to revision surgery. Two of the seven patients had existing MRIs and those images were used to determine whether OYL predated the previous instrumented lumbar fusion. Local kyphosis was measured and degenerative changes were confirmed at the involved segments. All seven patients underwent a neurologic examination and Japanese Orthopaedic Association (JOA) scoring.
Six patients underwent decompressive surgery with OYL removal at the involved segments. Among six patients undergone surgical intervention, instrumented fusions in two patients were extended (Fig. 1). The remaining four patients underwent decompression without additional instrumented fusions (Fig. 2). One of the seven patients rejected surgical intervention and was treated conservatively. The minimum follow-up after removal of OYL and laminectomy was 2 years (average 43.1 months, range 24–100 months). All patients were scored using the Japanese thoracic myelopathy system at every visit associated with their latest presentation (Table 1).
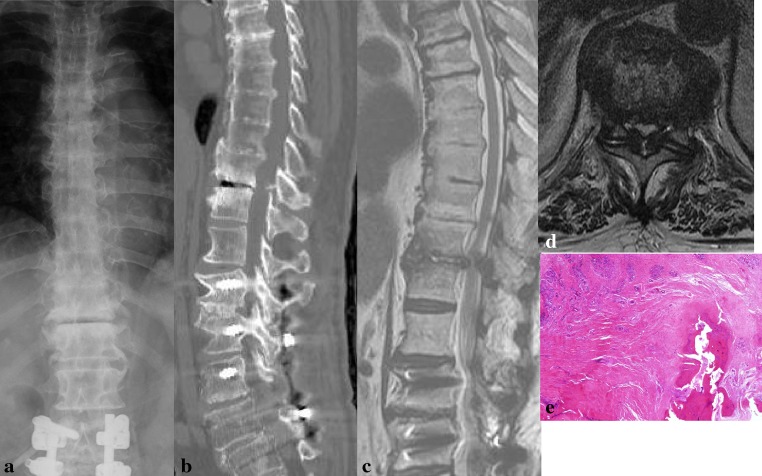
Fig. 1.
Case 4: An 84-year-old man. The patient had undergone instrumented lumbar fusion from L1 to L5 30 months previously. During follow-up, he did not complain of thoracic myelopathy. However, thoracic myelopathy developed progressively over 4 months. a AP plain radiograph of the lumbar spine revealed severe degenerative changes with a gas shadow at T10–11. b Sagittal reconstruction computed tomogram image showing severe degenerative changes and ossification of yellow ligament. c, d Sagittal and axial T2 weighted magnetic resonance images showing OYL at the T10–11 with severe degenerative changes at the OYL involved segments. We recommended surgical intervention, but the patient rejected the recommendation. Over 25 months of follow-up, his Japanese Orthopaedic Association score deteriorated somewhat from 5 to 4. e Histopathology shows ossification in the ligamentum flavum (H&E staining, ×50)
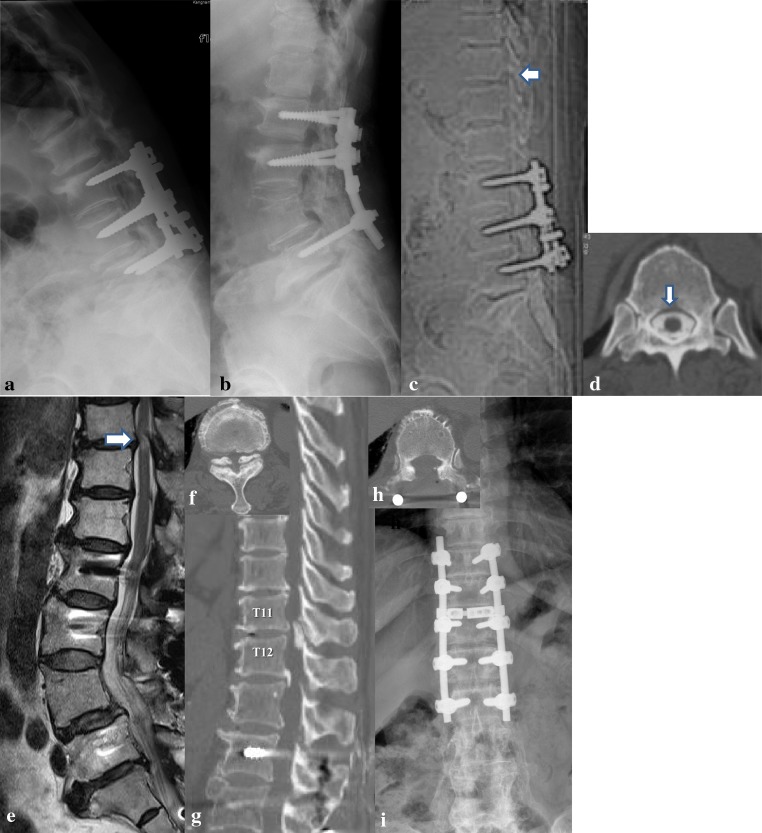
Fig. 2.
Case 6: A 67-year-old woman who visited our institute with complaints of a progressive myelopathic gait. a She had undergone posterior instrumented lumbar fusions for spinal stenosis about 17 years previously and developed adjacent segment failure with progression of kyphosis. b However, progression of kyphosis developed with severe low back pain (a). Revision surgery with pedicle subtraction osteotomy was performed 6 years ago. However, progressive neurological deficit and a myelopathic gait developed slowly over 12 months. c, d CT-myelography showed normal passage of the dyes at T11–12 (arrow) before osteotomy. e T2 weighted MRI (white arrow) illustrating intramedullary hyperintense signal changes. f Preoperative axial and sagittal CT (g) views depicting the OYL. h Anterior-posterior lumbar plain radiograph and an axial CT image (i) both taken after extended instrumentation and laminectomy. Her JOA score improved from 3 to 8
Table 1.
Summary of the Patient’s data
| Case | Age | Sex | Fusion level | OYL level | Duration of Sx (months) | Sx arising after op (months) | Local kyphosis (°) | CT findings | Cord signal changes on T2 | JOA score | OP method | F-U period (months) | Cx | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gas shadow | Asymmetric rotation | Pre op | Last FU | ||||||||||||
| 1 | 65 | M | L1–5 | T9–10 | 4 | 84 | −3.2 | + | + | − | 6 | 11 | Lam | 48 | |
| 2 | 54 | F | L2–5 | T9–10 | 6 | 60 | −4.1 | − | + | − | 2 | 6 | Lam | 100 | |
| 3 | 70 | M | L2–5 | T11–12 | 3 | 50 | −2.8 | − | + | + | 5 | 8 | Lam | 42 | Revision |
| 4 | 84 | F | L1–5 | T10–11 | 4 | 30 | 0.8 | − | + | − | 5 | 4 | – | 25 | |
| 5 | 64 | M | L–S1 | T11–12 | 10 | 50 | 0 | − | − | − | 3 | 8 | Fusion | 38 | |
| 6 | 67 | F | L2–5 | T11–12 | 12 | 70 | 0 | + | + | + | 3 | 8 | Fusion | 24 | |
| 7 | 66 | M | L–S1 | T9–11 | 6 | 100 | 15 | − | − | + | 3 | 7 | Lam | 25 | |
Lam laminectomy and removal of OYL; – at the local kyphosis represented lordosis
Case 6 symptoms arising after second operation
Results
Gait disturbance and sensory deficit of the lower extremity were the most frequent symptoms when patients first visited our institute. Three patients had previously undergone L2–5 instrumented fusions, L1–5 in two, L2–S1 in one, and L1–S1 in one. L1 was the most proximal end of the fused levels. Two patients were fused to S1. OYL with thoracic myelopathy developed on average at 63.4 months (range 30–100 months) after previous instrumented lumbar fusion. OYL was located at T11–12 in three patients, T9–10 in two, T10–11 in one, and T9–11 in one. Average symptom duration was 6.4 months (range 3–12 months). Mean local kyphosis at involved segments was 0.8°. All patients showed severe degenerative changes, which included severe facet arthritis combined with asymmetric rotation of facet joints in five patients. CT imaging showed a gas shadow in facet joints in two patients. Plain radiographs and MRI also showed severe degenerative changes at the involved segments. Unilateral OYL was present in two patients and bilateral OYL in five. The recovery scale was calculated using Hirabayshi’s equation [10]. Recovery scale (%) = (Postop score−preop score)/[11(full score)−preop score] × 100. Using this formula, postoperative conditions can be compared to preoperative conditions. Postoperatively, JOA scores improved to 8.0 (range 6–11) and the recovery rate averaged 58.9 % (range 50–100 %) at last follow-up examination in the six patients who underwent surgical intervention. Of these, one patient completely improved and had a JOA score of 11 after surgery. However, the patient treated conservatively deteriorated JOA score from 5 to 4 (Table 2). T2-weighted MRI showed a hyperintense signal in the spinal cord in three patients. Three patients exhibiting cord signal changes had JOA scores of 5, 3 and 3 (average 3.7), respectively, and improved to 8, 8 and 7, respectively (average 7.7 with 54.8 % recovery scale), after surgery. On the other hand, the three patients without signal changes on T2-weighted MRI had JOA scores of 6, 2, and 3 (average 3.7) and these improved to 11, 6, and 8 (average 8.3 with 63.0 % recovery scale), respectively, after surgery (Table 1). Therefore, the last follow-up JOA scores probably depended on the severity of preoperative JOA scores, signal changes of the cord on T2-weighted MRI, and the method of treatment.
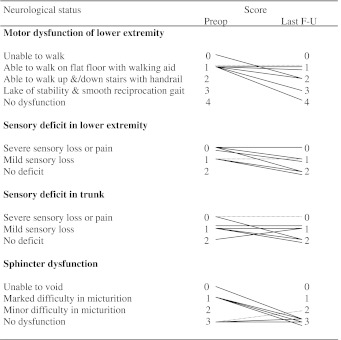
Table 2.
Changes of JOA score
Dotted lines are showing JOA score for a conservative case
Complication
No patients experienced a deterioration of neurological symptoms after surgery. One patient (Case 3) underwent revision surgery at 7 months after surgical intervention due to incomplete removal of the OYL. Although in this case, symptoms did not deteriorate after surgery we decided on revision surgery, because CT showed incomplete OYL removal and the patient’s JOA score was not improved. After revision surgery, the JOA score improved from 5 to 8 at final follow-up visit.
Discussion
Many clinical studies have been conducted on degeneration or disease adjacent to instrumented fusion segments. The length of fusion also plays an important role in the development of ASD or subsequent fractures as a longer lever arm produced by multi-segmental fusion causes more stress at adjacent segments [1, 11–13]. However, few reports have been released regarding OYL at proximal segments following long instrumented lumbar fusion. OYL is one of the causes of thoracic myelopathy reported, which has mainly been reported in Japanese patients, and it has been suggested that its pathogenesis might be correlated with mechanical stress concentration. Yoshida et al. [14] suggested that the yellow ligament is hypertrophied by mechanical stress and that the main constituent of hypertrophy is the proliferation of type 2 collagen at the enthesis. Kaneyama et al. [15] also suggested that repeated, localized rotator mechanical stress caused by a baseball pitching motion probably influences the development of OYL in their case reports of young baseball pitchers suffering from thoracic myelopathy. Recently, Chen et al. [16] reported that the local instability and repetitive stimulus of excessive stress could lead to development of OYL at the levels adjacent to the kyphotic apex in patients with thoracic tuberculosis of the spine.
In the present study, all patients underwent long instrumented lumbar fusions and showed severe degenerative changes by CT or MRI at the segments in which OYL developed. These severe degenerative changes at involved segments were likely to be ASD, and therefore it might be assumed that stress concentration at proximal segments after posterior long instrumented lumbar fusions could lead to OYL or aggravate existing asymptomatic OYL.
OYL at lower thoracic levels is often asymptomatic. Aizawa et al. [17] reported that the symptoms of OYL-induced myelopathy mimic those of other lumbar disorders, which often results in misdiagnosis, because half of their patients first noticed lower-extremity tingling and numbness or pain. These symptoms can be the chief complaints among patients with lumbar disorders. In the present study, it is uncertain whether the first surgery for instrumented lumbar fusion was performed as a result of misdiagnosis, but all patients had no history of thoracic myelopathy before lumbar fusion. Preoperative thoracic radiographs were available for two of the seven patients, and neither showed OYL at the first surgery. Additionally after the first surgery, all patients enjoyed normal daily activities, resumed previous jobs, and never complained of gait disturbance. These observations suggest that myelopathy by OYL might have developed after instrumented fusion or that existing asymptomatic OYL had been aggravated. Several studies have reported that radiologic instability in adjacent segments develops at 25 months after instrumentation and symptomatic changes in adjacent segments at 27 months after fusion in 5.2–18.5 % of patients [1, 4, 5, 18, 19]. In the present study, the average time to symptom presentation from instrumented fusion was 63.4 months. As compared with a previous ASD study [4, 5] symptom onset appeared later in our patients. Therefore prolonged mechanical stress concentration at proximal segments after instrumented fusion could have caused OYL or aggravated asymptomatic OYL in our patients, even though OYL progresses slowly.
The most common level of symptomatic OYL is at the thoracolumbar junction, because of facet orientation and the thoracic to lumbar transitional zone. Additionally, thoracic spines from T2 to T10 have unique stability from the surrounding thoracic rib cages. Thoracic levels of T-11 and T-12 allow more rotation and less extension, but no motion exists at higher thoracic levels, which are fixed to the ribs [20]. Smith and Gordersky [21] reported that OYL most commonly occurred at T9–T12, because these segments appeared to be particularly prone to degeneration due to the high tensile force present in the posterior column. In the present study, OYL developed at T11–12 in three patients, T9–10 in two, T10–11 in one, and T9–11 in one. After instrumented fusion, compensatory mechanics can occur at adjacent segments resulting from stress concentration, changes in the contact sites of facet joints, and alterations in biomechanics [1, 3, 13]. In particular, fusion to L1 or L2 should be avoided during long lumbar fusion to prevent proximal adjacent failure at the thoracolumbar junction [22]. In all our cases, the most proximal fusion level was L1 and L2, and OYL developed at the thoracolumbar junction. However, this does not warrant the extension of lumbar fusion to above the thoracolumbar level to prevent OYL and ASD, but myelopathy caused by OYL after long instrumented lumbar fusion is a concern. Pascal-Mousselard et al. [9] reported that OYL occurred in 58 % of adult patients combined with kyphosis. However, in the present study, six of the seven patients showed no local kyphosis.
The involvement of the dura by OYL makes safe removal difficult. Decompressive laminectomy or laminoplasty with excision of the OYL may still be the best available option of surgical treatment. Nishiura et al. [23] recommended early surgical intervention by partial laminectomy and foraminotomy, because the OYL is usually already quite large at time of detection in patients with motor weakness. Li et al. [24] postulated that posterior decompression combined with lateral fusion is the treatment of choice, because the pathogenesis of thoracic OYL is largely attributable to localized mechanical stress on the ligament.
In four of our cases, we performed decompressive laminectomy using a high-speed burr for decompression and en bloc excision of the OYL without additional instrumentation. This might not have been a reasonable surgical treatment in these patients if OYL had originated from mechanical stress after instrumented lumbar fusion. Extended instrumented fusions should have been conducted in patients in whom OYL development caused by stress concentration after long instrumented lumbar fusion was suspected to prevent further ASD and instability after laminectomy. However, only two patients underwent extended fusions. Had extended fusion been used, normal segments between OYL and upper-instrumented segments would have been sacrificed with removal of the previous instrumentation and it would have been an extensive revision surgery in the elderly. At the end of the study period, none of our patients who had undergone laminectomy alone had experienced a neurological deterioration or a worsening of JOA scores. Postoperative clinical outcomes are variable after removal of OYL.
Long-term follow-up studies indicate a poor prognosis [25, 26], and recovery that varies from 25 to 100 % [16, 25, 27]. Early diagnosis and surgical decompression could be related to a favorable outcome [26, 28–30]. However, outcomes after surgery may depend on the involvement of the spinal cord, irreversible changes, and duration of symptoms [29]. Sanghvi et al. [31] reported that the predictors of postoperative recovery are preoperative symptom duration and intramedullary signal size. In the present study, all six patients who had undergone surgical decompression showed JOA score improvement and an average recovery rate of 58.9 %. However, three patients with cord signal changes on T2 images showed poorer outcomes as compared with the three patients without signal changes.
Several limitations in this study require consideration. Firstly, thoracic images including MR or CT were not obtained prior to the initial instrumented lumbar fusions due to lack of thoracic pathology and symptoms. Secondly, the sample size was too small, because of the low incidence of OYL after long instrumented lumbar fusion. Thirdly, the incidence of OYL following lumbar instrumentation versus its incidence in the general population could not be adequately evaluated due to the lack of available data in the existing body of knowledge, a proper comparison could not be made. Therefore, further investigation is crucially required to define the risk factors of OYL and to determine whether OYL is related to stress concentration due to long instrumented lumbar fusion. However, although the occurrence of OYL after long instrumented lumbar fusion is rare, it may develop as ASD or further aggravate existing OYL. Whether the existence of mechanical stress concentration at proximal segments after long instrumented lumbar fusion is related to OYL remains uncertain.
In conclusion, despite the limitations of this study we report seven patients who suffered from thoracic myelopathy after instrumented lumbar fusion. Surgeons must be aware of the possibility of thoracic myelopathy caused by OYL at the thoracolumbar junction, especially in patients with a complaint of gait disturbance after long instrumented lumbar fusion.
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine (Phila Pa 1976) 2004;29(17):1938–1944. doi: 10.1097/01.brs.0000137069.88904.03. [DOI] [PubMed] [Google Scholar]
- 2.Ha KY, Lee JS, Kim KW. Degeneration of sacroiliac joint after instrumented lumbar or lumbosacral fusion: a prospective cohort study over five-year follow-up. Spine (Phila Pa 1976) 2008;33(11):1192–1198. doi: 10.1097/BRS.0b013e318170fd35. [DOI] [PubMed] [Google Scholar]
- 3.Ha KY, Schendel MJ, Lewis JL, Ogilvie JW. Effect of immobilization and configuration on lumbar adjacent-segment biomechanics. J Spinal Disord. 1993;6(2):99–105. doi: 10.1097/00002517-199304000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Aota Y, Kumano K, Hirabayashi S. Postfusion instability at the adjacent segments after rigid pedicle screw fixation for degenerative lumbar spinal disorders. J Spinal Disord. 1995;8(6):464–473. doi: 10.1097/00002517-199512000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Etebar S, Cahill DW. Risk factors for adjacent-segment failure following lumbar fixation with rigid instrumentation for degenerative instability. J Neurosurg. 1999;90(2 Suppl):163–169. doi: 10.3171/spi.1999.90.2.0163. [DOI] [PubMed] [Google Scholar]
- 6.Kim YJ, Bridwell KH, Lenke LG, Rhim S, Kim YW. Is the T9, T11, or L1 the more reliable proximal level after adult lumbar or lumbosacral instrumented fusion to L5 or S1? Spine (Phila Pa 1976) 2007;32(24):2653–2661. doi: 10.1097/BRS.0b013e31815a5a9d. [DOI] [PubMed] [Google Scholar]
- 7.Kumar MN, Baklanov A, Chopin D. Correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion. Eur Spine J. 2001;10(4):314–319. doi: 10.1007/s005860000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiltse LL, Radecki SE, Biel HM, DiMartino PP, Oas RA, Farjalla G, Ravessoud FA, Wohletz C. Comparative study of the incidence and severity of degenerative change in the transition zones after instrumented versus noninstrumented fusions of the lumbar spine. J Spinal Disord. 1999;12(1):27–33. doi: 10.1097/00002517-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Pascal-Mousselard H, Smadja D, Cabre P, Raynaud M, Catonne Y. Ossification of the ligamenta flava with severe myelopathy in a black patient. A case report. Spine (Phila Pa 1976) 1998;23(14):1607–1608. doi: 10.1097/00007632-199807150-00019. [DOI] [PubMed] [Google Scholar]
- 10.Hirabayashi K, Miyakawa J, Satomi K, Maruyama T, Wakano K. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine (Phila Pa 1976) 1981;6(4):354–364. doi: 10.1097/00007632-198107000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Nagata H, Schendel MJ, Transfeldt EE, Lewis JL. The effects of immobilization of long segments of the spine on the adjacent and distal facet force and lumbosacral motion. Spine (Phila Pa 1976) 1993;18(16):2471–2479. doi: 10.1097/00007632-199312000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Chen WJ, Lai PL, Niu CC, Chen LH, Fu TS, Wong CB. Surgical treatment of adjacent instability after lumbar spine fusion. Spine (Phila Pa 1976) 2001;26(22):E519–E524. doi: 10.1097/00007632-200111150-00024. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe K, Lenke LG, Bridwell KH, Kim YJ, Koester L, Hensley M. Proximal junctional vertebral fracture in adults after spinal deformity surgery using pedicle screw constructs: analysis of morphological features. Spine (Phila Pa 1976) 2010;35(2):138–145. doi: 10.1097/BRS.0b013e3181c8f35d. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida M, Shima K, Taniguchi Y, Tamaki T, Tanaka T. Hypertrophied ligamentum flavum in lumbar spinal canal stenosis. Pathogenesis and morphologic and immunohistochemical observation. Spine (Phila Pa 1976) 1992;17(11):1353–1360. doi: 10.1097/00007632-199211000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Kaneyama S, Doita M, Nishida K, Shimomura T, Maeno K, Tamura Y, Kurosaka M, Yonenobu K. Thoracic myelopathy due to ossification of the yellow ligament in young baseball pitchers. J Spinal Disord Tech. 2008;21(1):68–71. doi: 10.1097/BSD.0b013e31811dfc2d. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Lu XH, Yang LL, Chen DY. Ossification of ligamentum flavum related to thoracic kyphosis after tuberculosis: case report and review of the literature. Spine (Phila Pa 1976) 2009;34(1):E41–E44. doi: 10.1097/BRS.0b013e318189594f. [DOI] [PubMed] [Google Scholar]
- 17.Aizawa T, Sato T, Sasaki H, Kusakabe T, Morozumi N, Kokubun S. Thoracic myelopathy caused by ossification of the ligamentum flavum: clinical features and surgical results in the Japanese population. J Neurosurg Spine. 2006;5(6):514–519. doi: 10.3171/spi.2006.5.6.514. [DOI] [PubMed] [Google Scholar]
- 18.Lee CK. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine (Phila Pa 1976) 1988;13(3):375–377. doi: 10.1097/00007632-198803000-00029. [DOI] [PubMed] [Google Scholar]
- 19.Cheh G, Bridwell KH, Lenke LG, Buchowski JM, Daubs MD, Kim Y, Baldus C. Adjacent segment disease followinglumbar/thoracolumbar fusion with pedicle screw instrumentation: a minimum 5-year follow-up. Spine (Phila Pa 1976) 2007;32(20):2253–2257. doi: 10.1097/BRS.0b013e31814b2d8e. [DOI] [PubMed] [Google Scholar]
- 20.Oxland TR, Lin RM, Panjabi MM. Three-dimensional mechanical properties of the thoracolumbar junction. J Orthop Res. 1992;10(4):573–580. doi: 10.1002/jor.1100100412. [DOI] [PubMed] [Google Scholar]
- 21.Smith DE, Godersky JC. Thoracic spondylosis: an unusual cause of myelopathy. Neurosurgery. 1987;20(4):589–593. doi: 10.1227/00006123-198704000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Kim SS, Suk SI. Incidence of proximal adjacent failure in adult lumbar deformity correction based on proximal fusion level. Asian Spine J. 2007;1(1):19–26. doi: 10.4184/asj.2007.1.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishiura I, Isozumi T, Nishihara K, Handa H, Koyama T. Surgical approach to ossification of the thoracic yellow ligament. Surg Neurol. 1999;51(4):368–372. doi: 10.1016/S0090-3019(98)00051-2. [DOI] [PubMed] [Google Scholar]
- 24.Li KK, Chung OM, Chang YP, So YC. Myelopathy caused by ossification of ligamentum flavum. Spine (Phila Pa 1976) 2002;27(12):E308–E312. doi: 10.1097/00007632-200206150-00026. [DOI] [PubMed] [Google Scholar]
- 25.Okada K, Oka S, Tohge K, Ono K, Yonenobu K, Hosoya T. Thoracic myelopathy caused by ossification of the ligamentum flavumclinicopathologic study and surgical treatment. Spine (Phila Pa 1976) 1991;16(3):280–287. doi: 10.1097/00007632-199103000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Yonenobu K, Ebara S, Fujiwara K, Yamashita K, Ono K, Yamamoto T, Harada N, Ogino H, Ojima S. Thoracic myelopathy secondary to ossification of the spinal ligament. J Neurosurg. 1987;66(4):511–518. doi: 10.3171/jns.1987.66.4.0511. [DOI] [PubMed] [Google Scholar]
- 27.He S, Hussain N, Li S, Hou T. Clinical and prognostic analysis of ossified ligamentum flavum in a Chinese population. J Neurosurg Spine. 2005;3(5):348–354. doi: 10.3171/spi.2005.3.5.0348. [DOI] [PubMed] [Google Scholar]
- 28.Fong SY, Wong HK. Thoracic myelopathy secondary to ligamentum flavum ossification. Ann Acad Med Singap. 2004;33(3):340–346. [PubMed] [Google Scholar]
- 29.Miyakoshi N, Shimada Y, Suzuki T, Hongo M, Kasukawa Y, Okada K, Itoi E. Factors related to long-term outcome after decompressive surgery for ossification of the ligamentum flavum of the thoracic spine. J Neurosurg. 2003;99(3 Suppl):251–256. doi: 10.3171/spi.2003.99.3.0251. [DOI] [PubMed] [Google Scholar]
- 30.Shiokawa K, Hanakita J, Suwa H, Saiki M, Oda M, Kajiwara M. Clinical analysis and prognostic study of ossified ligamentum flavum of the thoracic spine. J Neurosurg. 2001;94(2 Suppl):221–226. doi: 10.3171/spi.2001.94.2.0221. [DOI] [PubMed] [Google Scholar]
- 31.Sanghvi AV, Chhabra HS, Mascarenhas AA, Mittal VK, Sangondimath GM. Thoracic myelopathy due to ossification of ligamentum flavum: a retrospective analysis of predictors of surgical outcome and factors affecting preoperative neurological status. Eur Spine J. 2011;20(2):205–215. doi: 10.1007/s00586-010-1423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]